Introduction
Macroalgae of the Caulerpa genus (Chlorophyta: Bryopsidales) are of interest in the marine environment for several reasons. The high growth rates that some species exhibit, and their ability to propagate from asexual fragments have generated serious negative impacts on natural communities (Biber & Irlandi, 2006; Glardon et al., 2008; Pérez-Ruzafa et al., 2012). Three species, C. taxifolia (M. Vahl) C. Agardh, C. brachypus Harvey and C. racemosa (Forsskål) J. Agardh, originally found in warm tropical waters grow rapidly and are therefore classified as invasive plants (Klüser et al., 2004; Lapointe & Bedford, 2010). The factors that determine growth have been evaluated for several Caulerpa species (Khou et al., 2007; Theil et al., 2007; Burfeind & Udy, 2009; Guo et al., 2015). Salinity and temperature, together with nutrients and light, are considered essential parameters for the further cultivation of seaweed on a large scale (Scrosati, 2001; Lapointe & Bedford, 2010). Furthermore, physiological responses of Caulerpa to those abiotic parameters are used to discuss the implications for the management of this green alga for possible invasions (West & West, 2007; Lirman et al., 2014). Little is known, however, about the impact of salinity on the growth of C. sertularioides in tropical environments, such as the Colombian Pacific, where oceanographical conditions are constantly changing due to extensive river discharges and high precipitation patterns along the coast (Tejada et al., 2003). Particularly, on the Pacific coast of Colombia, C. sertularioides inhabits intertidal and shallow subtidal areas (Peña, 1998; 2008). No seasonality of natural populations was registered; however, great biomass was observed during low rainfall season within creeks along the Bay of Tumaco (Marin & Peña, 2014). Unlike other species within this genus, such as C. taxifolia and C. racemosa, there are several studies that address their tolerance to different environmental conditions and their conditions as invasive plants (Piazzi et al., 2001; Lirman et al., 2014). Osmotic acclimatization in response to changes in salinity is a fundamental tolerance mechanism that conserves the stability of the intracellular medium and is therefore essential to maintain an efficient functional state in the cells (Kirst, 1990; Peña et al., 1999; Ospina et al., 2006). It has been suggested that algae can regulate their cell volume by modifying the internal water potential in response to changes in salinity (Goulard et al., 2001; Eggert et al., 2007). Although most marine algae can tolerate fluctuations in salinity over the short term, large variations of this parameter can significantly affect some biochemical processes involved in photosynthesis and growth, altering the biomass, distribution, and productivity of a great number of species (Sousa et al., 2007; Choi et al., 2006; Theil et al., 2007). Those results demonstrated the plasticity and adaptation of Caulerpa species to different salinity gradients compared to other siphonous algae and, therefore, their capacity to spread out in shallow coastal environments.
The aim of this study is to evaluate growth conditions under different salinity conditions of the green alga C. sertularioides and its effect on the distribution and colonization of the species in the estuary.
Materials and methods
Culture conditions and experimental design. Fragments of C. sertularioides were brought from Tumaco Bay, Pacific coast of Colombia (1° 45’ - 2° 00’ N; 78° 30’ - 78° 45’ W). The bay comprises a 350 km2 area with depths varying between 0 and 50 m (Tejada, 2002). Algal fragments were collected from the intertidal zone, during low tide and stored in paper towels moistened with seawater, packed in polyethylene bags and stored in a polystyrene icebox until transportation to the laboratory, according to West and Calumpong (1988). Fragments were cleaned of other benthic materials (sand, shell fragments, etc.) and kept in an environmental chamber with average temperatures of 27 ± 0.37 °C and controlled illumination (40-50 μmol photons m−2 s−1). A 12:12 cycle of light/darkness was maintained during two (2) weeks for their acclimation. Material for subsequent experiments was then selected from these fragments. Fragments of C. sertularioides consisted of a basal portion (stolon), rhizome and 3-4 erect axes (fronds); fresh weight was between 0.2 and 2.0 g and was calculated at the beginning of the experiments (West & West, 2007). Five ranges of salinity were used for the experiments (15, 20, 25, 30, and 35 ppt). These salinities were chosen after examination of historical data of salinity recorded for Tumaco Bay by the oceanographical and Hydrological Research Center of the Colombian Army at the Pacific coast, located in the Port of Tumaco (Tejada et al., 2003). Six replicates of each salinity range were set up and the experiment was repeated twice, with a culture period of 4 (four) weeks each time, and measurements taken every 8 days. Mini-aquaria of 0.5 L capacity were filled with artificially filtered seawater enriched with Provasoli, stirred with aerators (modified by West & McBride, 1999) (10 ml L-1), and changed weekly.
A one-way ANOVA (factor = salinity) was done to test for significant differences (5%) in total new growth. Data were tested for homogeneity of variances using Cochran’s test. Where significant differences were found, Tukey’s HSD test was used for means comparison. The R statistical package version 2.12.0 and SPSS 17.0 were used for the analyses.
Analysis of algal growth. The following growth variables were measured weekly: wet biomass, stolon length (cm), and number of new fronds and rhizomes. In the experimental cultures, growth (increase in wet biomass and stolon length) was calculated as the relative growth rate (RGR), expressed as percent daily growth, applying the following equation used by different authors (Areces, 1995; Anderson et al., 1997; Marinho-Soriano et al., 2002): RGR = [(Wf/Wi)1/t - 1] x 100, where RGR = relative growth rate, Wf = final wet weight, final length, Wi = initial wet weight, initial length; t = time interval elapsed between the two observations.
Results
Salinity had a significant effect on the growth of C. sertularioides (p <0,001; Table 1; Figs 1a- b) during the culture period. The highest growth rates in terms of wet biomass during the culture period occurred for salinities of 25 and 30 ppt, with mean values of 2.262 ± 0.242 % d-1 and 1.408 ± 0.215 % d-1, respectively (mean ± SD, n = 18), that remained constant during the experiment. The lowest growth rates were recorded for salinities of 15 and 35 ppt, which had growth rates of around 1% d-1 and lower. At a salinity of 15 ppt, growth rates were negative as a result of the progressive deterioration of the material at this salinity concentration (Fig. 1a).
Table 1: ANOVA results of differences in RGR (% d-1) of C. sertularioides fragments under different salinity ranges under laboratory conditions.
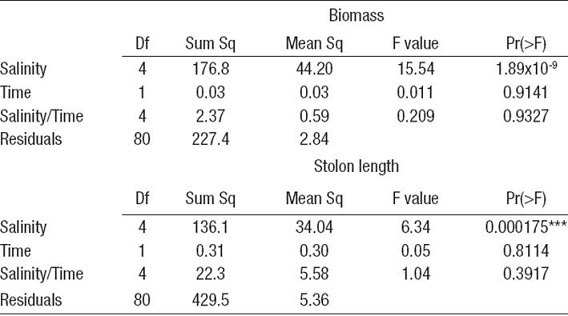
Significant codes (***) = 0, (**) = 0.001, (*) = 0.01, (.) = 0.05. Significant difference = 0.01.
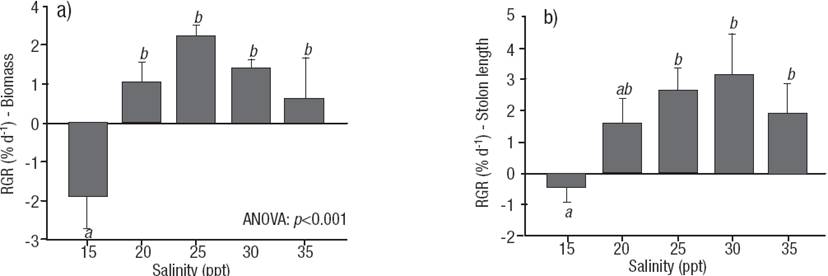
Figures 1a-b: Effect of salinity on Relative Growth Rate (% d-1) of C. sertularioides. a) Biomass. b) Stolon length. Means and standard deviations are shown (n = 18). Different letters represent significant differences between salinities as shown by Tukey´s test, p <0.05.
The fragments of C. sertularioides cultivated at different salinity concentrations produced growth of the existing stolons and new stolons, except at 15 ppt salinity (Fig. 1b). The highest stolon growth rates were observed at salinity concentrations of 25 and 30 ppt, with average values of 2.638 ± 0.712% d-1 and 3.177 ± 1.305% d-1, respectively (mean ± SD, n = 18). The lowest growth rates were observed at 15 ppt salinity (1.616 ± 0.760% d-1).
The appearance of new fronds and rhizomes was observed at all evaluated salinities, excepting 15 ppt salinity. The highest number of new fronds (mean = 2.111 ± 0.787) and rhizomes (mean = 5.111 ± 1.109) was obtained at 25 ppt. The lowest number of new fronds (mean = 1.111 ± 0.192) and rhizomes (mean = 1.167 ± 0.500) was obtained at 35 ppt.
The daily growth rate, determined at three time intervals (Fig. 2), showed that, from the first week of culture, the relative growth rate of C. sertularioides varied under the different salinity concentrations evaluated. This behavior was constant during the culture period and was more obvious at salinities of 25 ppt and 30 ppt than at 15 and 35 ppt. During the 24 days of culture, growth rates of 4.82% d-1 and 3.02% d-1 were recorded at salinities of 25 and 30 ppt, respectively. At salinities of 20 and 35 ppt, growth rates of only 2.8% d-1 and 2.6% d-1 were observed.
Discussion
According to these experimental results, C. sertularioides growth was significantly affected by changes in salinity. Growth increased with salinities over 20 and up to 30 ppt; the best growth response was obtained at 25 ppt. Variations in salinity can significantly affect the growth, distribution, and productivity of macroalgae (Chesnes & Montague, 2001). These results are consistent with the natural habitat of C. sertularioides in Tumaco Bay (Nariño, Colombia), where it is found in areas with average salinities of 24 ppt during its annual cycle (Fig. 3). Indeed, higher biomass of C. sertularioides showed a direct relationship with seasonal variation of salinity in the study area (Marin & Peña, 2014). Salinity is clearly one of the key variables influencing abundance and distribution of macroalgal meadows in shallow coastal environments, and it is the factor most easily manipulated through management decisions (Biber & Irlandi, 2006; Pérez-Ruzafa et al., 2012; Lirman et al., 2014).
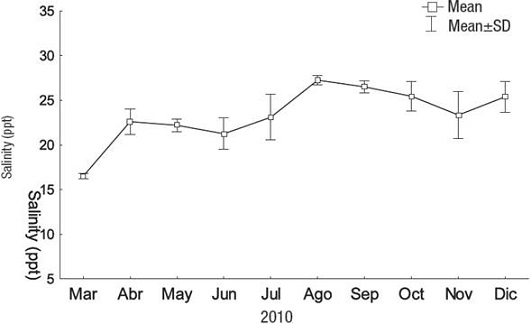
Figure 3: Salinity values recorded at sampling locations in Tumaco Bay (Nariño, Colombia), during 2010 (Marin & Peña, 2014).
Results of this study showed a progressive deterioration of C. sertularioides fragments at 15 ppt of salinity, demonstrating a negative growth rate in terms of biomass and stolon growth (Mosquera-Murillo, 2012). Other species of the Caulerpa genus, such as C. taxifolia (West & West, 2007) and C. lentillifera J. Agardh (Guo et al., 2015), exhibit reduced chlorophyll content and decreased Fv/Fm values, which could be the result of a disorganization of the cellular structure and chloroplasts in turgid cells. The rapid growth of C. sertularioides at salinities above 20 ppt observed in this study, as well as its deterioration at lower salinities, has also been reported for other species in this genus, such as C. taxifolia (Biber & Irlandi, 2006; West & West, 2007; Theil et al., 2007).
The fragments of C. sertularioides grown at higher salinity concentrations (35 ppt) also showed low growth rates (0.618 ± 1.047 % d-1), but maintained normal coloration. According to Kirst (1990), growth can be reduced near the salinity tolerance level in order to maintain osmotic regulation, which can guarantee survival. The reduction in growth can also be a consequence of the cumulative effect of enzymes and the reduction of turgidity pressure that inhibits cellular division (Lee & Liu, 1999; Liu et al., 2000; Kamer & Fong, 2000). West and West (2007) reported optimal growth rates of C. taxifolia at salinities between 22.5 and 30 ppt, with null growth at lower salinities. (Liu & Phang, 2010). Increases and decreases in salinity generate stress in macroalgae, and species that are tolerant to these conditions present different strategies for growth (Liu et al. 2000; Ospina et al., 2006; Choi et al., 2010; Guo et al., 2015).
These initial laboratory experiments demonstrated the effect of salinity changes on growth of C. sertularioides, and suggest that a range of experiments investigating other environmental factors, such as temperature, light, and nutrient conditions, would be beneficial in understanding the distribution of this species in the region.











 nueva página del texto (beta)
nueva página del texto (beta)



