Introduction
Among vertebrates, fish have the greatest number of species. Atheriniformes contains six, globally-recognized families, from which Atherinopsidae is exclusive to the New World (Nelson, 1994), and includes two genera restricted to inland waters of the Mexican plateau, Chirostoma and Poblana.
Chirostoma is the representative genus of the Atherinopsidae. It is endemic to Mexican ichthyofauna and includes the silversides "charales" and "peces blancos"; among the latter, C. humboldtianum (Valenciennes 1835) has the greatest but disjunct distribution, isolated in lakes and ponds from the Valley of Mexico to the Pacific Ocean following the Lerma-Chapala-Santiago basin system (Alvarez & Navarro, 1957; Barbour, 1973a, b). It also has high economic and cultural value (Jiménez & Gracia, 1995; Soria-Barreto et al., 1998; Barriga-Sosa, 2001; Barriga-Sosa et al., 2002, 2004, 2005; Rojas & Sasso, 2005).
Chirostoma humboldtianum faces several problems such as habitat reduction and modification (e.g., the groundwater extraction in the Basin of Mexico, Alvarez & Navarro, 1957), pollution, introduction of exotic species (Barbour, 1973a; Berlanga-Robles et al., 2002), and translocations. These in turn played important roles in the decline and extirpation of local populations (Lyons et al., 1998; Soria-Barreto et al., 1998; Soto-Galera et al., 1998; Barriga-Sosa et al., 2002, 2004, 2005; Rojas & Sasso, 2005; Mercado-Silva et al., 2006). In despite of all these threats to the species, it has not been designated as threatened in the Official Mexican Standard Norms (i.e., NOM-059-Semarnat-2010) or in the International Union for the Conservation of Nature (IUCN 2015).
Morphological and genetic studies on the "humboldtianum group" (a group mainly made up of "peces blancos", C. humboldtianum included) at the intra and interspecific level (Barriga-Sosa, 2001; Barriga-Sosa et al., 2002), found the presence of significant morphological and genetic differences between populations of C. humboldtianum. These studies suggest that habitat diversity and geographic isolation might have played important roles on their heterogeneity. However, species distribution (populations within the same region) has been of limited interest. Only recently, García-Martínez et al. (2015), using mitochondrial DNA sequences and including samples from six locations along the entire disjunct distribution of the species, have found evidence of a strong genetic structure of the species and have established the presence of at least six populations.
Where differences in the number and structure of chromosomes have been detected, cytogenetic analysis of fish have been used to characterize both populations (i.e., rainbow trout, Thorgaard, 1983; Ocalewicz & Dobosz, 2009) and species (Ariids, Uribe-Alcocer, 1988; cichlids, Uribe-Alcocer et al., 1992, 1999; Hodaňová et al., 2014), contributing to the knowledge and understanding of the evolutionary history and relationships of organisms (White, 1973; Huxley, 1974; Mank & Avise, 2006). For the members of Chirostoma, there is no information regarding intraspecific chromosome characterization, and of 18 recognized species, only five have been described as karyotypes (Uribe-Alcocer et al., 2002; Uribe-Alcocer & Díaz-Jaimes, 2003). Thus, the aim of this study is to describe the karyotype of C. humboldtianum along its distribution in the Lerma-Chapala-Santiago basin in order to solve one important question: Does the species possess a conserved karyotype along its disjunct distribution?
Materials and Methods
Fish sampling. We obtained twenty-two C. humboldtianum adults from stocks maintained in culture conditions in the Planta Experimental de Producción Acuícola at the Universidad Autónoma Metropolitana-Iztapalapa campus. The original stocks were collected from four natural locations (Table 1, Fig. 1) that correspond to what hereinafter we refer to as "populations" according to García-Martínez et al. (2015). Our populations originated on the high, middle, and low Lerma-Chapala-Santiago basin and were identified by their morphological characters according to Barbour (1973b).
Table 1 Collecting sites for the analyzed Chirostoma humboldtianum.

OBS = Original brood stock collected in the referred site; PExPA = Planta Experimental de Producción Acuícola; MASL = Meters above sea level; n = Number of organisms analyzed.

Figure 1 Map showing the collected localities (populations) from Chirostoma humboldtianum specimens.
Chromosome preparation. We obtained chromosome spreads from gill epithelium using a 1 % sodium citrate hypotonic solution following Denton (1973). Slides with chromosomes were stained with 10 % Giemsa Sörensen solution, pH 7 for 15 min. Mitotic chromosomes were screened in an Olympus CX31 microscope equipped with a digital Infinity 1 camera and 7.5X zoom. All images were processed using Adobe Photoshop CS5 Version 8.0.1. Ten mitoses of Las Tazas, Tiacaque dam population, four of Villa Victoria dam, six of Tepuxtepec dam and five of San Pedro Lagunillas, were measured to determine chromosome relative length. Centromeric index and arm ratios of chromosomes were used to classify them following Levan et al. (1964): a ratio from 1.0 to 1.7 for short to long arms (p and q) corresponded to metacentric (m); 1.71 to 3.0 to submetacentric (sm); between 3.1 and 7.0 to subtelocentric (st) and higher than 7.0 to telocentric (t) chromosomes. The fundamental number (FN) was determined as the total number of chromosome arms (Matthey, 1973). Ideograms were prepared according to the relative lengths of the short and long arms, as well as the centromere position (Denton, 1973) of each chromosome pair.
We analyzed differences between relative lengths of the most variable chromosome pairs in morphology of different cytotypes by a Wilcoxon-Mann-Whitney test (STATISTICA Version 10).
A UPGMA dendrogram was constructed in order to form groups based on cytotype similarities. A similarity matrix was built containing the following characters: 1) fundamental number; 2) numbers of metacentric, submetracentric, subtelocentric, and telocentric chromosomes; 3) morphology of pairs 1, 2, 4, and 5; and 4) the relative lengths of each chromosome pairs (1 to 24) and using the Bray-Curtis coefficient (MVSP Version 3.0, Kovach, 1998). We used the Similarity Percentage Analysis (SIMPER) (Clark, 1993) to determine the relative contribution of populations to dissimilarity between the resolved groups and average dissimilarity; the analysis was conducted in Past 3.03 (Hammer et al., 2001).
Results
From 429 mitotic spreads analyzed from the four populations of C. humboldtianum, 70.39 % revealed a diploid number of 2n = 48 chromosomes. One specimen from Tepuxtepec dam exhibited a tetraploid karyotype (4n = 96) 57.14 % of the analyzed mitosis (12 out of 21 mitosis) for that specimen (Table 2).
Table 2 Number of individuals, preparations and mitosis analyzed per population of C. humboldtianum.
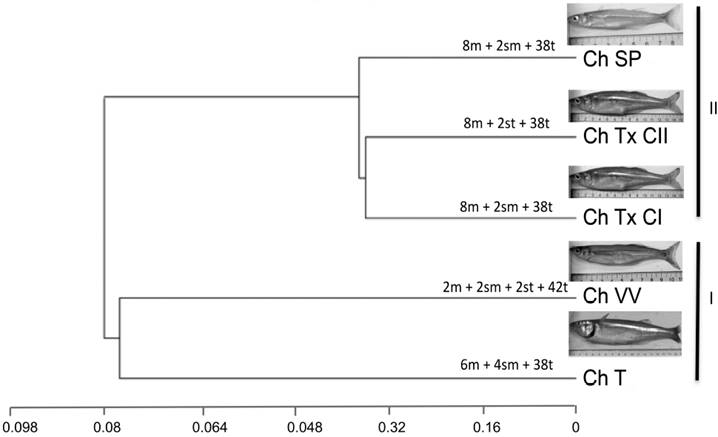
Five cytotypes were resolved in the four populations analyzed: one cytotype for each one of the populations, Las Tazas, Tiacaque, and Villa Victoria dams and San Pedro Lagunillas lagoon, and two cytotypes in Tepuxtepec dam (Tables 2, 3, and Fig. 2).
Table 3 Chromosomal characters of the five cytotypes described for C. humboldtianum.
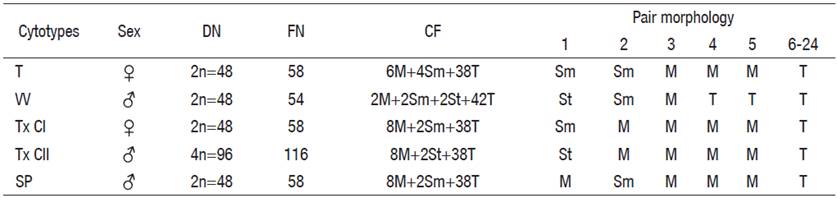
T = Las Tazas Tiacaque dam; VV = Villa Victoria dam; Tx CI = Tepuxtepec dam, Cytotype I; Tx CII = Tepuxtepec dam, Cytotype II; SP = San Pedro Lagunillas lagoon; DN = Diploid number; FN = Fundamental number; CF = Chromosome formulae; M = Metacentric; Sm = submetacentric; St = subtelocentric; T = telocentric; ♀ = Female; ♂ = male.
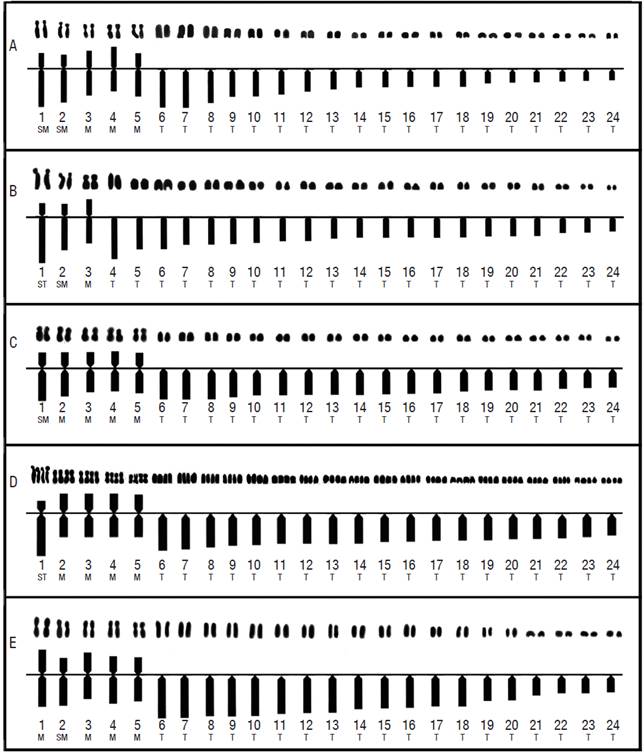
Figure 2 Karyotypes and idiograms of the five cytotypes resolved for Chirostoma humboldtianum. A. Female karyotype and idiogram from Las Tazas Tiacaque dam (2n = 48, FN = 58); B. Male karyotype and idiogram from Villa Victoria dam (2n = 48, FN = 54); C. Female karyotype and idiogram from Tepuxtepec dam (CI, 2n =48, FN = 58); D. Male karyotype and idiogram from Tepuxtepec dam (CII, 4n = 96, FN = 116); E. Male karyotype and idiogram from San Pedro Lagunillas lagoon (2n= 48, FN = 58).
No pairs of heteromorphic chromosomes that could correspond to sex chromosomes were identified in any of the studied populations. All chromosome pairs were ordered by size, from the largest to the smallest (Table 4 and Fig. 2).
Table 4 Relative chromosome length averages of the cytotypes of Chirostoma humboldtianum.
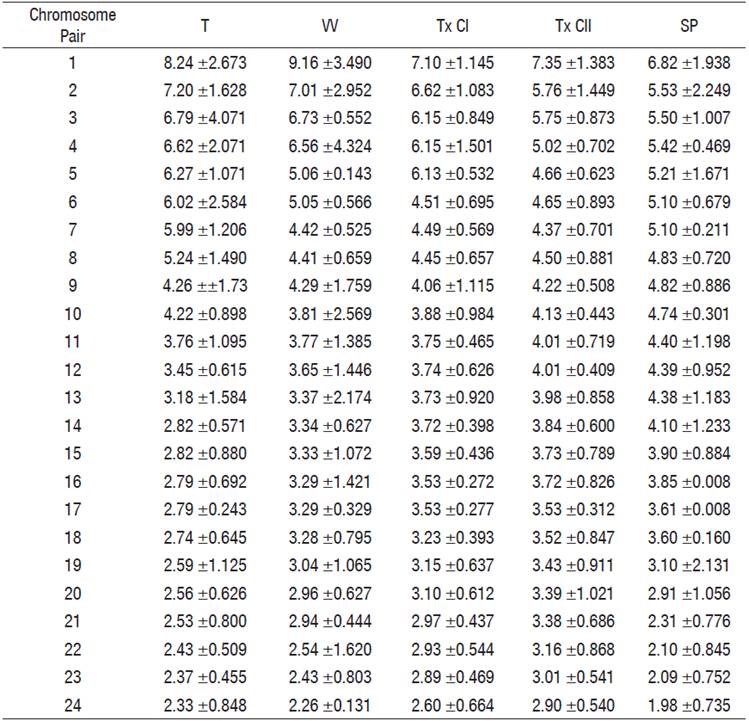
Las Tazas Tiacaque dam (T), Villa Victoria dam (VV), Tepuxtepec dam, Cytotype I (TxCI), Tepuxtepec dam, Cytotype II (TxCII), San Pedro Lagunillas lagoon (SP).
Chromosome pairs 1, 2, 4, and 5 were the most variable in morphology. Pair 1 was submetacentric and subtelocentric for the cytotypes from Tepuxtepec. Pair 2 was metacentric in both cytotypes from Tepuxtepec dam and submetacentric in the cytotypes from Las Tazas, Tiacaque, and Villa Victoria dams and San Pedro Lagunillas lagoon. Pairs 4 and 5 were metacentric in Las Tazas, Tiacaque, and Tepuxtepec dams and San Pedro Lagunillas lagoon, and acrocentric in Villa Victoria dam. However, their relative lengths did not show differences (P = 0.591, 0.9079, 0.591, and 0.6099, for pairs 1, 2, 4, & 5, respectively).
The constructed dendrogram using 33 chromosome characters allowed the identification of three groups. Based on their similarity, Las Tazas, Tiacaque, and Villa Victoria dams made up group I. The cytotypes from Tepuxtepec dam and San Pedro Lagunillas lagoon were placed in group II (Fig. 3). Geographically, group I corresponds to individuals from the high Lerma-Chapala-Santiago basin, and group II to individuals from the middle and lower basin. The SIMPER analysis identified the population that contributed most strongly to the configuration of groups. Average dissimilarity for both groups was 35%. The contribution of dissimilarity of the populations of C. humboldtianum from Villa Victoria dam (35.42%) and San Pedro Lagunillas (35.46%) are close to the overall average dissimilarity, thus, these populations appear to contribute the most to the conformation of Groups I and II.
Discussion
The four populations of C. humboldtianum analyzed in this study showed a diploid number (2n = 48), which concurs with the most frequent diploid number found in Atheriniformes (Atherinopsidae, in Labidesthes, Membras and Menidia, Jeffrey & Fitzsimons, 1987; Warkentine et al., 1987; Odontesthes, Sola et al., 1988; Basilichthys,Gajardo, 1992; and Atherinella,Da Silva Cortinhas et al., 2003; Sczepanski et al., 2007). It is also considered to be the ancestral number for several freshwater teleost fish (i.e., salmonids, Amaro et al., 1996; cichlids, Thompson, 1979; Arias-Rodríguez et al., 2006) and even marine fishes (Galetti et al., 2000). Overall, within the family, the diploid number 2n = 48 is a conserved characteristic, except for C. patzcuaro (2n = 44) (Uribe-Alcocer et al., 2002; Uribe-Alcocer & Díaz-Jaimes, 2003).
On the other hand, the fundamental number (FN) in Chirostoma humboldtianum populations was variable (FN = 54, 58 and 116). Variation in FN can be explained either by the occurrence of chromosomal rearrangements consistent with pericentric inversions and/or heterochromatin additions (Jackson, 1971; Thitiot-Quiévreux, 1994; Appels et al., 1998; Sobti et al., 2002). These rearrangements can affect only the fundamental number, but not the diploid number (Appels et al., 1998; Sobti et al., 2002). These patterns are common in fish and play a significant role in fish chromosomal evolution (i.e., Cyprinidae, Uyeno & Miller, 1973; Cichlidae, Thompson, 1979; Atherinopsidae, Jeffrey & Fitzsimons, 1987; Uribe-Alcocer et al., 2002; Muñoz et al., 2006; Carangidae, Lobotidae and Sciaenidae, Tripathy & Das, 1988; and Batrachoididae, Merlo et al., 2005). Particularly within Atheriniformes the FN is very variable, with chromosome arms recorded from 44 to 86, and such variation has been related to pericentric inversion rearrangements (Jeffrey & Fitzsimons, 1987; Warkentine et al., 1987; Sola et al., 1988; Gajardo, 1992; Uribe-Alcocer et al., 2002; Da Silva Cortinhas et al., 2003; Sczepanski et al., 2007).
Although morphological variation was detected in chromosomes pairs 1, 2, 4, and 5 of Chirostoma humboldtianum, non-significant differences were observed when their relative lengths were compared; hence we explain the changes in the morphology of these pairs by rearrangements of the pericentric-inversion type rather than by heterochromatin addition.
Considering that pericentric inversions are the most frequent rearrangements detected on the resolved karyotype variation of C. humboldtianum, then the hypothesis of a process of orthoselection, in which only one type of chromosome rearrangement occurs repeatedly within a species (White, 1973, 1978, & 1978a), could be proposed as the predicted mutation model for the species. Although karyotype analysis with a larger sample size is suggested to estimate chromosome mutation rate (King, 1993), the presence of at least two different cytotypes in the population in the Tepuxtepec dam population allows us to support the proposed hypothesis.
The arrangement observed in the inferred dendrogram, conformed by two resolved groups, supported by the SIMPER average dissimilarity concurs with the preliminary layout of disjunct distribution of the species, where each population possesses a different cytotype, indicating geographic isolation and a process where intraspecific karyotype diversification might promote allopatric speciation (Key 1968, fide in King 1993). This proposal is supported by intraspecific variation previously reported for the species at the morphological, meristic, and allozyme level, which were also explained by the geographic isolation of the studied populations (Barriga-Sosa 2001; Barriga-Sosa et al., 2002), and more recently with sequences of the mitochondrial genome by García-Martínez et al. (2015), where a high degree of genetic divergence between the studied populations was confirmed.
The results presented here allow us to suggest that the cytotype from the Villa Victoria dam could represent the most primitive karyotype described so far for Chirostoma humboldtianum and for the genus; this is so basically because it contains the largest number of single-armed chromosomes, it coincides with the diploid number of 48, also considered the primitive teleost karyotype in about 200 fish species (Nayyar, 1966; Gyldenholm & Scheel, 1971; Thompson, 1979; Rab et al., 1983; Feldberg & Bertollo, 1985; Salas & Boza, 1991; Martins et al., 1995; Uribe-Alcocer et al., 1992, 1999; Arias-Rodriguez et al., 2006), and is supported by the relative contribution of this population to the SIMPER average dissimilarity.
With respect to the polyploid organism that we found at the Tepuxtepec dam, we explain its presence by a possible autopolyploid mechanism, according to Leggatt & Iwama (2003) and Vasil'ev (2009), where the entire chromosomal complement could be derived from only one parental species. In this species, the fertilization of the haploid egg occurs by haploid sperm with subsequent errors in meiotic reduction, due to abrupt changes in experimental conditions, such as temperature or exposure to high hydrostatic pressure (Arai, 2001), which in turn, leads to spontaneous autotetraploids, that are not morphologically different from their diploid counterparts. The tetraploid specimen described in the present study was a male and showed no apparent morphological differences to their diploid counterparts from the same population (Table 1). Spontaneous autopolyploids have been reported in a closely related silversides, Menidia sp, Odontesthes x Patagonina (Schultz, 1980; Strüssmann et al., 1993, 1997), and in other teleost fish, (i.e., cyprinids, Machordom & Doadrio, 2001; Leggatt & Iwama, 2003; Steven & Smith, 2004; and acipenserids, Schreier et al., 2013); however, in order to test this hypothesis, further studies are required (i.e., crosses between populations and/or polyploid induction).
In summary, the most common diploid number in the genus Chirostoma is 2n = 48. The intraspecific karyotype variation encountered in C. humboldtianum was caused by rearrangements of the pericentric-type inversions and could be associated to its disjunct distribution. The chromosome changes observed within the species could respond to a process of karyotype orthoselection. The tetraploid organism encountered in Tepuxtepec is likely to have resulted from an autopolyploid mechanism.











 nueva página del texto (beta)
nueva página del texto (beta)