INTRODUCTION
Petroleum is a super complex mixture of chemical compounds with an estimated minimum of 50,000 different substances (Marshall & Rod gers, 2004). The main groups of compounds are aliphatic hydrocar bons, polyaromatic hydrocarbons (PAHs), heterocyclic of nitrogen and sulphur, endocrine compounds, and saturated fatty acids (Benassi et al., 2013). In addition, alkenes and heavy metals have been identified (Cote, 1976). Petroleum and its products are common pollutants in the aquatic environment (Crunkilton & Duchrow, 1990). As an example of the impacts of crude oil spills during one such spill in 2010, approxima tely 200 million gallons of South Louisiana crude oil were released into the northern Gulf of Mexico over the course of 87 days (Crone & Tolstoy 2010). Communities of aquatic macro-invertebrates have been suitable for environmental risk assessment in streams and rivers following spills of crude oil (Poulton et al., 1998). Recently, numerous studies have re ported that snails are appropriate for toxicity testing because they are benthic and have reduced mobility (Ma et al., 2014a). In addition, it is possible to perform tests under laboratory conditions to evaluate the potential damage provoked by environmental pollutants (Whitehead, 2013). One of the most common toxic effects in aquatic organisms is related to oxygen metabolism. Under aerobic conditions, cells can produce reactive oxygen species (ROS); in addition, the antioxidant sys tem in aquatic animals includes specific enzymes to reduce ROS levels (Hermes-Lima, 2004; Lushchak, 2011). Nevertheless, if antioxidant sys tems do not reduce ROS, their concentrations could be deleterious and induce oxidative stress. Oxidative stress is characterized by oxidation of biomolecules such as lipids, proteins, and nucleic acids (Lushchak, 2011). These oxidative damages caused by crude oil or its components could compromise energy provision as well as high energy demanding organs, such as the nervous system (Beal, 1995). Fatty acids (FA) are essential as energy sources and their concentrations could be suitable as biomarkers (Kowalczyk-Pecka et al., 2017). The acyl-CoA oxidase (AOX) is an enzyme that belongs to peroxisomal β-oxidation, which is the metabolic pathway to obtain energy from fatty acids (Cajaraville et al., 2003). Further, some enzymes, such as acetylcholinesterase (AChE) and glutamate decarboxylase (GDA), are involved in neurotransmission, i.e., a key function in the nervous system (Basu, 2015). In addition, carboxylesterases (CbE) are enzymes involved in the detoxification of compounds that can inhibit the activity of AChE (Fukuto, 1990).
The tegogolo Pomacea patula (Baker, 1922) is an edible freshwater mollusk, endemic to Catemaco Lake in Veracruz, Mexico (Carreón-Palau et al., 2003). However, due to the economic importance of this species, its culture has been developed in Central and Southern Mexico. The southern region of Mexico is an important supplier of oil resources (CNH, 2017). In addition, pollution associated with the petroleum indus try has been documented in this area (PROFEPA, 2017). The objective of this study was to select and assess a series of biomarkers involved in oxidative stress (contents of O2-* and H2O2, lipid peroxidation, and pro tein oxidation), antioxidant defense activity (SOD, CAT, and GPx), fatty acid metabolism indicators (fatty acid levels and acyl-CoA oxidase), and neurotransmission enzymes in the Pomacea patula mollusk exposed under controlled conditions to the WAF of Maya crude oil. The study compared some of the widely documented aspects related to toxic effects caused by petroleum, such as the oxidative stress response and other less-studied aspects, such as fatty-acid metabolism and neuro transmission indicators.
MATERIALS AND METHODS
Animals and experimental design. Cultured specimens of the Mexi can freshwater snail tegogolo (Pomacea patula) were obtained from an aquaculture center located in Zacatepec (Morelos, México). Snails were maintained in glass aquariums with a capacity of 145 L using semi-hard synthetic water (0.22 g MgSO4, 0.18 g NaHCO3, 0.08 g KCl and 0.13 g CaSO4.2H2O per L) with constant aeration at 24±1 °C un der natural light-dark cycle at Mexico City latitude for three months until the experiments started. The snails were feed twice a week with lettuce obtained from a local supermarket. Healthy tegogolos of similar size (51.5 ± 2.96 mm) and weight (26.85 ± 3.87 g) were selected for the test. Groups of seven tegogolos were exposed for 96 h to the four concentrations of WAF obtained from different loads (0.1, 1, 10 and 100 mg/L) of Maya crude oil. The WAF fraction was obtained by the Singer et al. (2000) method. Maya crude oil was supplied by the Instituto Mexi cano del Petróleo (Mexico). Specimens of P. patula were treated in glass aquariums protected from the light in a total volume of 10 L. The control group was exposed to semi-hard synthetic water.
Chemical analysis. A sample of 1 L of exposure medium was collected after the snails were euthanized. The quantification of PAHs was un dertaken with a Biotek SynergyMX spectrofluorometer, using certified analytical standards obtained from Chem Service, Inc. (West Chester, PA), as previously documented (Dzul-Caamal et al., 2016).
Biomarker evaluation. Test specimens were measured with a Vernier caliper and weighed in analytical balance with a sensitivity of 0.1 g. Organisms were euthanized by fast-freezing them at -20 °C/30 min, as previously reported (Nica et al., 2015). Tissues of tegogolo (foot, head, intestine, mantle, digestive gland, and kidney) were obtained according to the anatomy of gastropods (Barnes, 1980). All tissues were weight and homogenized in a Glas-Col™ homogenizer in an ice bath as fo llows: foot and head in 10 mL of PBS 1X; the rest of the tissues in 3 mL of PBS 1X. 1.0 mL of the homogenates was centrifuged at 4,980 X g (9,000 rpm) and 4° C for 15 min in a Hermle Labnet Z216MK centrifuge to obtain the cytosolic fraction. The uncentrifuged and cytosolic frac tions were stored at -70°C until the biomarker assay was performed (less than 2 weeks).
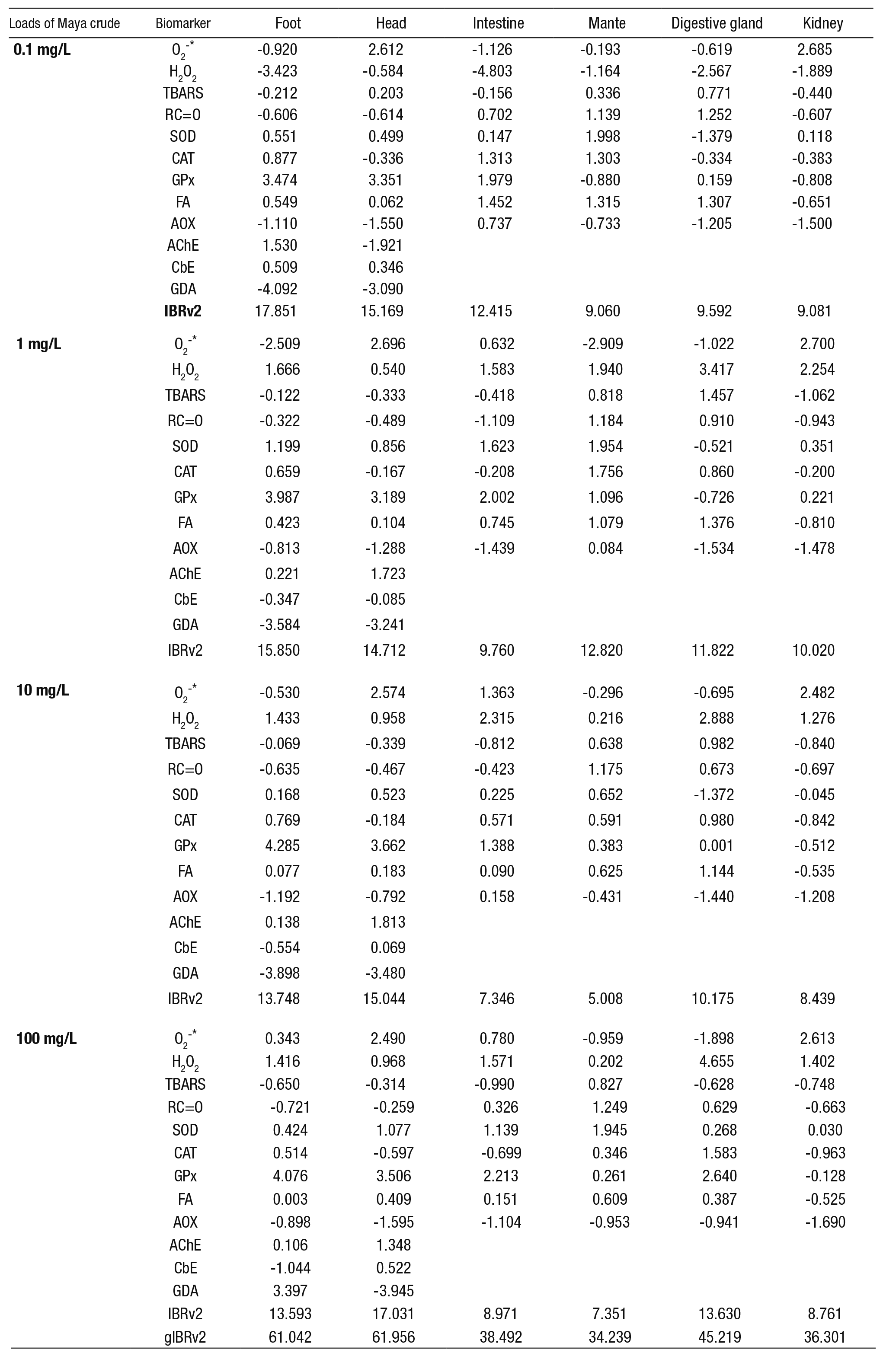
Table 1 summarizes the methods used to assess biomarkers.
Table 1 Methods for biomarker evaluation in Pomacea patula (Baker, 1922) exposed to the water-accommodated fraction (WAF) of Maya crude oil
O2-*: superoxide anion; H2O2: hydrogen peroxide; TBARS: lipid peroxidation evaluated as thiobarbituric acid reactive substances; RC=O: protein oxidation evaluated as carbonyls (RC=O) content; SOD: superoxide dismutase activity (CuZn-SOD plus Mn-SOD); CAT: catalase activity; GPx: selenium-dependent glutathione peroxidase activity; FA: fatty acids; AOX: acyl-CoA oxidase activity; AChE: acetyl cholinesterase activity; GDA; glutamate decarboxylase activity; CbE: carboxylesterase activity. EC: Enzyme Commission number; NA: not applicable. F: foot; H: head; I: intestine; M: mantle; DG: Digestive gland; K: kidney.
Statistical analysis. Biomarker results were compared to controls and treatments by ANOVA, followed by post-hoc Dunnet´s Comparison Test. Statistical significance was set at p≤0.05 for all tests. Analyses were done in GraphPad Prism version 5.00 for Windows (GraphPad Software, San Diego, California, USA, http://www.graphpad.com). To determine the im pact of the WAF of MCO in tissues of tegogolos, we calculated the IBRv2 according to Sanchez et al. (2013) and employed the general IBRv2 (gIBRv2) proposed by Dzul-Caamal et al. (2016) to integrate the IBRv2 values by tissue and treatment.
RESULTS
PAHs concentrations in the exposure medium. The concentration of the PAHs increased in relation to the load of Maya crude oil at the end of the experiment (Table 2). The proportion of individual compounds between loads did not show large variations, except for phenanthrene, benzo[a]pyrene (BaP), and fluoranthene. BaP was the PAH with the hig hest proportion in all loads. There was a lower percentage of total low molecular weight (LMW) PAHs than the high molecular weight (HMW) PAHs, due to their volatility and the characteristics of heavy crude oil.
Table 2 Concentration of polyaromatic hydrocarbons (PAHs) in µg/L medium of exposure after 96 h of exposure. Mean ± standard deviation.
LMW: Low molecular weight; HMW: High molecular weight.
Biomarker responses. Most of the treatments show that the content of ROS was higher in the tissues of P. patula exposed to the WAF of Maya crude oil, as compared to controls. The tendency of O2-* and H2O2 was similar in the tissues under study. Wider differences were observed in the higher concentration of WAF particularly for the foot and kidney (p <0.05, Fig. 1a, f). A higher content of ROS was found in the head at 1 mg/L load (p <0.05, Fig 1b). In the intestine, the higher concentration of H2O2 was recorded at 1 mg/L; meanwhile, the high levels of O2-* was detected at 10 mg/L (Fig. 1c). In the tegogolo mantle, the content of ROS was irregular in treatments and was higher at the lower load of Maya crude oil (0.1 mg/L, Fig. 1d). Notably, in the digestive gland, the levels of ROS were similar to controls at 0.1 and 1 mg/L. However, from 1 to 100 mg/L, a slight reduction was observed (Fig. 1e).
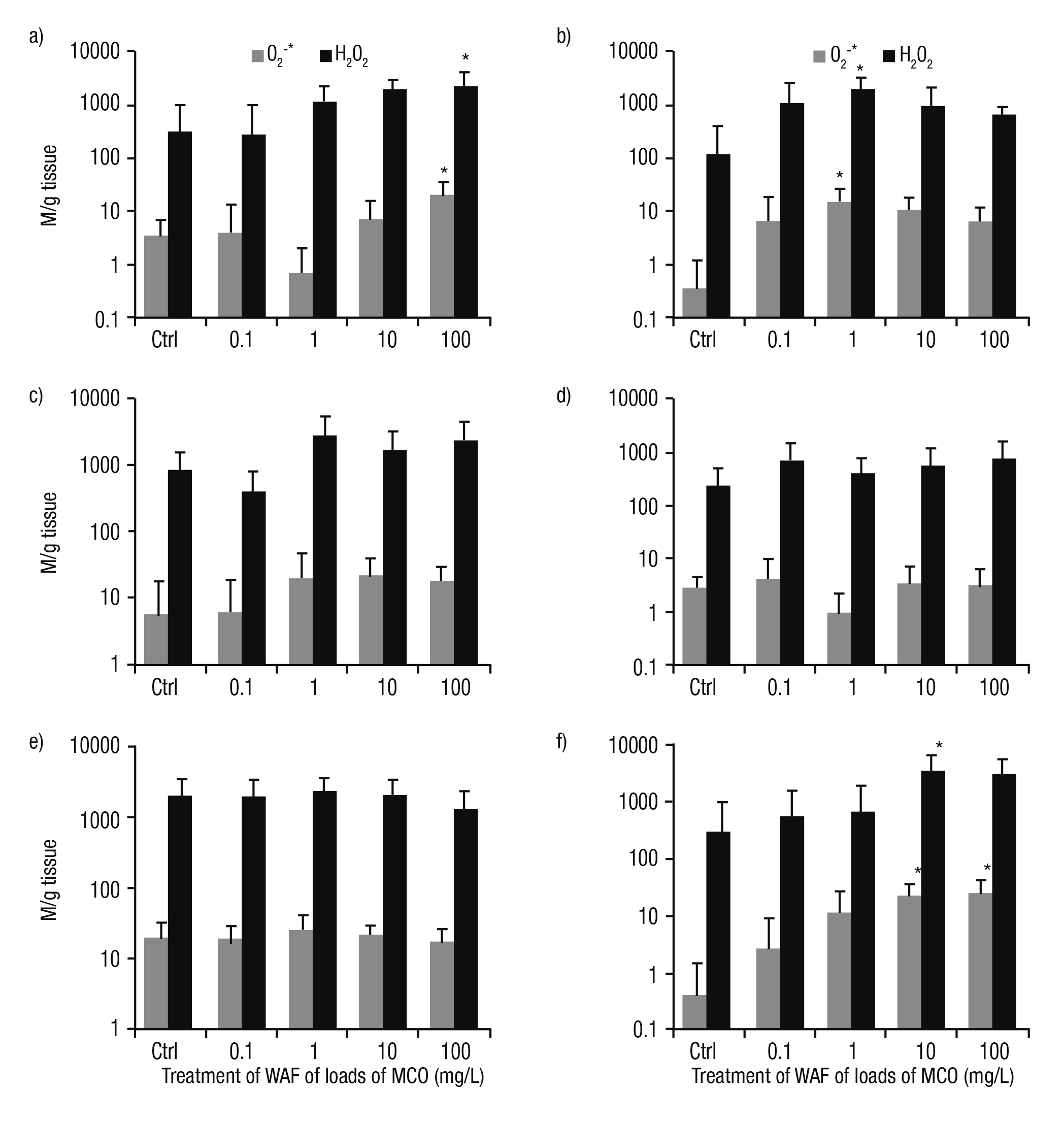
Figures 1a-f Content of ROS (O2-* y H2O2) in tissues of Pomacea patula exposed under controlled conditions to the WAF of MCO for 96 hours. Bars represent standard error of the mean. a) Foot, b) Head, c) Intestine, d) Mantle, e) Digestive gland, f) Kidney.
Oxidative stress. The response of lipid peroxidation and protein oxida tion in WAF treated gastropods was inconsistent compared to controls. In addition, the responses of these biomarkers were erratic among tis sues. We observed no significant differences in the foot (Fig. 2a). In the head, differences among TBARS and RC=O were found. The lipid peroxidation was higher at 0.1 mg/L; however, this oxidative damage diminished at greater WAF concentrations. Likewise, protein oxidation was similar to controls (Fig. 2b). Of importance, in the intestine, mantle, and digestive gland of P. patula treated at 1 mg/L, we detected higher oxidative damage (Figs. 2c-e). In the kidney, the greater level of lipid peroxidation was observed at 0.1 mg/L of crude oil load. In contrast, the protein oxidation in this tissue was lower than controls in all treatments (Fig. 2f).
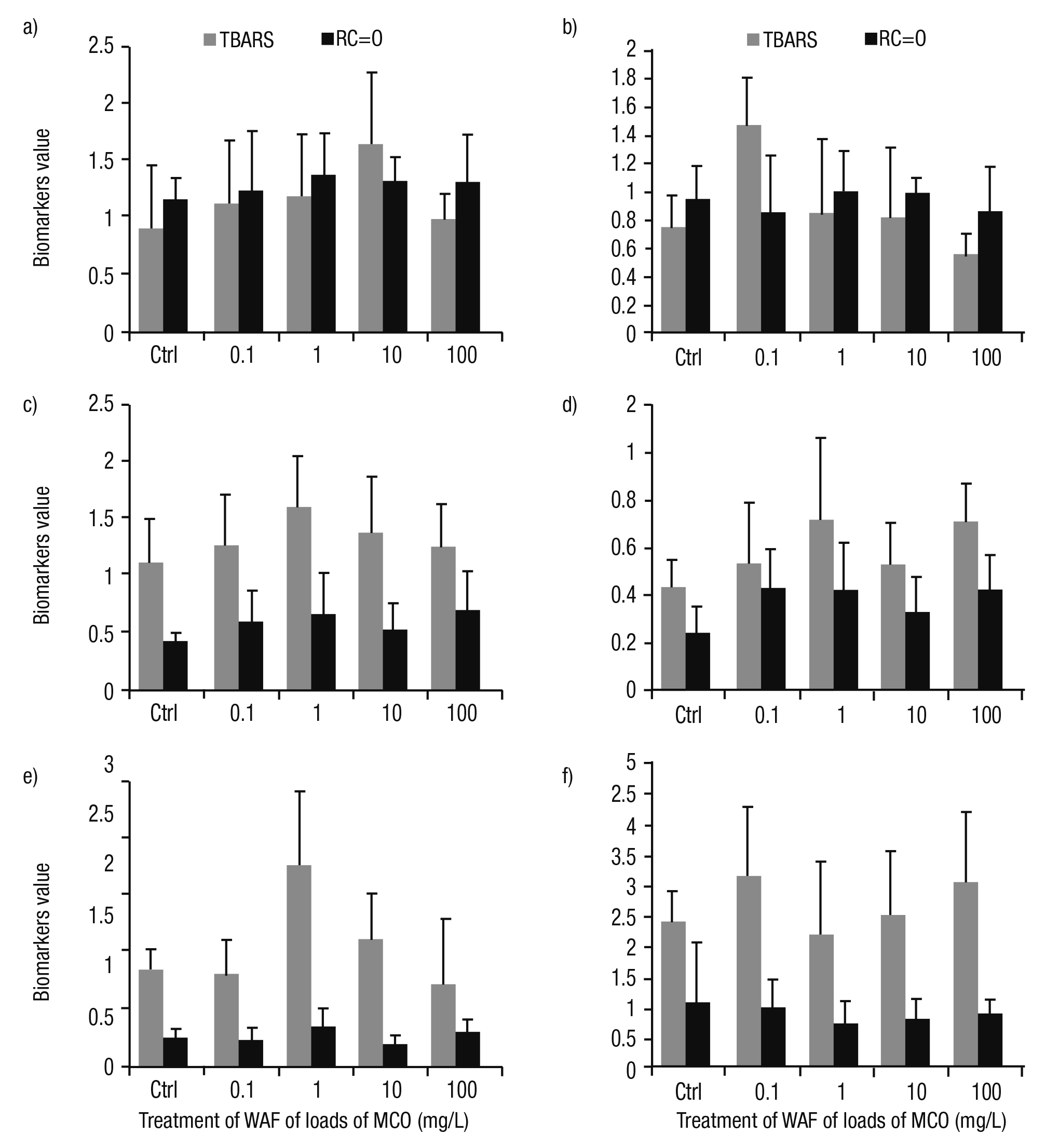
Figures 2a-f Oxidative damage measured as lipid peroxidation (TBARS) and protein oxidation (RC=O) in tissues of Pomacea patula exposed under controlled condi tions to the WAF of MCO for 96 hours. TBARS were presented as mmol TBARS/g tissue and RC=O as mmol RC=O/mg protein/g tissue. Bars represent standard error of the mean. a) Foot, b) Head, c) Intestine, d) Mantle, e) Digestive gland, f) Kidney.
Activities of enzymes involved in antioxidant defense. Several pat terns of responses were detected in the activities of enzymes involved in antioxidant defense (SOD, CAT, and GPx) in P. patula exposed to the WAF of Maya crude oil. In the tegogolo foot and head, the SOD activity did not show a trend compared to controls and treatments. In the foot, the activity of CAT increased with WAF concentration. In contrast, in the head, this response was inversely linked with the load of crude oil from 1 to 100 mg/L. The activity of GPx was clearly induced by exposure to the WAF of Maya crude oil compared to controls. Significant differences were found in this snail’s foot treated at 10 and 100 mg/L (p <0.05); in the head, significant differences were documented at 10 mg/L (p <0.05; Figs. 3a-b). In the intestine, the activities of CAT and GPx were higher than control specimens with the exception of CAT at 100 mg/L; however, in this tissue, the catalysis of SOD was irregular compared to controls and treatments (Fig. 3c). CAT activity in the mantle was indu ced at 0.1 and 1 mg/L. Nevertheless, at high concentrations (10 and 100 mg/L), CAT activity was reduced. SOD and GPx showed an irregular tendency in their metabolisms (Fig. 3d). In the digestive gland, higher activities of CAT and GPx were observed at 100 mg/L; meanwhile, SOD was similar and lower than controls (Fig. 3e). In the kidney, the catalysis of these enzymes reached a peak at 1 mg/L of a load of Maya crude oil. However, these activities were reduced at 10 and 100 mg/L (Fig. 3f).
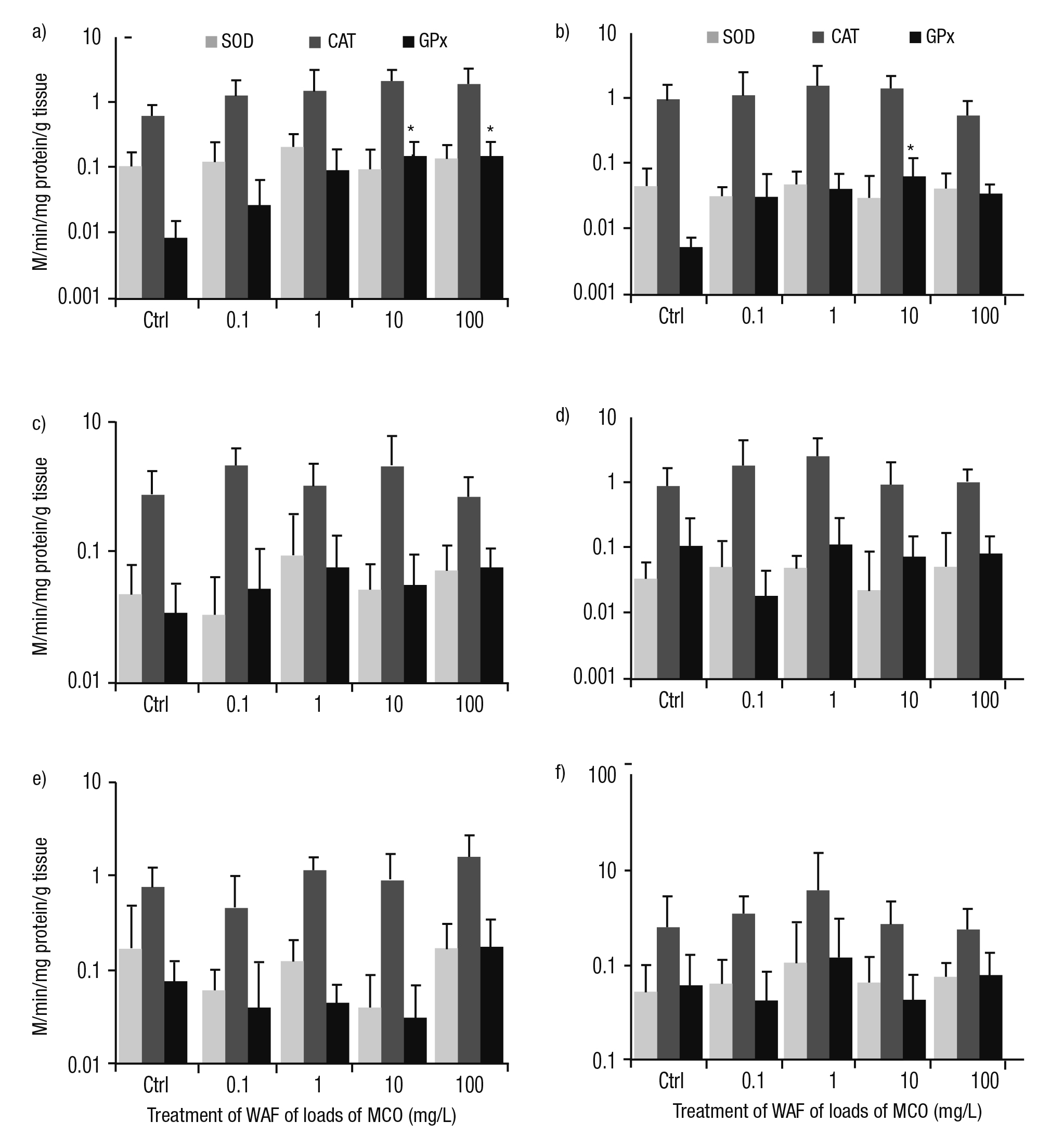
Figures 3a-f Activity of enzymes involved in antioxidant defense (SOD, CAT, and GPx) in tissues of Pomacea patula exposed under controlled conditions to WAF of MCO for 96 hours. Bars represent standard error of mean. a) Foot, b) Head, c) Intestine, d) Mantle, e) Digestive gland, f) Kidney.
Fatty acid metabolism. The concentration of FA in the foot and intes tine of P. patula exposed to the WAF of Maya crude oil were higher than in controls (Figs. 4a, c). However, in the head, levels of FA were similar among exposed and unexposed snails (Fig. 4b). In the digestive gland and kidney of P. patula treated with the WAF, the concentration of FA was lower than controls, except those observed in the treatment with the WAF obtained from 1 mg/L of Maya crude oil (Figs. 4e-f). In the mantle, the content of these biomolecules was irregular compared to treatment (Fig. 4d).
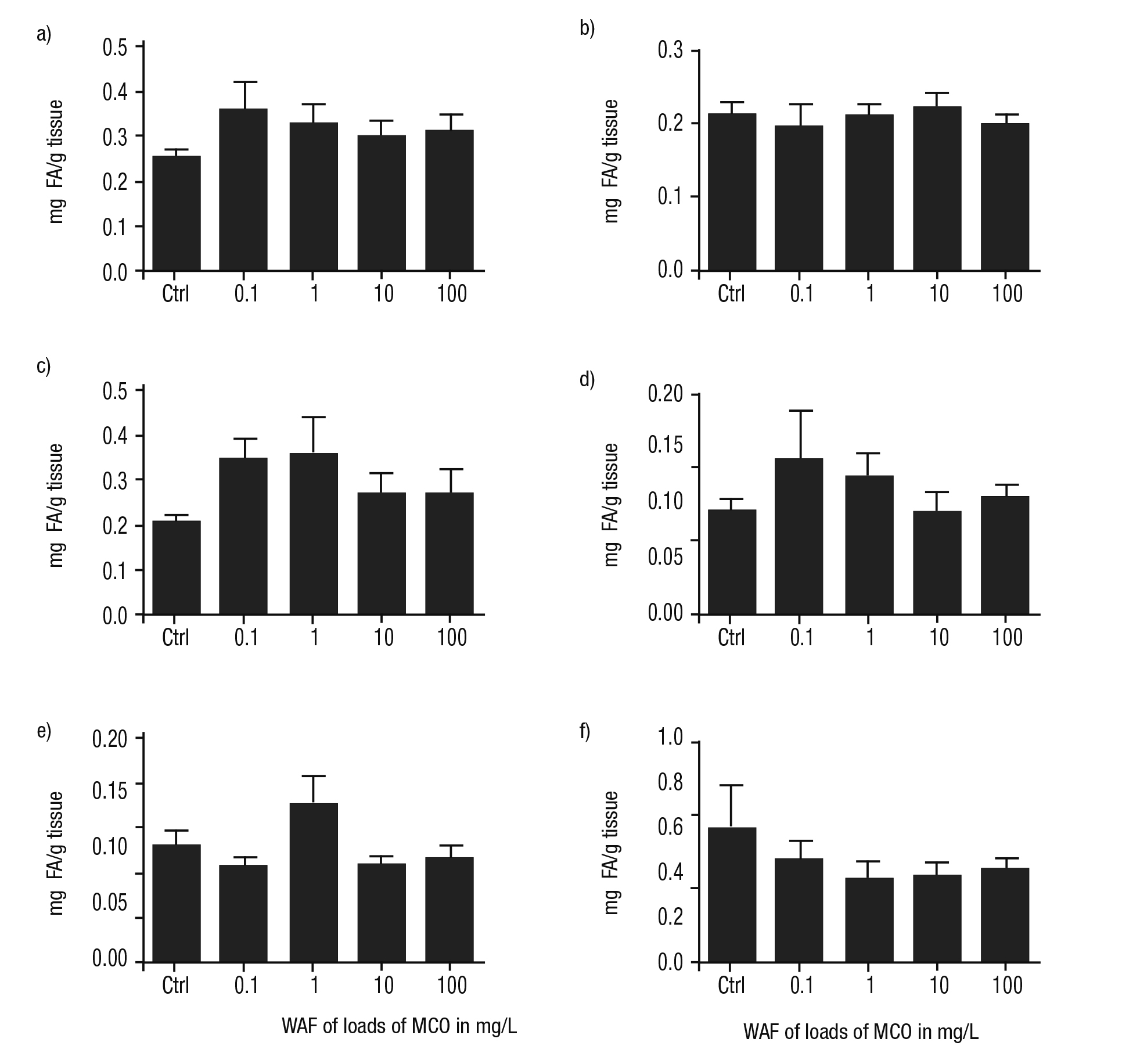
Figures 4a-f Concentration of FA in tissues of Pomacea patula exposed under controlled conditions to WAF of MCO for 96 hours. Bars represent standard error of the mean. a) Foot, b) Head, c) Intestine, d) Mantle, e) Digestive gland, f) Kidney.
The activity of AOX was higher in the foot and in the kidney of P. patula than in the control in all treatments, with exception of the cataly sis of this enzyme detected in the heads of snails exposed to the WAF of 0.1 mg/L of MCO (Figs. 5a-f). In contrast, in the mantle and in the digestive gland, an irregular activity of AOX was observed compared to controls (Figs. 5d-e). The maximum activities of AOX were detected at the WAF at the higher load of MCO (100 mg/L) solely in the snails’ foot and digestive gland.
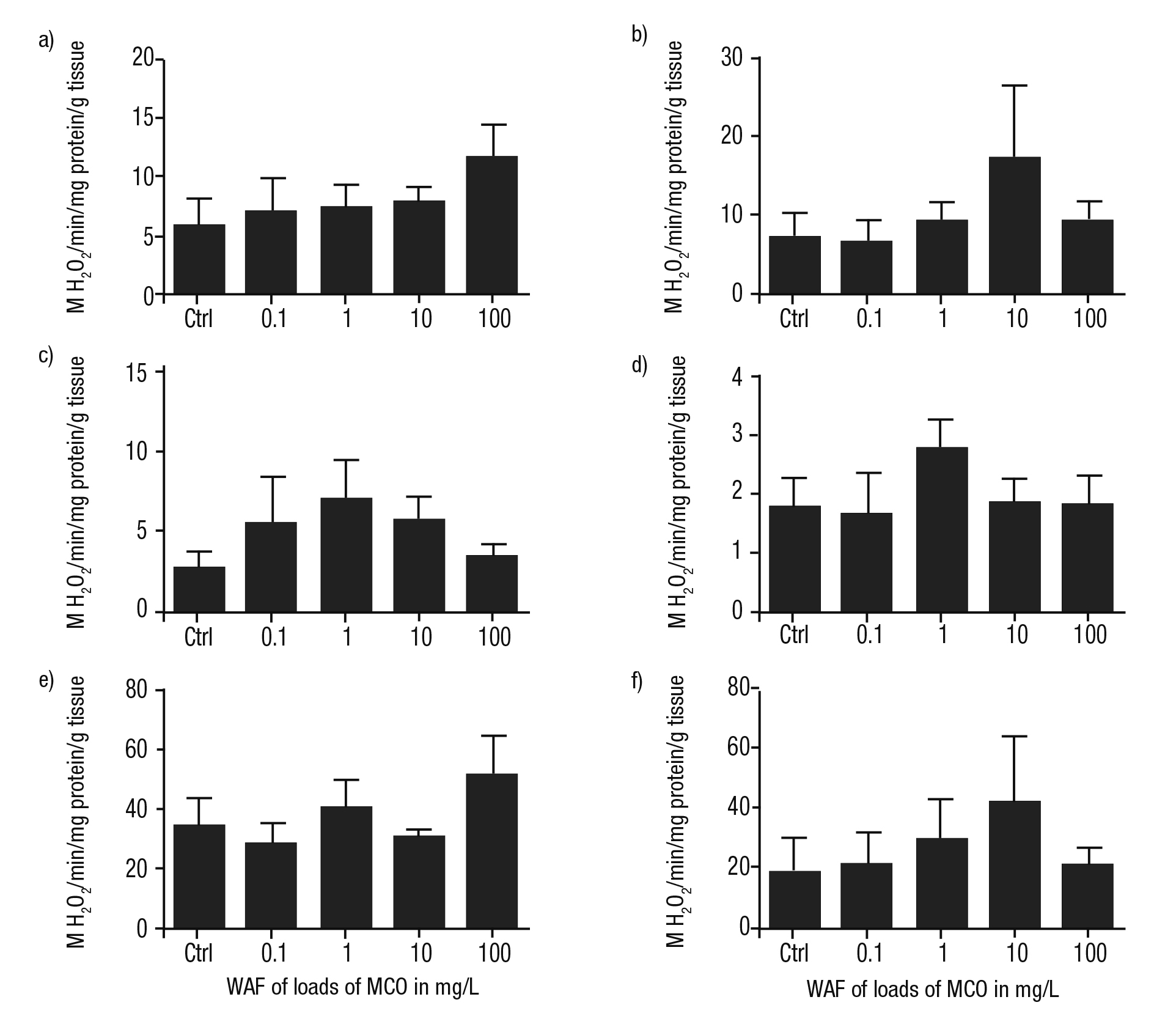
Figures 5a-f Metabolism of AOX in tissues of Pomacea patula exposed under controlled conditions to the WAF of MCO for 96 hours. Bars represent standard error of mean. a) Foot, b) Head, c) Intestine, d) Mantle, e) Digestive gland, f) Kidney.
Activity of enzymes involved in neurotransmission. In the head and foot of P. patula exposed to the WAF of MCO, AChE was higher than con trols in all cases and was related with WAF concentration. In addition, significant differences compared to controls were noted at WAF loads of MCO at 1, 10, and 100 mg/L (p ≤0.05) (Figs. 6a-b).
The catalysis of GDA in the head was lower than controls with a concentration-dependent response with statistical differences at WAF of 10 and 100 mg/L of MCO (Fig. 6d). Similarly, the activity of this en zyme in the foot was lower than control; however, at the WAF of 100 mg/L of MCO, an increase in this enzyme was found, even greater than controls (Fig. 6c).
In general, the activity of CbE in the head and foot of P. patula treated with the WAF of Maya crude oil was irregular compared to the treatments. Nevertheless, at the WAF of the lower load of MCO, an in crease of this enzyme was detected in both tissues (Figs. 6e-f).
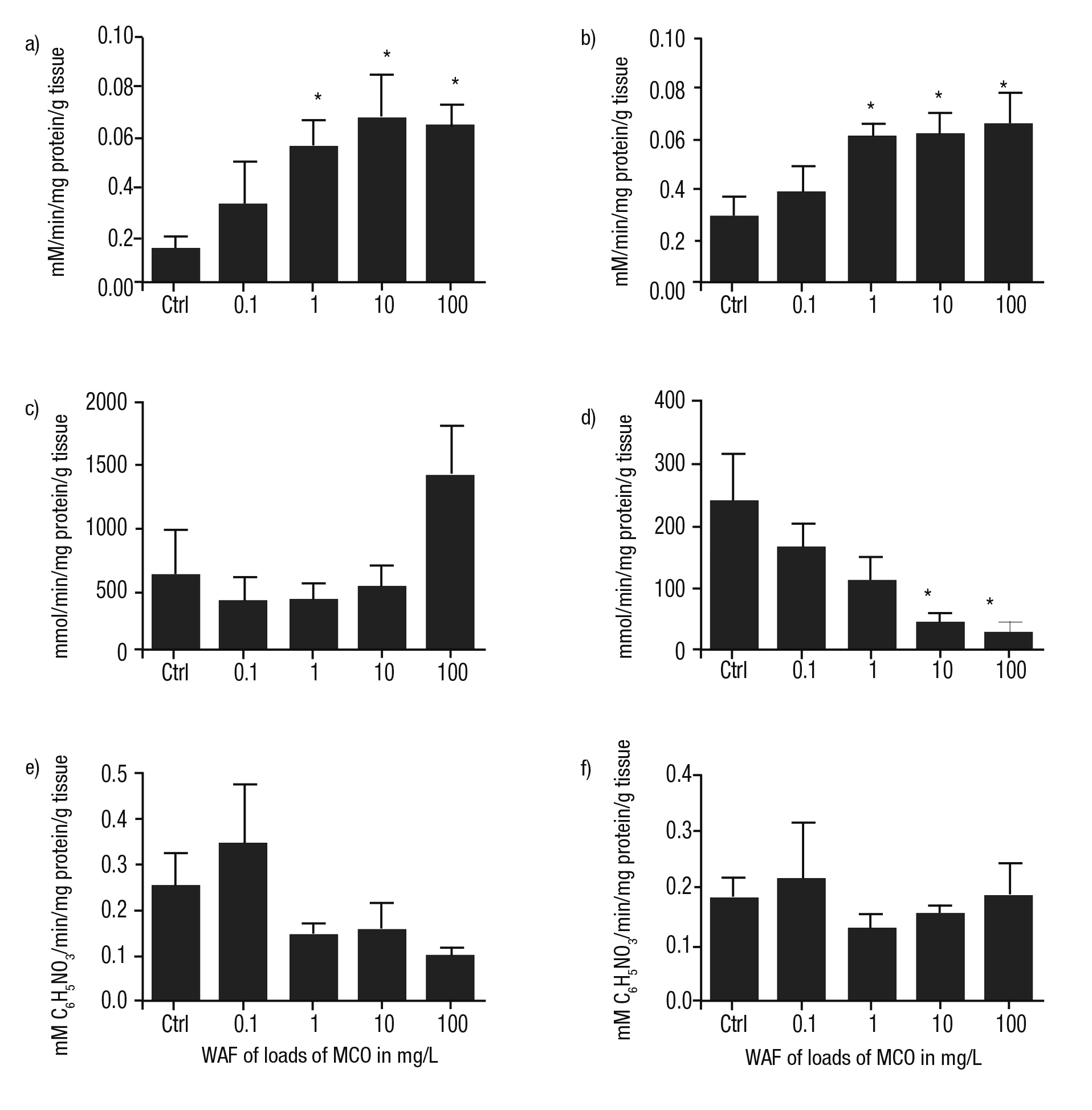
Figures 6a-f Metabolism of enzymes involved in neurotransmission of Pomacea patula exposed under controlled conditions to the WAF of MCO for 96 hours. Bars represent standard error of mean. a) Activity of AChE in the foot, b) Activity of AChE in the head, c) Activity of GDA in the foot, d) Activity of GDA in the head, e) Activity of CbE in the foot, f) Activity of CbE in the head.
Integrated biomarker response. Higher values of gIBRv2 were found in the head and foot of P. patula exposed to the WAF of MCO followed by the digestive gland, intestine, kidney, and mantle. In terms of treatments, the higher value of gIBRv2 was found in specimens ex posed to the WAF at 1 mg/L and by treatments of the WAF at 0.1, 100, and 10 mg/L, respectively (Table 3).
DISCUSSION
Several natural sources of pro-oxidants forces have been documented in aquatic organisms such as the electron-transport chain, oxygenases, auto-oxidation, and dependent systems of NADPH oxidases (Lushchak, 2011). Although the pro-oxidant/antioxidant balance in snails exposed to diverse pollutants has been studied, a lack of information about the content of ROS prevails. In this study, following exposure to the WAF of Maya crude oil, the levels of ROS in the tissues under study were higher than in their respective controls in the head of P. patula treated with the WAF from 1.0 mg/L, followed by the kidney (10 and 100 mg/L), and the foot (100 mg/L). Since crude oil contains more than 50,000 chemicals (Marshall & Rodgers, 2004), it is not possible to attribute the generation of ROS solely by decoupling the electron flux during the catalysis of specific isoforms of the CYP450 superfamily (Arzuaga & Elskus, 2010). The WAF has high bioavailability to organisms and its chemical charac teristics are related to the type of crude oil. There is a large variation in the chemical composition of different oils. The WAF of heavy crude oil, such as the Maya type, contains large amounts of water-soluble heavy molecules and microscopic oil droplets that are associated with HMW PAHs (Couillard et al., 2005). Chemical transformations occur in the so luble molecules allowing the decrease of the concentrations of the LMW compounds within a period of 24 h (Nebo et al., 1998), because of these transformations; in this study, the amounts of HMW PAHs overcame the temporary decline. The low variation of most individual PAHs between the different loads showed a reduced WAF weathering during the ex periment. Besides, crude oil contains transition metals such as Fe, Mn, and Cr, among others, which are involved in ROS induction by interfe rence of a metal-related process also brought about by generation of free radicals (Lushchak, 2011). Thus, we may speculate that HMW PAHs in addition to transitions metals and other compounds were responsible for ROS generation. With regard to the organ-specific response, it has been documented that some organs of the central nervous system are located in the head of the snails (Battonyai et al., 2012; Battonyai et al., 2014). In addition, the foot is responsible for locomotion (Miyamae et al. 2010; Longley, 2014). Thus, the cells which make up this system require large amounts of energy in order to function (Rigon et al., 2010; Panov et al., 2014). However, during the generation of energy, ROS could be induced in the mitochondria which is the principal organelle related to energy production (Sharp & Haller, 2014). The results in this study could be associated to these events; however, more studies are needed to clarify this point. Despite the lack of information about ROS levels in the snails’ kidneys, in fish a positive and negative selection of hematopoietic progenitor cells occurs (Davidson & Zon, 2004; Stachura et al., 2009) that involve the generation of ROS required for the extrinsic pathway of apoptosis. Besides, ROS induction is a defense mechanism of some immunocompetent mature cells, which are plentiful in the kidney (Janeway & Medzhitov, 2002).Oxidative stress response is the most reported biological response in snails exposed to several chemical compounds. In this study, the lipid peroxidation assessed as TBARS and protein oxidation evaluated as carbonyl proteins were greater in the tissues of P. patula exposed to the WAF of Maya crude oil compared to controls. Nevertheless, the differences observed were not statistically significant. Similar responses were found in some gastropods exposed to compounds different from crude oil (Cochón et al., 2007; Zheng et al., 2013). However, in other mollusk species, contrasting findings have been documented (Ansaldo et al., 2005; Kaloyianni et al., 2009; Itziou et al., 2011a; Itziou et al., 2011b; Ali et al., 2012; Ma et al., 2014a; Wang et al., 2014). Results of this study indicate the presence of efficient processes to reduce the induction of ROS in the foot, head, and kidney of P. patula, probably by unspecific antioxidant systems, as well as an efficient process mediated through the ATP-dependent ubiquitination, via endogenous proteases such as cathepsin c, calpain, and trypsin for degradation of RC=O (Hermes-Lima, 2004). This process is aimed at auto-regulating the oxidative damage induced by the WAF of Maya crude oil.
No significant changes in the catalysis of enzymes involved in antioxidant defense (SOD, CAT, and GPx) compared to controls were observed in tissues of tegogolos exposed to the WAF of MCO, with the exception of GPx in the foot and head of P. patula exposed to high con centration of WAF (10 and 100 mg/L). In addition to oxidative stress, the activity and presence of antioxidant systems are also widely studied in snails treated with pollutants (Ismert et al., 2002; Li et al., 2008; El-Gendy et al., 2009; Radwan et al., 2010; Ali et al., 2012; Bouétard et al., 2013; Zheng et al., 2013; Ma et al., 2014a; 2014b; Wang et al., 2014). Inactivity of SOD, particularly at high concentrations of the WAF of Maya crude oil, could be due to the oxidative stress induced by the accumulation of ROS (Liesivuori & Savolainen, 1991). Additionally, the damage to this enzyme could be due to reactive and oxidant meta bolites produced by biotransformation of many compounds, as is the case of PAHs (Gao et al., 2005; Vondrácek et al., 2009). In contrast, significant increases in GPx activity in the foot and head of P. patula exposed to high concentrations of WAF are likely due to the presence of H2O2 in the cells, since this ROS is the main substrate for these enzymes (Hermes-Lima, 2004). The induction of ROS could be different among tissues due to contact with the environment, but also to their energy de mands obtained through fatty-acid metabolism, among others sources. Consequently, the activity of enzymes involved in antioxidant defense could be linked to these pro-oxidant forces. It has not been possible to substantiate that oxidative stress participates in depleting the activity of these antioxidant defenses.
In this study, we found different patterns of response due to the concentration of FA in P. patula exposed to the WAF of MCO; however, in no case did we find significant results. In contrast, in other mollusk spe cies, significant results were found (El-Wakil & Radwan, 1991; Radwan et al., 1993; Radwan et al., 2008; Lyssimachou et al., 2009). Research suggests that the changes of FA content in snails exposed to stres sing agents could be explained by their synthesis to repair and prevent damage to organelle and cells, whereas its decrease could be due to utilization of energy requirements (Padmaja & Rao, 1994). Similarly to FA, the activity of AOX showed a different pattern of response among treatments and tissues, even though significant differences were not found. However, in other snail species exposed to inducers of the pe roxisome proliferator activated receptor alpha (PPARα), the response was irregular under laboratory conditions (Lyssimachou et al., 2009) or amplified in specimens from polluted sites (Cajaraville et al., 2003; Regoli et al., 2006). The lack of response to FA levels and AOX activity in P. patula treated with the WAF of Maya crude oil may have an adap tive significance as documented in other snail species (Arakelova et al., 2004) as a protective mechanism for reducing the toxic effects of WAF, as suggested by Padmaja & Rao (1994) in other species. The current results and previous reports denote the need of more studies aimed at increasing knowledge about fatty-acid metabolism in snails exposed to pollutants.
CbE activity, which is present in a range of organism including Bac teria, Eukaryota, and Archaea, is responsible for the hydrolysis of car boxylic esters, carboxylic thioesters, and esters of about 1684 substra tes (BRENDA, 2017) in the head and foot of P. patula exposed to the WAF of Maya crude oil, was irregular with regard to treatments and tissues. However, these findings were not significant, probably due to the varia ble bioavailability of carboxylic compounds in the WAF of Maya crude oil, as well as to the role of CYP450 isoenzymes involved in metabolism of PAHs as documented in snails (Wilbrink et al., 1991; Ismert et al., 2002). However, it is more likely that the specific aging or stimulation of the AChE will occur after the exposure to soluble compounds present in Maya crude oil. Increases in the catalysis of AChE in head and foot of P. patula treated with the WAF of MCO were found. Similar findings were documented in the Senegal sole Solea senegalensis (Kaup, 1858) exposed to the WAF of “Prestige” crude oil under laboratory conditions (Solé et al., 2008). These results suggest that compounds present in the WAF of Maya crude oil stimulate the activity of this enzyme. Likewise, it is probable that the degradation of acetylcholine overcomes the ba sal levels provoking deficiencies in this neurotransmitter. Since the acetylcholine participates in the activation of neuromuscular function, it is likely that this function in P. patula is inactive. Little information is available regarding the activity of AChE in snails exposed to petro leum hydrocarbons. However, inhibition has been documented in the catalysis of this enzyme in some snail species exposed mainly to pes ticides (Singh & Agarwal, 1983; Coeurdassier et al., 2001; Radwan & Mohamed, 2013; Khalil et al., 2015; Zheng & Zhou, 2017). The different responses documented in previous reports and in this study could be attributed to the presence of bioavailable compounds in WAF that are able to stimulate this enzyme, despite the lack of information about the complete characterization of the WAF obtained from Maya crude oil. Nevertheless, it is probable that the degradation of acetylcholine cau sed by WAF exposure could modify the response of P. patula, probably provoking the apparent lack of sensitivity of this snail species linked to reduced motility.
The catalysis of GDA in the head and foot of P. patula was reduced compared to the control, with exception of the activity detected in the foot at the higher WAF concentration. There are few reports regarding the activity of this enzyme in snails exposed to hydrocarbons. However, in the ganglia of a feral freshwater mussel Elliptio complanata (Lightfoot, 1786) exposed to dilutions of primary-treated effluent, decreases were docu mented in GABA catalysis, suggesting glutamatergic stimulation (Gag né et al., 2007), which exerts excitatory effects. This neurophysiological process probably occurs as a compensatory mechanism for depression of locomotion activity related with low levels of acetylcholine. Neverthe less, more studies are required to explore the neurotoxicity of petroleum hydrocarbons in freshwater snails as well as specific studies about the composition of the WAF obtained from Maya crude oil.
Comparing the tissues and concentrations of WAF, it is probable that two factors are involved in increased values of gIBRv2 in the head and foot of P. patula detected in this study: i) both are the main tissues in contact with the medium that contains petroleum hydrocarbons, and ii) the numerical effect of the three additional biomarkers involved in neurotransmission which was only measured in these tissues, mainly through their high nervous innervation in the foot and by the presence of some organs of the central nervous system in the head. However, the first hypothesis is the more likely, considering their regular contact with the polluted medium. Yet, the digestive gland showed higher va lues of gIBRv2 compared to intestine, mantle, and kidney. This could de due to its high capacity to uptake and concentrate contaminants, which suggests the usefulness of this organ for monitoring biochemical responses (Abdel-Halim et al., 2013).
Given the results of this study, we can conclude that the tegogolo foot was the most sensitive organ in terms of the biological response to exposure to the WAF of Maya crude oil. However, more studies are required in order to clarify the biotransformation, bioaccumulation, and detoxification involved in oxidative stress in gastropods exposed un der controlled conditions to diverse pollutants. The alterations of some enzymes involved in neurotransmission (AChE and CbE) seem to be suitable biomarkers for monitoring the toxic effects of hydrosoluble compounds present in the Maya crude oil found in this type of organism that also possesses mechanical defenses (shell and operculum) against environmental pressures. Research also confirms that crude oil is one of the most complex contaminants in the aquatic environment and the knowledge of its effects in aquatic organism should be increased.











 nova página do texto(beta)
nova página do texto(beta)