INTRODUCTION
Surfaces of materials used in marine environments rapidly become coated by a conditioning biofilm, also referred to as microfouling, which is highly variable in time and heterogenous in its composition (Patil & Anil, 2008). These biofilms consist mainly of attached bacteria and diatoms, with all components enmeshed in a matrix of extracellu lar polymers secreted during the construction of biofilms (Cooksey et al., 1980). Microfouling modifies the substrate surface chemistry and strongly influences the subsequent colonization by macrofouling orga nisms. Moreover, it is generally accepted that exopolymer films secre ted by diatoms promote the onset of macrofouling by conditioning the original substrate for the settlement of invertebrate larvae (Characklis & Cooksey, 1983; Qian et al., 2003; Patil & Anil, 2005a).
Benthic diatoms usually have been identified as major microfoulers of artificial substrata placed in the marine environment, although the source may be the water column itself or nearby surfaces that may function as islets (Fernandes et al., 1999). However, many diatom spe cies, which turn out to be abundant in artificial substrata, are not consi dered typically from rocky or other hard substrata but are epipelic forms (Siqueiros-Beltrones, 2002).
The structure and composition of the microfouling community ex hibits wide temporal and regional variations that are also influenced by the substratum (Cooksey et al., 1984; Patil & Anil, 2005b). These changes in community structure are influenced by various biotic and abiotic factors and play an important role in the temporal dynamics of microfouling and macrofouling. Eventually, any diatom assembla ge (regardless of the substratum) may reach a climax; then the film may degenerate and clumps of the more abundant taxa detach and drift away, becoming potential colonizers of any available surface, thus disrupting the classical colonizing sequence from pioneer (prostrated) forms to erect climax forms (Siqueiros-Beltrones, 2002). Hence, it is important to understand the species composition of diatoms during bio film formation. Such information will be useful in environmental studies, such as pollution abatement and the design and operation of industrial equipment which are prone to biofouling.
Fouling diatoms. Extensive qualitative studies on the fouling diatom are important in order to gain understanding about autoecological pro cesses and behavior of certain taxa under changing environmental con ditions. Few studies provide precise taxonomic information at a species level in the development of microfouling processes. Of the hundreds of existing diatom genera, only a few have been documented as cons tant components of the microfouling, such as Amphora, Licmophora, Navicula, Nitzschia, Cocconeis, and Achnanthes. The most commonly reported species include Halamphora coffeiformis (C. Agardh) Levkov, Achnanthes longipes C. Agardh, Craspedostauros australis E. J. Cox, Toxarium undulatum J. W. Bailey, and Navicula perminuta Grunow (Mo lino & Wetherbee, 2008).
Studies reveal that diatoms have specific preferences among artifi cial substrata (Mitbavkar & Anil, 2000), e.g., higher diatom recruitment has been observed on fiberglass (hydrophobic) than on glass (hydrophi lic) surfaces (Patil & Anil, 2005a), although heavy fouling was inciden tally documented on silicon treads used to fix aluminum foil collectors (Siqueiros-Beltrones, 2002).
Glass fiber reinforced polymer (GFRP) is one of the structural com posite materials widely used in engineering applications in seawater for building and repairing boats, offshore structures in the oil industry, and many others. It is composed of two phases: the plastic one is termed the matrix, which is continuous and surrounds the fiber reinforcement embedded and dispersed into a matrix that holds it together (Loewens tein, 1973; Gupta & Kothari, 1997).
Most of these early applications have been driven by the need to overcome the corrosion problem experienced with steel and aluminum alloys. Another reason for using GFRP has been to reduce weight, par ticularly the topside weight of ships. Over 95% of all composite marine craft are built with GFRP because of its low cost and excellent degra dation (corrosion) resistance in seawater (Loewenstein, 1973). GFRP is usually almost free of defects, with high fiberglass content for maxi mum stiffness, strength, and fatigue resistance.
However, it has been recognized for several decades now that the use of anti-fouling paints on different surfaces to create a toxic environ ment may aid in precluding development of the initial microfilm leading to macrofouling (Robinson et al., 1985), but the effectiveness of anti-fouling paints may vary. Thus, because diatoms are opportunistic fast-growing microalgae that proliferate on almost any substrate, given ade quate conditions of humidity and nutrients, the concomitant hypothesis for this study posits that diatom assemblages growing on fiberglass surfaces coated with antifouling paint will not differ in their structure from assemblages growing on uncoated surfaces. To our knowledge, this is the first study on fouling diatoms growing on fiberglass surfaces, a common material used in fishing boats in the Caribbean Sea, whose duration is significantly diminished by fouling processes.
MATERIALS AND METHODS
Exposure of samples and their characterization. Biofilms grew for 2, 4, and 18 months on 30 (100x40x2 mm) glass-fiber-reinforced polymer plates (GFRP), with only one side coated with antifouling acrylic paint (as commercially sold for boat construction) immersed in Caribbean seawater. The polymer plates were fixed on PVC stands submerged at a 10-m depth, 10 km off the Telchac marine station of CINVESTAV-Mé rida, located in Yucatán, Mexico between 21°7’ N and 89°25’ W. The microfouling assays began in March 2011. After different periods of exposure, triplicate samples of each material were removed from the sea for observation to determine the type of biofouling adhered to the material surfaces. At the end of the essays in August 2012, the rest of the samples was removed for assessment. Likewise, next to the stands a sediment sample was collected with a spatula (using the top of a box of Petri as a mold); this was kept in the same Petri dish in ice and in darkness.
Seawater chemistry. Physical and chemical data were measured in the laboratory a day after each sampling. Seawater is a very aggressive medium for materials and can cause severe damage in a very short time. Usually seawater contains ions (in decreasing quantities) of Cl-, Na+, SO42-, Mg2+, Ca2+, K+, HCO3-, Br-, B3+, Sr2+, F-, and dissolved gases, such as O2 and CO2. Thus, seawater at 10-m depth was analyzed for total salinity, dissolved oxygen, temperature specific sea nutrients, am monium, silicates, phosphates, nitrites, and nitrates.
Diatom flora. Three plates were recovered after two months and pro cessed 24 hours after being removed from the stands. Plates obtai ned after four and 18 months were stored for 4 weeks to separate the diatoms. The two-month microalgae film that was detached from both sides of the fiberglass plates (coated and uncoated) and those that were separated from the sediments were preserved in ethanol (96%). Microalgae detached from plates submerged four and 18 months were treated as a compound sample, without differentiating coated from un coated surfaces, and were used solely for floristic purposes. Part of the samples was observed under the microscope to ensure the existence of live diatoms.
In order to clean the diatom frustules by removing all organic mat ter, the samples were oxidized following the technique by Siqueiros-Beltrones and Voltolina (2000) in which a mild exothermic reaction is carried out mixing a portion of the sample with commercial ethanol (96%) and nitric acid at a 1:3:5 ratio. The oxidized material was then rinsed with tap water until a pH >6 was reached. Afterwards, six per manent preparations were mounted using Pleurax and Zrax (IR 1.7). Cleaned material was also mounted on stubs and covered with a film of graphite spray (Aerodag® G, PELCO®) for observation in a scanning electron microscope (Jeol JSM-7600F). Diatom identification was done at 400X and 1000X under a Zeiss compound microscope equipped with phase contrast, following the works of Schmidt et al. (1874-1959), Pe ragallo and Peragallo (1897-1908), Navarro (1982), Foged (1984), Wi tkowski et al. (2000), Siqueiros-Beltrones (2002), and López-Fuerte et al. (2010). The floristic list was constructed according to Round et al. (1990) and updated in http://www.algaebase.org (Guiry & Guiry, 2014).
Assemblage structure. To determine the relative abundances of the diatom taxa, a sample size of 150 individuals (frustules) per slide was chosen. Numerical analyses were done twice, examining one slide from each plate and one from the sediments.
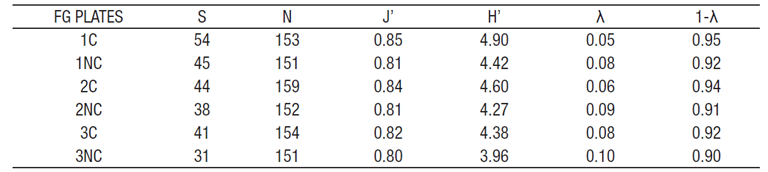
Species diversity of the diatom assemblages was estimated ba sed on information theory (log2) only on the plates submerged for two months, inasmuch as the four- and 18-month samples were not diffe rentiated by surface (coated and uncoated), but rather as a compound sample, and thus were used for species composition analysis of the assemblages and similarity measurement by date. Values of diversity based on information theory were computed using Shannon’s H’ and Pielou’s evenness (J’). Simpson’s diversity index (1-λ) and dominance (λ) were also estimated (Brower & Zar, 1984) to better interpret our esti mates of diversity by considering criteria that weight rare and common species differently (Siqueiros-Beltrones, 1990).
To measure similarity among diatom assemblages, samples were compared on the basis of presence/absence of species using Jaccard index, considering also their relative abundances using Bray Curtis In dex (Magurran, 1988). These were fed into Program Primer V.5 based on an agglomerative classification module with flexible algorithm (Clar ke & Gorley, 2001).
RESULTS
Seawater chemistry. The seawater had total salinity of 37.48, a pH of 7.69, dissolved oxygen 1.1 ppm, and a temperature of 21°C, at a depth of 10 m. Specific nutrients were (expressed in μM L-1): 1.75 ammonium, 2.61 silicates, 0.28 phosphates, 0.04 nitrites, and 1.84 nitrates.
Diatom flora. In general, the diatoms recruited on the fiberglass plates during the study were abundant and diverse (Figures 1, 2, 3).
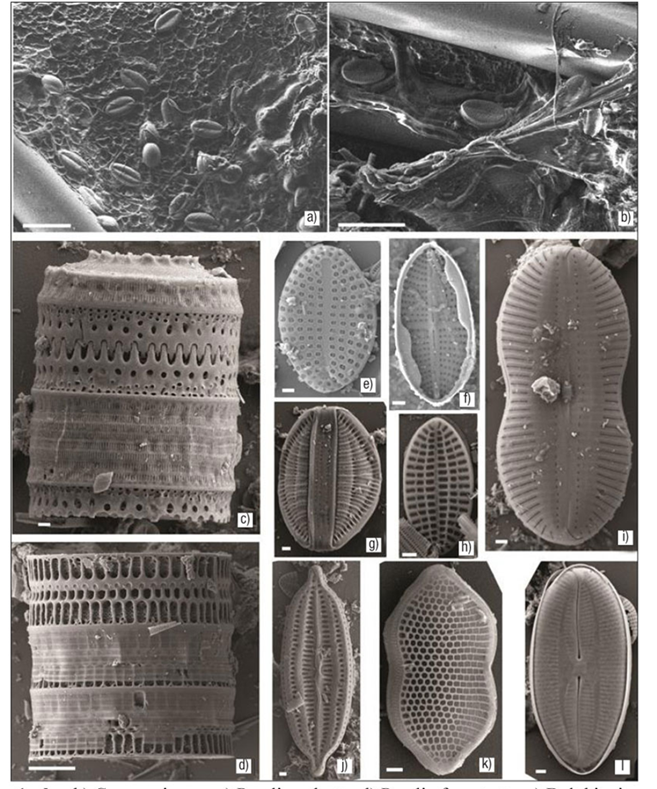
Figures 1a-l a-b) Cocconeis sp. c) Paralia sulcata. d) Paralia fenestrata. e) Delphineis surirella. f) Mastogloia crucicula var. alternans. g) Rhopalodia musculus. h) Cocconeis discrepans. i) Diploneis bomboides. j) Mastogloia corsicana. k) Psammodictyon panduriforme var. minor. l) Fallacia forcipata. Escala: Figs. a-b, d = 10 μm; Figs. c, e-l = 1 μm.
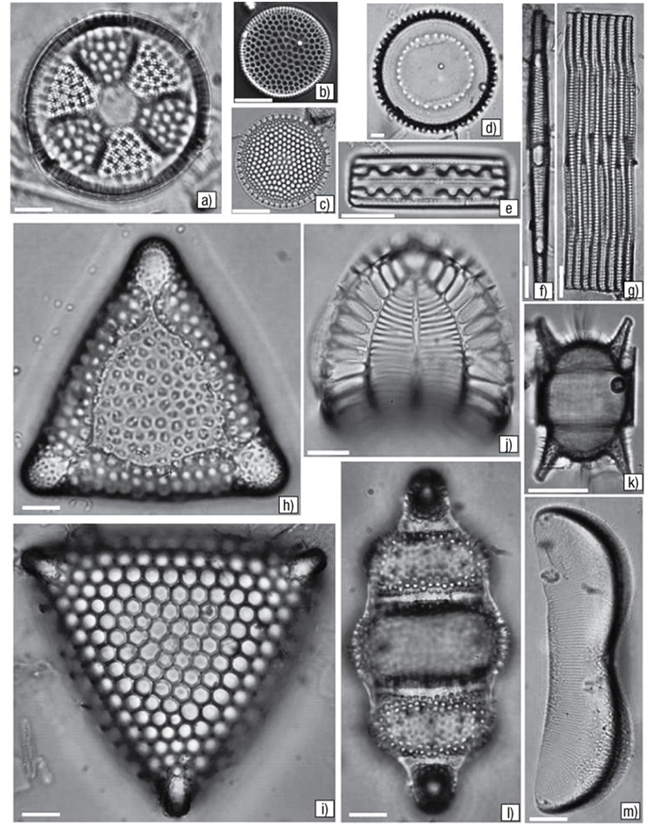
Figures 2a-m a) Actinoptychus senarius. b) Coscinodiscus nitidus. c) Shionodiscus oestrupii. d) Paralia sulcata var. coronata. e) Grammatophora serpentina. f-g) Rhabdonema adriaticum. h) Triceratium reticulum. i) T. favus. j) Campylodiscus samoensis. k) Odontella aurita. l) Terpsinoë americana. m) Auricula intermedia. Escala = 10 μm.
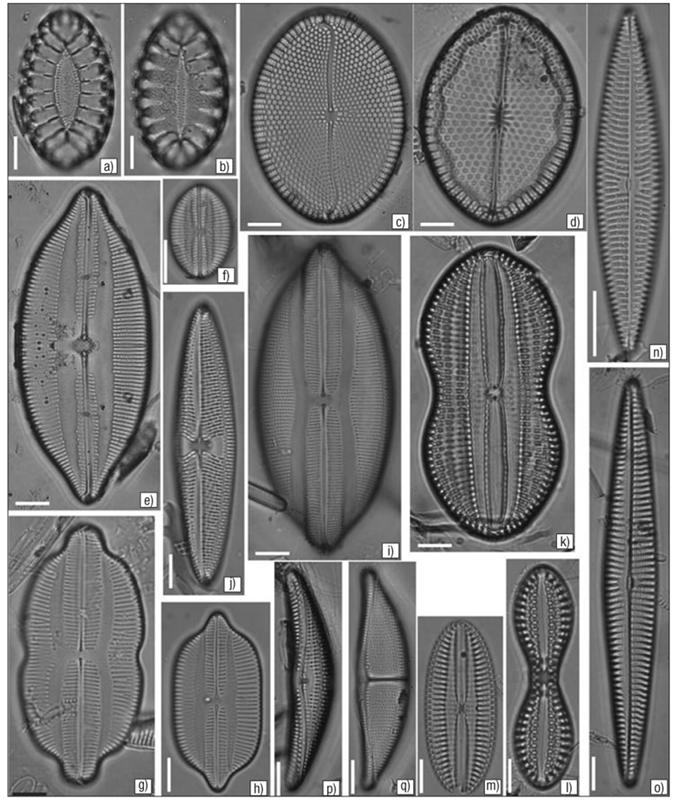
Figures 3a-q a) Surirella fastuosa. b) S. fastuosa var. cuneata. c) Mastogloia splendida. d) M. fimbriata. e) Lyrella approximata. f) L. barbara. g) Navicula (Lyrella) clavata var. distenta. h) Navicula (Lyrella) caribaea. i) Lyrella lyra. j) Trachyneis aspera. k) Diploneis bomboides. l) D. crabro var. dirhombus. m) D. papula var. papula. n) Navicula arenaria var. arenaria. o) N. longa. p) Amphora proteus. q) A. ostrearia var. vitrea. Escala = 10 μm.
Taxono mic observations yielded 170 diatom taxa including species, varieties, and forms within 61 collected genera of diatoms from the fiberglass plates and sediments (Table 1). During the quantitative analysis, a total of 1,117 valves were counted. The Bacillariophyceae were the most diverse class with 129 taxa, the Coscinodiscophyceae yielded 22, and the Fragilariophyceae 19. As in other similar studies, diatom assembla ges were dominated by several pennate species (Cassé & Swain, 2006; Molino et al., 2009; Zargiel et al., 2011; Sweat & Johnson, 2013). Out of the 60 identified genera, the one with most species were Amphora and Nitzschia with 13 and Diploneis and Mastogloia with 11. Meanwhile, 35 genera were represented by a single species, mostly uncommon. Sixty-nine taxa were recorded only once during the quantitative phase, while 35 taxa were recorded only from the two-month submerged plates, nine from the four-month plates, four from the 18-month submerged plates, and 28 were recorded exclusively from sediments. Thus 83% of the recorded taxa in the sediments were present in the fiberglass plates. Only four taxa occurred in the three periods: Amphora turgida Gregory, Mastogloia crucicula (Grunow) Cleve, Rhopalodia musculus (Kützing) O. Müller, and Grammatophora serpentina (Ralfs) Ehrenberg.
Table 1 Overall floristic list of diatoms observed growing on submerged fiberglass plates during 2 months (FG2), 4 months (FG4), and 18 months (FG18), and in sediments (SED), 10 km off the coast of Telchac, Yucatán, Mexico. Recorded only from sediments (▲) .

Overall, the estimated diversity for three of the four plates sub merged for the two- month period was high; these values indicate that, besides the high species richness, there was also a somewhat homogeneous distribution of individuals among species with no single taxon being clearly dominant (Table 1). This is reflected in high-mean estimates of diversity using two indices (Hʼ and 1-λ). Diversity differen ces between the values of the coated and uncoated side (Table 2) were minimal (higher for the assemblage from the coated side). However, said differences do not support rejection of the proposed hypothesis, i.e., structure is similar and thus diatom assemblages develop likewise on both surfaces.
Table 2 Diversity values describing the structure of the diatom assemblages found growing on fiberglass plates (FG PLATES) off the coast of Yu catán, Mexico. C = coated, NC = Uncoated. S = Species richness; H’ = Shannon´s species diversity; J’ = Equitatibility; 1- λ = Simpson´s diversity; λ = Dominance.
Conspicuous diatom taxa, such as Cocconeis thalassiana Romero et López-Fuerte, Delphineis surirella (Ehrenberg) G. W. Andrews, Mas togloia corsicana Grunow, M. crucicula (Grunow) Cleve, and Rhopalodia musculus (Kützing) O. Müller showed a noticeable affinity to the fiber glass plates, either coated or uncoated, both in terms of frequency and abundance. Similarity measurements using Jaccard´s index segregated three associations of diatoms on the fiberglass plates (Fig. 4). However, the samples from the 4- and 18-month plates showed only a 31% si milarity because they had only five species in common, suggesting two distinct assemblages. In contrast, between diatom assemblages from plates submerged 2 months and from the sediments, there was 69% si milarity, since they share 89 species and were clearly segregated from the other two (Fig. 4).
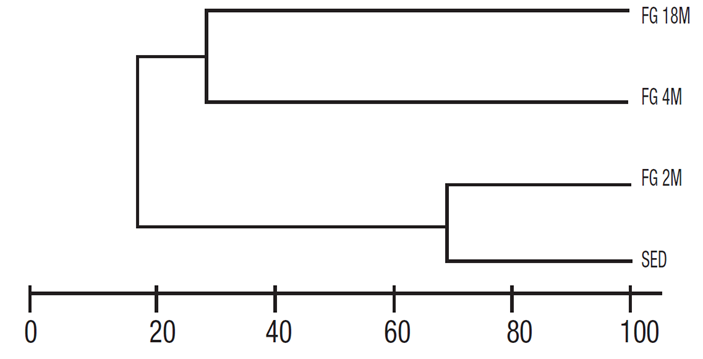
Figure 4 Similarity of diatom assemblages grown on fiberglass (FG) plates and sediments (SED), based on Jaccard’s similarity index. M = Months.
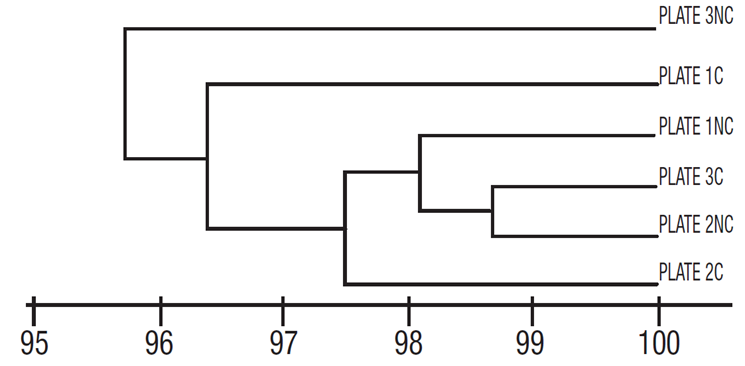
Based on quantitative data, however, the Bray Curtis similarity index shows no group segregation, either between plates or according to the side of the plate (coated and uncoated). In this case, the similarity values between the three plates were very high (>95%), even between coated and uncoated surfaces of the number two and three plates (Fig. 5).
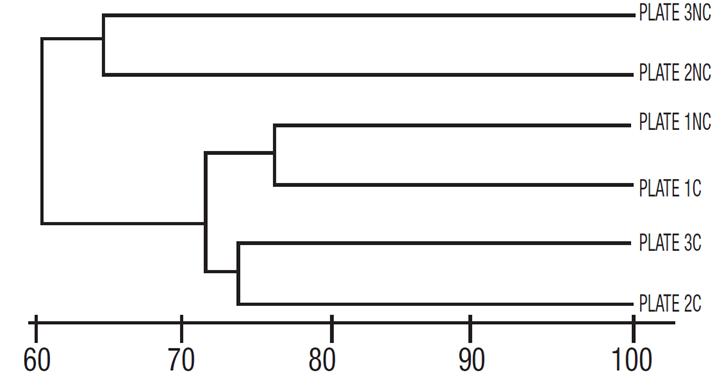
Figure 5 Similarity (Bray Curtis) of diatom assemblages grown only on fiberglass plates, with no group segregation. C = coated, NC = uncoated, surfaces.
On the basis of qualitative similarity (Jaccard´s index), the apparent group formation could be deceiving (Fig. 6). However, because this type of similarity measurement tends to yield much lower values than the qualitative approach, in our experience said differences do not evidence distinct groups; accordingly, the values are relatively high and segrega tion is due to heterogeneous distribution of taxa. Therefore, the overall similarity analysis indicates that no effect exists due to the use of an tifouling paint in terms of deterring taxa typical of a diatom film taxo coenosis, either qualitatively or quantitatively, thus supporting the Ho.
DISCUSSION
Our results reveal that the fouling populations were heterogenous and included epipsammic, epiphytic, and epipelic (tychoplankton) species with different affinities (marine, brackish, and even freshwater). The species composition and structure (diversity, dominance, and equita bility) of the diatom assemblage growing on the surface coated with antifouling paint was very similar to that of the uncoated surface, re gardless of the time the plate had been submerged. Even though the computed diversity values were high, they do fall within the range-esti mated values for this type of taxocoenosis (Siqueiros-Beltrones, 2005). They do, however, suggest that the colonized substrata (coated and uncoated) may be compared to those that favor the growth of diatoms such as macroalgae and seagrasses, which harbor abundant epiphytic forms. Although the number of taxa per plate appeared to be the main component of the high diversity values estimated, the distribution of individuals among the taxa was also uniform, deriving in high values of equitability.
Many of the species found in this study belong to genera from estuarine environments that occur commonly as biofouling (Molino & Wetherbee, 2008), including Amphora, Nitzschia, Diploneis, Masto gloia, and Navicula. As in similar studies (Characklis & Cooksey, 1983; Cooksey et al., 1984), the assemblage in the biofilm was dominated by pennate diatoms, irrespective of the nature of the substratum and the exposure period. Anil et al. (2006) observed that pennate diatoms such as Amphora, Navicula, and Nitzschia species often dominate the fouling assemblage as well as epibiotic populations, since they are able to attach to substrates. Our observations partly agree with Amphora, Nitzschia, Mastogloia, and Diploneis being the dominant taxa; the latter two taxa are very common in sediments.
The examination of the sediment adjacent to the structure holding the fiberglass plates rendered an accurate reference of the origin of the diatoms found on the plates, because many of the diatom taxa have been recorded from sediments (83 %). These observations indicate that the dominance of certain species in the biofilm can be attributed to their dominance in the adjacent sediments, resulting in a higher subs tratum-encountering probability. Benthic diatoms that are frequently re-suspended by hydrodynamic or biotic processes may, after a whi le, colonize a different available substrate (Breznak et al., 1985). Once attached, the growth and assemblage structure is further dependent upon the physicochemical and biological nature of both the ambient water and the substrate surface. Studies reveal that diatoms have spe cific substrate preferences (Mitbavkar & Anil, 2000), and higher diatom recruitment has been observed on fiberglass (hydrophobic) than on a hydrophilic surface such as glass (Patil & Anil, 2005a). Due to its physi cal characteristics, the fiberglass plates allow initially for the settlement of small forms (<20 µm) of diatoms (Figures 1a-b), which in turn make the substrate favorable for large forms (Figures 1c-l). Also, the presence of sand on both sides of the plates may be a factor because diatoms from the sediments (epipelic and epipsammic) may migrate from the sediments (Round et al., 1990) to the available space in the plates, finding it suitable for growth and colonizing it, as observed in this expe riment. Heavy diatom growth has been known to occur as thick films in certain artificial substrata such as PVC surfaces and silicon, where up to 178 taxa have been recorded for colonization periods of three weeks (Siqueiros-Beltrones, 2002).
The assemblage structure was similar to that recorded for many studies on benthic diatoms, i.e., few abundant species, many common species, and many more rare species, but once either in the qualitative or quantitative analysis. The number of identified taxa in this investi gation is similar to diatom assemblages from natural substrata, e.g., López-Fuerte et al. (2013), in which 106 taxa were recorded living as epiphytes of Thalassia testudinum K. D. Koenig in Yalahau lagoon, Quin tana Roo, Mexico. On the other hand, in comparison with other studies that used artificial substrata, the number of diatom taxa recorded here is much higher, e.g., Fernandes et al. (1999) and Patil & Anil (2005b) reported 60 and 51 taxa, respectively, growing on glass slides.
The marked difference in the number of taxa recovered from the fiberglass plates from one period of submergence to another, i.e., 135 taxa in the two-month plates vs. 11 and 21 taxa for the four- and eighteen-month plates, may be explained by the fact that when diatom films reach a certain degree of growth, lumps then begin to detach from the films and so provide colonizing material for other substrata (Siqueiros-Beltrones, 2002). Thus, much of the diatom flora from the late phases of succession would be lacking in the plates submerged for longer periods.
Because exopolymer films secreted by diatoms promote the onset of macrofouling by conditioning the original substrate for invertebrate larvae, it is necessary to acquire a precise knowledge of the micro fouling species from the initial phases of microfouling in order to better understand the ecological processes that may be helpful for controlling fouling events. Likewise, the development of anti-fouling paints should focus on avoiding the settlement and growth of diatoms, thus delaying the onset of macrofouling that generally depends on the modulation of the substrate by pioneer microfouling diatoms.
Overall, the above results suggest a greater ability of benthic dia toms to colonize fiberglass surfaces, which is probably explained by a higher degree of contact between the cells and the surface. Moreover, as observed in natural substrata, the structure of diatom assemblages showed variations that may be attributed to how long the plates were submerged. In order to sustain this, intermediate submersion times should be implemented in order to allow other successional phases to be detected.
Because the microfouling diatom assemblages may provide sui table conditions for the onset of macrofouling, we suggest further re search be undertaken in order to better understand their successional processes, which may aid in developing an efficient strategy for pre venting or delaying the settlement of macrofouling organisms.











 nueva página del texto (beta)
nueva página del texto (beta)