Introduction
There is a need for an updated checklist of Laurencia (Rhodophyta, Ceramiales) in the Atlantic Ocean that accounts for recent morphological and phylogenetic studies that have modified our current knowledge of the group. For instance, the “Laurencia complex” has replaced the former concept of Laurencia sensu lato. The genus Laurencia sensu stricto (s.s.) (Garbary & Harper, 1998; Nam, 2006; Gil-Rodríguez et al., 2012), and the following seven genera belonging to the complex (mentioned above):Osmundea Stackhouse, Chondrophycus (Tokida et Saito) Garbary et J. T. Harper, Palisada K. W. Nam, Yuzurua (K.W. Nam) Martin-Lescanne, Laurenciella Cassano, Gil-Rodríguez, Sentíes, Díaz-Larrea, M. C. Oliveira et M. T. Fujii, Coronaphycus Metti and Ohelopapa F. Rousseau, Martin-Lescanne, Payri et L. Le Gall. These genera differ in the number of pericentral cells cut off by the vegetative axial segments, the origin of spermatangial branches, and the origin of tetrasporangia from determined cells (Saito, 1967; Nam et al., 1994; Garbary & Harper, 1998; Nam, 1999, 2006, 2007). Molecular phylogenetic data also support distinction among them (Martin-Lescanne et al., 2010; Cassano et al., 2012a; Metti et al., 2015; Rousseau et al., 2017).
Laurencia comprises 130 taxonomically recognized species worldwide (Guiry & Guiry, 2017). In particular, 18 species of the genus have been recorded for the Western Atlantic (from North Carolina to Brazil) and 21 for the Eastern Atlantic (Europe and Africa). These represent 23% of the species richness worldwide. Phylogenetic studies of some Laurencia species in the Atlantic have shown the artificiality of the wide ranges of distribution of some species, such as L. microcladia and L. obtusa (Cassano et al., 2012a) that have been eliminated from the Bra zilian flora.
The area of endemism is defined as the sympatric congruence between two or more endemic species, based on the fact that the se species share a common spatial history (Platnick, 1991; Morrone, 2007). Other areas that can be documented are the secondary areas, which have only one endemic species or the so-called relic species that are important because these areas may be later isolations where spe cies radiation has not yet occurred (Ippi & Flores, 2001; Vargas et al., 2008). The PAE (Parsimony Areas of Endemism) analysis is a method that allows us to identify areas of endemism from area cladograms. In a manner similar to phylogenetic systematics, at least two synapo morphic characters (restricted species) will define areas of endemism (Morrone, 2013).
One important outcome of this taxonomic updating is to provide a better understanding of the biogeographical patterns. With the present updated checklist, we will discuss endemism and the widespread geo graphic distribution of Laurencia species in the Atlantic.
Materials and methods
Records for Laurencia for the Atlantic Ocean were obtained from pri mary sources (these sources are indicated for each species in Results section). We complemented information with the online database Algae Base (Guiry & Guiry, 2017). Parsimony Analysis of Endemicity (PAE; Rosen, 1988) was used in a biogeographical analysis. For this analysis, an r x c binary matrix (presence and absence) was constructed, where r (rows) displays the 17 Atlantic ecoregions proposed by Spalding et al. (2007) (Figure 1) and c (columns) contains 24 of the 30 species recorded for the Atlantic Ocean. Because they could be found practically all over the Atlantic, we did not include L. brongniartii J. Agardh, L. caducira mulosa Masuda et S. Kawaguchi, L. dendroidea J. Agardh, L. intricata J. V. Lamouroux, L. microcladia Kützing, and L. obtusa (Hudson) J.V. Lamouroux. In addition, the Macaronesian Islands were excluded from the analysis because they are considered to be a transition zone with species from other areas of Atlantic (see Haroun & Prud’homme van Reine, 1993; Tuya & Haroun, 2009), and may affect our distributional patterns (Medina, 2007). Parsimony analysis was done through a heu ristic search using TBR+TBR routines with the NONA program, using the WinClada software (Nixon, 1999). A strict consensus tree was built from the most parsimonious trees.

Figure 1 Marine ecoregions used in the PAE analysis, modified from Spalding et al., (2007): Adriatic Sea (AdS), Aghulas Bank (AghB), Bermuda (Ber), Eastern Brazil (EBr), Eastern Caribbean (Eca), Greater Antilles (GA), Gulf of Guinea Central (GGC), Gulf of Guinea Upwelling (GGU), Gulf of Guinea Western (GGW), Levantine Sea (LevS), Northeastern Brazil (NEBr), Southern Caribbean (Sca), South European Atlantic Shelf (SEAS), Southeastern Brazil (SEBr), St. Helena and Ascension Islands (SHAI), Western Caribbean (WCa), and Western Mediterranean (WMe).
Six “species inquirenda” (L. alsidiidormis Zanardini ex Fraudenfeld, L. alsidioides P. L. Crouan et H.M. Crouan, L. botryocephala Kützing, L. canariensis Montagne ex Kützing, L. moriformis Kützing and L. trifaria Kützing) did not provide information about their geographical distri bution and were recorded only once by their respective authors. Null or limited herbarium material prevented us from being totally certain about these taxa. Categories of areas (patterns) were defined: Those containing an endemic species (Secondary Area); those with congru ence in geographical distribution of two or more endemic species (Area of Endemism), that is, clades defined by two or more synapomorphies; and widespread species (amphi-Atlantic Areas). In addition, we defined areas containing two or more endemic species without total congru ence in the geographic distribution of these, known as areas of Partial Congruence.
Results
Checklist of Laurencia species
Laurencia aldingensis Saito et Womersley
Type locality: Aldinga Reef, South Australia.
Distribution: Tropical Southwestern Atlantic: Brazil: Espírito Santo (Carvalho et al., 2006; Fujii et al., 2011) and Rio de Janeiro (Fujii et al., 2011).
Laurencia brachyclados Pilger
Type locality: Annobon Island, Equatorial Guinea, West Africa.
Distribution: Gulf of Guinea: Equatorial Guinea: Annobon Island (John et al., 2004).
St. Helena and Ascension Islands: Ascension Island (John et al., 2004).
Laurencia brongniartii J.Agardh
Type locality: Martinique, West Indies.
Remarks: Dizerbo & Herpe (2007) and Stokes et al. (2004) considered this an introduced species in southeast France and Ireland, respectively.
Distribution: Gulf of Guinea:Ghana (John et al., 2004).
Luisitanian: Canary Islands: El Hierro and Lanzarote (uncertain record) (Gil-Rodríguez et al., 2012). France: Brest (Dizerbo & Herpe, 2007).
Tropical Northwestern Atlantic: Costa Rica: Punta Coclas (Fernández & Alvarado, 2004). Cuba: Havana (Suárez, 2005). Martinique (Womersley, 2003). Mexico: Quintana Roo (Sentíes & Fujii, 2002).
Northern European Seas: Ireland (Stokes et al., 2004).
Laurencia caduciramulosa Masuda et Kawaguchi
Type locality: Hon Tre Island, Tien Hai Islands, Hatien, Kien Giang Province, Vietnam.
Distribution: Lusitanian: Canary Islands: Tenerife (Gil-Rodrí guez et al., 2012).
Mediterranean Sea: France: Mediterranean coast (Klein & Ver laque, 2005). Greece: Zakynthos Island (Tsikira & Haritonidis, 2005). Italy: Linosa Island (Furnari et al., 2001; Serio et al., 2006).
St. Helena and Ascension Islands: Ascension Island (Tsiamis et al., 2014a).
Tropical Northwestern Atlantic: Cuba: Havana (Sentíes et al., 2010) and Rincón del Guanabo (Suárez et al., 2015). USA: Florida (Collado-Vides et al., 2014).
Tropical Southwestern Atlantic: Brazil: Bahia (Torrano-Silva & Oliveira, 2013) and Rio de Janeiro (Cassano et al., 2006).
Laurencia caraibica P.C. Silva
Type Locality: Abraham Bay, Mariguana (Mayaguana), Baha mas.
Distribution: Tropical Northwestern Atlantic: Bahamas: Abraham Bay (Schneider et al., 2010). Belize: Carry Bow Cay (Norris & Bucher, 1982) and Pelican Cays (Littler & Littler, 1997). Cuba: Bucunayagua, Guanahacabibes and Havana (Suarez, 2005). Jamaica (Taylor, 1960). Lesser Antilles (Taylor, 1969). México: Campeche, Quintana Roo, Tamaulipas, and Veracruz (Ortega et al., 2001; Sentíes & Fujii, 2002). Venezuela (Ganesan, 1990).
Tropical Southwestern Atlantic: Brazil: Rio Grande do Norte (Villaça et al., 2010) and Bahia (Creed et al., 2010).
Warm Temperate Northwest Atlantic: Bermuda: Bermuda Island, Gibbet Island, and Somerset Island (Schneider et al., 2010).
Laurencia catarinensis Cordeiro-Marino et M.T. Fujii
Type locality: Mole Beach, Santa Catarina Island, Brazil.
Distribution: Lusitanian: Canary Islands: El Hierro, Fuerteventura, Gran Canaria, La Gomera, La Palma, Lanzarote and Tenerife (Machín-Sánchez et al., 2012).
WarmTemperate Southwestern Atlantic: Brazil: Santa Catarina (Fujii & Sentíes, 2005; Machín-Sánchez et al., 2012) and São Paulo (Fujii et al., 2006).
Tropical Southwestern Atlantic: Brazil: Rio Grande do Norte (Fujii & Sentíes, 2005; Machín-Sánchez et al., 2012), Espírito Santo (Machín-Sánchez et al., 2012) and Rio de Janeiro (Fujii & Sentíes, 2005).
Laurencia chondrioides Børgesen
Type locality: Saint John Island, U. S. Virgin Islands.
Remarks: Only one record outside the Atlantic (Philippines) by Silva et al., (1987). In the Mediterranean Sea (France and Israel) it is regarded as an invasive species (Klein & Verlaque 2011; Hoffman et al., 2014).
Distribution: Lusitanian: Canary Islands: Lanzarote (Gil-Rodríguez et al., 2012).
Mediterranean Sea: France: Hyères and Porquerolles Island (Klein & Verlaque, 2011). Greece: Zakynthos Island (Tsirika & Haritonidis, 2005). Israel: Achziv Beach, Achziv Reserve, Hai fa-Bat Galim Beach, Rosh Hanika and Shavei Zion (Hoffman et al., 2014). Italy: Aeolian Islands, Lachea Island (Gómez-Garreta et al., 2001), Linosa Island (Serio et al., 2006), Tremiti Islands (Gómez-Garreta et al., 2001) and Tuscany (Rindi et al., 2002). Spain: Balearic Islands and Columbretes Island (Gómez-Garreta et al., 2001).
Tropical Northwestern Atlantic: Cuba: Canarreos Archipelago and Alonzo Cay (Suárez, 2005). Mexico: Quintana Roo (Ortega et al., 2001). Virgin Islands: St John (Klein & Verlaque, 2011).
Laurencia coronopus J. Agardh
Type locality: “in mare Nigro ad littusTauriae” (Black Sea).
Remarks: Gómez-Garreta et al., (2001) suggested that a revision using recent taxonomic criteria should be made to corroborate if this species actually belongs to Laurencia or whether it belongs to Chondrophycus or Osmundea.
Distribution: Black Sea: Bulgaria: Athopol (Gómez-Garreta et al., 2001; Kamenarska et al., 2006; (Kamenarska et al., 2009). Romania Littoral: (Caraus, 2012; Gómez-Garreta et al., 2001).
Mediterranean Sea: France: Herault (Gómez-Garreta et al., 2001).
Laurencia corymbosa J. Agardh
Type locality: Cape of Good Hope, South Africa.
Distribution: South Africa: Cape of Good Hope (Silva et al., 1996).
Laurencia decumbens Kützing
Type locality: New Caledonia.
Distribution: Tropical Northwestern Atlantic: Bermuda: Bermuda Island (Schneider & Lane, 2005). Venezuela (Ganesan, 1990).
Tropical Southwestern Atlantic: Brazil: Atol das Rocas (Villaça et al., 2010).
Laurencia dendroidea J.Agardh
Type locality: Brazil.
Remarks: According to Cassano et al. (2012b), using molecular and morphological characters, the taxa that have been identified as L. filiformis, L. majuscula, and L. obtusa in Brazil correspond to L. dendroidea. Similarly, L. majuscula in the Canary Islands corresponds to L. dendroidea.
Distribution: Gulf of Guinea: Cameroon, Gabon, and Ghana (John et al., 2004).
Lusitanian: Canary Islands: Fuerteventura, Gran Canaria, La Palma, Lanzarote, and Tenerife (Gil-Rodríguez et al., 2012). Madeira Archipelago: Madeira (John et al., 2004). Mediterra nean Sea: France [uncertain record (Gómez-Garreta et al., 2001)]. Greece: Zakynthos Island (Tsikira & Haritonidis, 2005). Italy: Lecce and Linosa Island (Gómez-Garreta et al., 2001; Serio et al., 2006).
Tropical Northwestern Atlantic: Barbados (Taylor, 1960, Wynne et al., 2014). Bermuda (Taylor, 1960). Costa Rica (Taylor, 1960). Jamaica (Taylor, 1960). Lesser Antilles (Taylor, 1960). Nether lands Antilles, Trinidad and Tobago, and Venezuela (Taylor, 1960).
Tropical Southwestern Atlantic: Brazil: Bahia (Oliveira et al., 2013). Espírito Santo (Oliveira et al., 2013; Fujii et al., 2006) and Rio de Janeiro (Cassano et al., 2012b, Oliveira et al., 2013)
West African Transition Zone: Cape Verde Islands and Salvage Islands (John et al., 2004), Gambia (John et al., 2004). Senegal: North of Senegal (John et al., 2004).
Warm Temperate Northwest Atlantic: USA: North Carolina (Taylor, 1960).
Warm Temperate Southwestern Atlantic: Brazil: Santa Catari na (Creed et al., 2010) and São Paulo (Cassano et al., 2012b).
Laurencia epiphylla F. Boisset et J. C. Lino
Type locality: Alicante: La Granadella, Jávea, the Mediterra nean coast of Spain.
Distribution: Mediterranean Sea: Spain: Alicante (Gómez-Ga rreta et al., 2001).
Laurencia flexuosa Kützing
Type locality: “Ad Caput Bonae Spei”, South Africa.
Distribution: South Africa: Cape Town (Stegenga et al., 1997).
West African Transition: Mauritania [uncertain record (John et al., 2004)].
Laurencia foldatsii N. Rodríguez de Rios
Type locality: Taguao, Distrito Federal, Venezuela
Distribution: Tropical Northwestern Atlantic: Venezuela (Gane san, 1990).
Laurencia galtsoffii M. Howe
Type locality: Pearl and Hermes Reef, Hawaiian Archipelago.
Distribution: Gulf of Guinea: Gabon, Ghana, and Liberia (John et al., 2004).
West African Transition: Cape Verde Islands (John et al., 2004).
Laurencia griseaviolacea M.J. Wynne
Type locality: Clovelly, Cape Peninsula, South Africa.
Distribution: South Africa: Cape Town (Stegenga et al., 1997, as Laurencia peninsularis H. Stegenga, J. J. Bolton et R. J. Anderson not L. peninsularis Taylor).
Laurencia intricata J.V. Lamouroux
Type locality: Antilles.
Remarks: According to Machín-Sánchez et al. (2012), records of L. intricata from the Canary Islands correspond to L. catarinensis. Therefore, we believe that a taxonomic study of this species from the rest of Macaronesian Archipelago and Western Africa is needed to verify the identity of L. intricata from these areas.
Distribution: Gulf of Guinea: Sao Tomé and Principe, and Sierra Leone (John et al., 2004).
Mediterranean Sea: Greece: Messolonghi (Christia et al., 2011) and Zakynthos Island (Tsikira & Haritonidis, 2005). Italy. Lino sa Island (Furnari et al., 2001; Gómez-Garreta et al., 2001). Libya: Cyrenaica (Gómez-Garreta et al., 2001).
Tropical Northwestern Atlantic: Bahamas (Taylor, 1960). Belize: Carrie Bow Cays (Norris & Bucher, 1982) and Pelican Cays (Littler & Littler, 1997). Bermuda: (Taylor, 1960). Cayman Island and Costa Rica (Taylor, 1960). Cuba: Canarreos Archipelago, Sabana-Camagüey Archipelago, Nuevitas Bay, Bocas de Alonzo, Coco, Gulf of Batabanó, Guanacahabites, Guardalavaca, Havana, Matanzas, and Villa Clara (Suárez, 2005; Gil-Rodriguez et al., 2010). Hispaniola Island and Jamaica (Ta ylor, 1960). Martinique (Rodriguez-Prieto et al.,1999). Mexico: Campeche (Fujii et al., 2006) and Quintana Roo (Sentíes & Fujii, 2002, Cassano et al., 2010; Gil-Rodríguez et al., 2010). Panama (Taylor, 1960). Trinidad and Tobago (Duncan & Lee-Lum, 2006). USA: Florida (Collado-Vides et al., 2011; Fujii et al., 2006). Venezuela: Miranda State (Wynne, 2017). Virgen Islands: St. Croix (Taylor, 1960).
West African Transition: Cape Verde Islands [uncertain record (John et al., 2004)] and Senegal (John et al., 2004).
Warm Temperate Northwest Atlantic: USA: Texas (Wynne, 2008).
Laurencia laurahuertana Mateo-Cid, Mendoza-González, Sentíes et Díaz-Larrea
Type locality: Punta Herrero, Quintana Roo. México
Distribution: Caribbean Sea and Gulf of Mexico. Mexico: Quin tana Roo (Mateo-Cid et al., 2014).
Laurencia microcladia Kützing
Type locality: West Indies
Remarks: Records of L. microcladia from Brazil containa mis applied name, which correspond to L. dendroideaCassano et al. (2012a).
Distribution: Lusitania: Azores Islands: Santa María Island and Pico (Tittley et al., 2009). Canary Islands: El Hierro, Fuerteven tura, Gran Canaria, La Palma, Lanzarote, and Tenerife (Gil-Rodríguez et al., 2012). Madeira Archipelago: Porto Santo (Neto et al., 2001). Salvages Islands (John et al., 2004).
Mediterranean Sea: Algeria: Alger (Gómez-Garreta et al., 2001; Gil-Rodríguez et al., 2012). Cyprus: Akamas, GaziMağusa, Girne, Karpasia, Liopetri, and Salamis (Taskin et al., 2013; Tsiamiset al., 2014b). Egypt: El Dabaa (Gómez-Garreta et al., 2001). France: Corsica (Gómez-Garreta et al., 2001) and Hérault (Verlaque, 2001). Greece: Zakynthos Island (Tsikira & Haritonidis, 2005). Italy: Linosa Island (Serio et al., 2006), Si cilia (Gómez-Garreta et al., 2001), Tuscan Archipelago (Rindi et al., 2002), Italian Adriatic Sea (Gómez-Garreta et al., 2001), Gulfof Taranto (Gómez-Garreta et al., 2001), Cherad iIslands, and Sardinia (Gómez-Garreta et al., 2001). Malta: Gozo Island and Malta Island (Comarci et al., 1997). Morocco: Alhucemas, Cabo de Agua, Karia Arkemanne, Muelle Colorado, Punta Ne gri, Punta de Rostrogordo, and Punta de Sabinilla (Gómez-Garreta et al., 2001). Spain: Almería (Soto & Conde, 1989), Balearic Islands (Gómez-Garreta et al., 2001), Catalunya (Gómez-Garreta et al., 2001), and Murcia (Gil-Rodríguez et al., 2012). Turkey: Akdeniz, Ízmir Bay, Gulf of Gökova, Karadeiz and Mersin (Taskin et al., 2008).
Tropical Northwestern Atlantic: Bahamas (Taylor, 1960). Belize: Carrie Bow Cays (Norris & Bucher, 1982) and Pelican Cays (Littler & Littler, 1997). Bermuda (Taylor, 1960). Caicos Islands, Cayman Islands, Costa Rica, Jamaica, Lesser Antilles, Netherlands Antilles, and Panama (Taylor, 1960). Cuba: Gulf of Batabanó (Suárez, 2005). Puerto Rico: Arecibo and Santiago Cay (Gil-Rodríguez et al., 2012). Trinidad and Tobago (Duncan & Lum-Lee, 2006). USA: Florida (Taylor, 1960). Venezuela: Aves Island (Taylor, 1960) and Cumana (Gil-Rodríguez et al., 2012). Virgin Islands (Taylor, 1960).
West African Transition: Cape Verde Islands: Ilheu Branco and San Nicolau (John et al., 2004, Gil-Rodríguez et al., 2012). Mauritania (John et al., 2004). Senegal: North of Senegal (John et al., 2004).
Laurencia minuscula Schnetter
Type locality: Puerto López (Alta Guajira), Guajira Department, Colombia.
Distribution: Tropical Northwestern Atlantic: Colombia: Guajira (Schnetter, 1976). Cuba: Havana (Sentíes et al., 2010).
Laurencia minuta Vandermeulen, Garbary et Guiry
Type locality: Eilat, Israel.
Distribution: Lusitanian: Canary Islands: Tenerife (“taxa inqui renda”Haroun et al., 2002; John et al., 2004). Spain: Galicia (Bárbara et al., 2005).
Mediterranean Sea: Cyprus: Girne (Taskin et al., 2013). Spain: Alicante (Gómez-Garreta et al., 2001). Greece: Zakynthos Is land (Tsikira & Haritonidis, 2005). Italy: Apulia, Gulf Taranto, NorwesternItaly, and Sicily (Gómez-Garreta et al., 2001), Li nosa Island (Serio et al., 2006), and Tuscany (Rindi et al., 2002). Malta: Malta Island (Gómez-Garreta et al., 2001).
Laurencia natalensis Kylin
Type locality: Isipingo Beach, near Durban, South Africa
Distribution: South Africa: Pearly Beach to Agulhas (Stegenga et al., 1997)
Laurencia nidifica J. Agardh
Type locality: Hawaiian Islands
Distribution: Gulf of Guinea: Ivory Coast,Ghana, and Liberia (John et al., 2004).
Lusitania: Madeira Archipelago: Deserta Grande [uncertain re cord (Neto et al., 2001)].
St. Helena and Ascension Islands: St. Helena (John et al., 2004).
West African Transition: Cape Verde Islands (John et al., 2004).
Laurencia obtusa (Hudson) J. V. Lamouroux
Type locality: England.
Remarks: Cassano et al. (2012b) confirmed through molecu lar and morphological characters that records of L. obtusa from Brazil correspond to L. dendroidea.
Distribution: Black Sea: Bulgaria: Ahtopol area (Dimitrova-Konaklieva, 1981). Romania: Romanian Littoral (Caraus, 2012). Turkey: Antalya, Dikili Gelibolu, Gokçeada Island, Izmir Bay, Kirklareli, Korfezi, Mersin, and Zonguldak (Gómez-Garreta et al., 2001; Taskin et al., 2008).
Gulf of Guinea: Cameroon. Equatorial Guinean: Annobon Island. Ghana. Sao Tome and Principe. Sierra Leone (John et al., 2004).
Lusitania: Azores Islands: Faial, Pico, Sao Miguel, Terceira, and Santa Maria (Neto, 1994; Tittley & Neto, 1994). Canary Islands: El Hierro, Fuerteaventura, Gomera, Gran Canaria, La Palma, Lanzarote Island, and Tenerife (John et al., 2004; Gil-Rodríguez et al., 2012). France: Britanny (Feldmann,1954), Guernsey (Sentíes & Fujii, 2002), Herault (Ben Maizet al., 1988), and Normandia (Dixon, 1961; Dizerbo & Herpe, 2007). Madeira Archipielago: Deserta Grande, Ilhéu de Fora, Madeira, Porto Santo, Selvagem Grande, and Selvagem Pequena (John et al., 2004). Salvage Islands (John et al., 2004). Mauritania (John et al., 2004). Portugal: Beira Litoral, Douro Litoral, and Minho (Araujo et al, 2009). Spain: Asturias (Cires-Rodríguez & Cuesta-Moliner, 2010), Basquecoast (Gorostiaga et al., 2004), Cantabria (Martínez-Gil et al., 2007), Galicia (Bárbara et al., 2005; Peña & Bárbara, 2008), and Vigo (Hamel, 1928).
Mediterranean Sea: Algeria: Alger, Annaba, Bab El Oued, Bo loghine, El Marsa, Rais Hamidou, Sidi Fredj, Southwest of Cap Bordj El Bahri, and Tipaza (Gómez-Garreta et al., 2001). Croatia: Istria Coast (Munda, 1979). Cyprus: Dip Karpaz, Far magusta, Gazi Mağusa, Girne, Karpasia, Kumyali, Koruçaim, Kyrenia, Liopetri, Salamis, and Yeşilirmak (Taskin et al., 2013; Tsiamis et al., 2014b). Egypt: Alexandria (Gómez-Garreta et al., 2001). France: Corsica (Gómez-Garreta et al., 2001; Sales & Ballesteros, 2010), Hyéres (Augier et al., 1971), Pyrenees Orientales (Gómez-Garreta et al., 2001), and Var (Coppejans, 1972). Greece: Ionan Islands (Tsikira & Haritonidis, 2005), Kleisova (Gómez-Garreta et al., 2001; Christia et al., 2011), Rhodos Island (Diapoulis et al., 1986), and Sporades du Nord (Diannelidis, 1953). Israel: Habonim (Gómez-Garreta et al., 2001). Italy: Adriatic Sea (Furnari et al., 1999; Gómez-Garreta et al., 2001), Gulf of Taranto (Cecere et al., 1996), Napoli (Cinelli, 1971; Feoli & Bressan, 1972), Salerno (Edwards et al., 1975), Sicilia (Serio et al., 2006; Gómez-Garreta et al., 2001), Tuscany (Rindi et al., 2002), and Sardinia (Furnari et al., 2003). Libya: Bengazi-Sabri, Cyrenaica, Derna, and Tripoli (Gómez-Garreta et al., 2001). Malta: Gozo Island (Cormaci et al., 1997). Morocco: Cabo de Agua, Cabo Quilates, Cal Iris, Cala Bonita, Cala Charranes, Cala Viñas, Cazaza, Chafarinas Islands, Karia Arkemanne, Mar Chica, Playa del Quemado, Punta Negri, Punta de Rostrogordo, and Sammar (Gómez-Garreta et al., 2001). Spain: Andalusia (Conde et al., 1996; Conde & Flores-Maya, 2000), Balearic Islands (Gómez-Garreta et al., 2001), Catalunya (Ballesteros, 1981; Rodríguez-Prieto & Polo-Albertí, 1988, 1998), Murcia (Pérez-Ruzafa & Honrubia, 1984; Pérez-Ruzafa, 1990), and Valencia (Barce lo & Seoane, 1982). Tunisia: Bahiret, Bechateur, Bizerte, Cap Blanc, Cap Farina, Cap Serrat, Cathage, Djerba, El Bibane, Gabès, Gammarth, Ghar El Khebir, Gulf of Gabès, Iles Cani, Kelibia, Kerkennah, Korba, Korbous, La Goulette, La Marsa, Mer de Bou Grara, Monastir, Le Galiton, Raf Raf, Raouad, Ras el Fartass, Salambô, Sidi Bou Said, Sfax, Sídi Raïs, Tabarka, Zarzis, and Zembra (Gómez-Garreta et al., 2001).
Northern European Seas: England: Sussex and Devon (Lipkin & Silva, 2002). Ireland: Antrim (Morton, 1994), Clare (De Valéra et al., 1979), Derry (Morton, 1994), Donegal (Morton, 1994), Down (Morton, 1994), Cork, Dublin, Galway, Kerry, Leitrim, Mayo, and Wexford (Guiry, 1978). Sweden (Athanasiadis, 1996).
Tropical Northwestern Atlantic: Bahamas: Berry Island (Sentíes & Fujii, 2002). Barbados (Wynne et al., 2014). Belize: Carrie Bow Cay (Norris & Bucher, 1982) and Pelican Cays (Littler & Littler, 1997). Bermuda: St. Georges Island (Sentíes & Fujii, 2002; Taylor, 1960). Caicos Island, Cayman Islands, and Colombia (Taylor, 1960). Cuba: Havana City (Sentíes & Fujii, 2002), Camagüey, Juventud Island, and Matanzas, and Villa Clara (Suárez, 2005). Dominican Republic: Santo Domingo (Sentíes & Fujii, 2002). Guadeloupe: Pointe de la Verdure (Fujii et al., 2006). Jamaica (Taylor, 1960). Martinique: Pointe des Salines and Ste. Anne (Rodríguez-Prieto & Michanek, 1999). Mexico: Campeche, Quintana Roo, and Tamaulipas (Ortega et al., 2001; Sentíes & Fujii, 2002), Veracruz and Yucatán (Ortega et al., 2001). Netherlands Antilles: Bonaire and Curaçao (Sentíes & Fujii, 2002). Panama: Galeta Point (Sentíes & Fujii, 2002). Puer to Rico: Guanica and Jaobos (Sentíes & Fujii, 2002). Trinidad and Tobago: Tobago (Taylor, 1960; Sentíes & Fujii, 2002)). USA: Florida (Littler et al., 2008). Venezuela: Aves Island, Cabagua, Margarita Island, and Pelona Island (Taylor, 1960; Sentíes & Fujii, 2002); Fujii et al., 2006). Virgin Islands (Taylor, 1960).
Warm Temperature Northwestern Atlantic: USA: Texas (Wynne, 2008).
West African Transition: Cape Verde Archipielago (John et al., 2004). Gambia (John et al., 2004). Senegal: North Senegal (John et al., 2004).
Laurencia oliveirana Yoneshigue
Type locality: Ponta da Cabeça, Cabo Frio, Rio de Janeiro State, Brazil.
Distribution: Tropical Southwestern Atlantic: Brazil: Rio de Janeiro (Fujii et al., 2012) and Bahia ((Fujii & Sentíes, 2005).
Warm Temperate Southwestern Atlantic: Brazil: Rio Grande do Sul and São Paulo (Fujii & Sentíes, 2005).
Laurencia pyramidalis Bory ex Kützing
Type locality: Granville, Normandy, France.
Distribution: Lusitania: Azores: Pico, Santa Maria, and Sao Miguel (Machín-Sánchez et al., 2014). Canary Islands: Fuerteventura, La Gomera, Lanzarote, and Tenerife (Machín-Sánchez et al., 2014). France: Finistère (Bouxin & Dizerbo, 1971) and Normandy (Machín-Sánchez et al., 2014). Madeira Archipelago: Ponta de São Jorge-Casi, Porto Moniz-Piscinas, and Seixal-Praia da Laje (Machín-Sánchez et al., 2014). Portugal: Minho (Araujo et al., 2009). Spain: Asturias (Cires-Rodríguez & Cuesta-Moliner, 2010; Díaz et al., 2008), BasqueCoast (Go rostiaga et al., 2004), Cantabria (Martínez-Gil et al., 2007), Galicia (Bárbara et al., 2005) and Sisargas Island (Veiga et al., 1998).
Mediterranean Sea: Cyprus: Dip Karpaz and Gazi Mağusa (Taskin et al.,2013). Italy: Adriatic Sea (Furnari et al., 1999) and Sardinia Island (Serio et al., 2004). Spain: Andalusia (Conde et al., 1996), Catalunya (Ballesteros, 1981), and Murcia (Pérez-Ruzafa & Honrubia, 1984).
Nothern European Seas: Great Britain and Ireland (Hardy & Guiry, 2003).
Laurencia tenera C. K. Tseng
Type locality: Shek-O, Hong Kong.
Distribution: Gulf of Guinea: Ivory coast, Ghana, Liberia, Sierra Leone, and Togo (John et al., 2004).
West African Transition: Cape Verde Islands, Gambia, Mauritania, and Senegal (John et al., 2004).
St Helena and Ascension Islands: St. Helena (John et al., 2004).
Laurencia translucida M. T. Fujii et Cordeiro-Marino
Type locality: Padres beach, Aracruz, Espírito Santo State, Brazil.
Distribution: Warm Temperate Southwesern Atlantic: Brazil: São Paulo (Creed et al., 2010).
Tropical Southwestern Atlantic: Brazil: Bahia, Ceará, and Pernambuco Espírito Santo (Fujii et al., 2006), Rio de Janeiro (Creed et al., 2010).
Laurencia venusta Yamada
Type locality: Koshiki-jima, Kagoshima Prefecture and Goto-retto, Nagasaki Prefecture, Japan.
Distribution: Tropical Northwestern Atlantic: México: Quintana Roo (Sentíes & Fujii, 2002).
Tropical Southwestern Atlantic: Brazil: Espírito Santo (Fujii & Sentíes, 2005).
Laurencia viridis Gil-Rodríguez et Haroun
Type locality: Canary Isands: Tenerife: Punta Hidalgo-Baja Negra.
Distribution: West African Transition: Cape Verde Islands (John et al., 2004).
Macaronesian Archipelago. Azores: Santa María (Machín-Sánchez et al., 2014). Canary Islands: El Hierro, Fuerteventura, Gran Canaria, La Gomera, La Palma, Lanzarote, and Tenerife (John et al., 2004; Gil-Rodríguez et al., 2012; Machín-Sánchez et al., 2014). Madeira Archipelago: Ilhéu de Fora, Ponta de Sao Jorge, Porto Santo, and Salvagem Pequena (John et al., 2004; Machín-Sánchez et al., 2014).
Species inquirenda
Laurencia canariensis Montagne ex Kützing
Remarks: Gil Rodríguez et al. (2012) suggested that it should be considered an uncertain species. According to John et al. (1994), L. canariensis and L. caespitosa are synonyms of Osmundea hybrida (De Candolle) K. W. Nam.
Type locality: Canary Islands.
PAE Analysis. The area cladogram obtained had the following parameters: Length = 28, Consistency Index=0.85 and Retention Index=0.88. The strict consensus tree of the 3 equally parsimonious trees is presented (Figure 2). Cladogram topology suggests four areas of endemism: South Africa (AghB) with four restricted species, Brazil (EB, NEB, and SEB) with four restricted species, Northeastern Atlantic (WMe, SEAS, LevS, and AdS) with three restricted species, and Gulf of Guinea (SHAI, GGU, CV, GGW, and GGC) with four restricted species (Figure 3). Some of these species, however, are also distributed in the Indo-Pacific (see Table 1). In addition, we have defined three categories of areas: partial congruence (areas with partial congruence between the areas of distribution) composed by GA, ECa, WCa, and SCa (Figure 2), secondary areas (area with an endemic species, Ippi & Flores, 2001) and widespread species (Table 1).
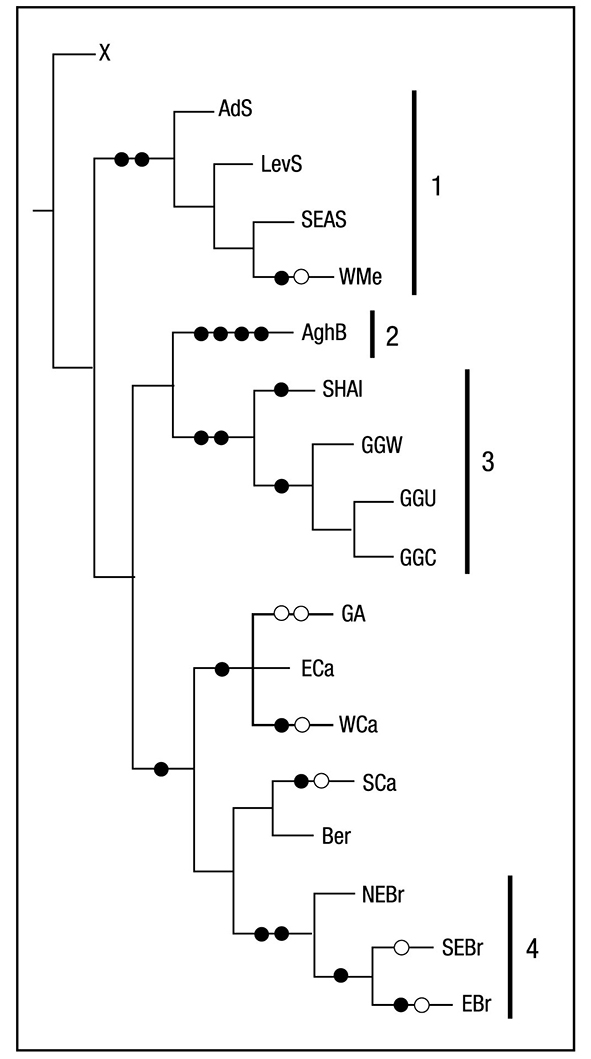
Figure 2 Areas of endemism defined in the PAE analysis. 1) Northwestern At lantic, 2) South Africa, 3) Gulf of Guinea, and 4) Brazil. The black dots represent restricted species or synapomorphies and the white dots represent homoplasies.
Discussion
The nomenclature from genus to species, valid names, and taxonomic synonyms of Laurencia are a fundamental part of the checklist here proposed and a topic of continuing discussion among taxonomists. Checklists allow for the documentation of species distributions, the biodiversity within certain areas, and offer the possibility of using this information to carry out a biogeographical analysis; thus, considering the taxonomic remarks, uncertain records, reports of invasion, and in troduction of species allows us to obtain biogeographical natural pat terns or approximate reality. Taxonomic validity of the records and the recognition of the distribution limits of the species area are essential requirements in biogeographic works (Morrone, 2013). In turn, areas of endemism and secondary areas can be defined, and widespread spe cies identified.
On endemism. Areas of endemism (non-endemic species) are defined as the sympatric distribution congruence of two or more taxa belon ging to a given category (e.g., order, family, genus or species) (Morrone, 2013). However, when we take Laurencia, the Caribbean Sea is defined by partial congruence in the distribution of two or more species (group GA, ECa, WCa, and SCa; see Figure 2). Endemic species inhabit this region, such as L. laurahuertana in the western Caribbean, L. foldatsii in the southern Caribbean, L. chondrioides, and L. minuscula in the Caribbean Sea in general, but their distributional congruence is not total, and the PAE analysis does not define this area for two or more synapormor phies. Furthermore, the Caribbean Sea is the Atlantic biotic area with the highest species richness of Laurencia (13 spp). This biogeographic pattern is also shared by the distributional diversity of several unrelated taxa, such as coastal fishes, mangroves, coral reefs, and seagrasses in the Atlantic Ocean (Tittensor et al., 2010).
According to Tapia-Silva et al. (2015), a mathematical analysis of the geographic distribution of the macroalgal species richness can reveal high diversity spots that, in the case of macroalgae, coincide well with the major distribution of the great marine environments in the area (mangroves, coral reefs and seagrasses): the Mexican Caribbean barrier reef, the Veracruz reef system, and the Alacran reef system at Puerto Progreso, Yucatan (Vilchis et al., in press). Studies have shown higher macroalgal diversity in the Indo-Pacific area than in the Atlantic (Kerswell, 2006), and when comparing worldwide Laurencia data (Tapia et al., 2015) this pattern is confirmed. Areas of endemism in the south Atlantic were also found in Brazil, Gulf of Guinea, and South Africa, which are also reported to contain endemic species (Brown & Lomolino, 1998); furthermore, it was found that the Gondwana breakup is the geological process that explains this endemism.
Other areas such as the Northwestern Atlantic, Europe, and the Caribbean Sea share Laurencia species with other regions (i.e., the Indo-Pacific), and the influence of vicariance events such as the closing of Isthmus of Panama and the final closure of the Tethys seaway in the Eastern Atlantic (Cowman & Bellwood, 2013) has not been studied.
Secondary areas. These areas are defined by the presence of one endemic species. When this happens, for example, in the Macaronesian Archipelago with L. viridis, the area is inhabited mostly by species that are also distributed in other places (9 spp.), and the number of endemic species is smaller compared to the defined areas of endemism. In the Macaronesian Archipelago, the phycofloristic composition reveals elements in common with the littoral of continental Europe and the North of Africa (Haroun and Prud’Homme van Reine, 1993; Tuya & Haroun, 2009). This is because the geological origin of the Macaronesian islands dates to the early Miocene (20 my) (Brown & Lomolino, 1998), allowing the colonization of species from other areas. Thus, this region is a spe cial biotic area that could be researched using an island-biogeography approach.
Widespread species. The genus Laurencia in the Atlantic Ocean extends from the coast of Ireland to South Africa, including the Mediterranean Sea and Black Sea; in the western Atlantic, the genus extends from North Carolina, USA, to southern Brazil. Seventeen of these species are also distributed in the Indo-Pacific, of which five are widely distributed in the Atlantic Ocean.
Recent phylogenetic studies have been useful in detecting misidentifications in the Atlantic, especially in records from Macaronesia, Brazil, and the Mexican Caribbean, although only nine widespread species of Laurencia in the Atlantic have a molecular characterization in these places. The use of the molecular-phylogenetic approach on these species in the Atlantic Ocean should allow us to identify new lineages over a wide distribution range, as has happened with other groups of red algae (Skage et al., 2005; Nuñez-Resendiz et al., 2015), and achieve a much better understanding of the historical biogeography of Laurencia in the Atlantic Ocean.
According to Miranda and Marques (2011), the two principal obstacles in the biogeographical works are (1) the reliability of species identifications and the consequent uncertainty of the presence of species in a given area, and (2) the difficulty in producing reliable cladograms from phylogenies.
In conclusion, it has been possible to establish a biogeographic pattern in Laurencia from the definition of areas of endemism and partial and secondary areas. This pattern has been linked to geological events that occurred in the past. Therefore, the recognition of new species from systematics studies and the clarification of taxonomic problems will allow researchers to continue developing a biogeographic hypothesis for the genus in the Atlantic Ocean.











 text new page (beta)
text new page (beta)




