Introduction
Freshwater cyanobacteria form blooms and scums containing millions of cells per liter and often are the predominant division of phytoplankton in eutrophic environments. It is a world-wide problem because may cause anoxic conditions and frequently high mortalities among aquatic animal populations, furthermore these blooms affect directly the abundance and distribution of phytoplankton and zooplankton in freshwater environments; some of these cyanobacteria form colonies and synthesize numerous secondary metabolites with toxic properties (WHO, 1999; Pietsch et al., 2001; Hudnell, 2008).
In Mexico, Microcystis is one of the most common cyanobacterium that inhabit tropical and temperate waterbodies, also is the most abundant bloom-forming throughout the year (Ramírez-García et al., 2002; Arzate-Cardenas et al., 2010; Vasconcelos et al., 2010). The principal secondary metabolites produced by Microcystis are a family of more than 80 cyclic peptide toxins, collectively known as microcystins (MCs). In freshwater environments, MCs are the most frequently reported and monitored toxins; these are characterized based on the arrangement of seven amino acids in a ring structure containing five fixed D-amino acids and two variable L-amino acids which differ mainly at positions 2 and 4 (Fig. 1), the most common MCs isoforms are MC-RR, MC-YR, MCLR and MC-LA (Harada et al., 1996; WHO, 1999; Metcalf et al., 2009).
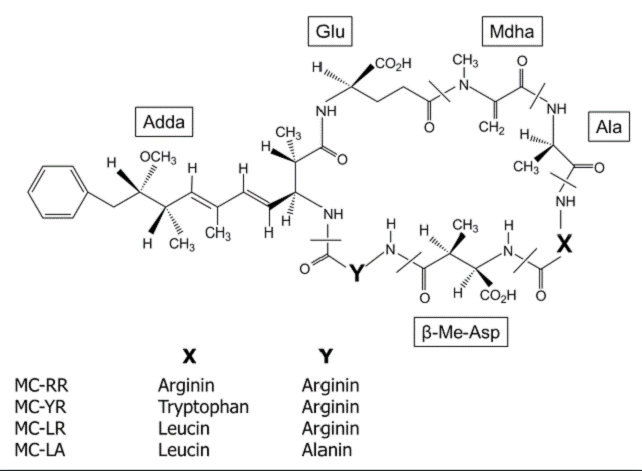
Figure 1 General structure of microcystins (MCs). X and Y are variable L-amino acids (Harada et al., 1996).
Biotic and abiotic factors control the production of MCs, further some authors have reported differences in the production of MCs at strain-level for the same species of Microcystis (Hisbergues et al., 2003; Metcalf et al., 2009); among biotic factors, competition from other phytoplankton species is one of the possible factors responsible for the induction of MCs in Microcystis, but the most documented is the impact of zooplankton grazing on Microcystis (Jang et al., 2003; 2007; 2008; Reynolds, 2006; Hudnell, 2008).
Several species of zooplankton, including copepods, cladocerans, rotifers, and protozoans are reported from waterbodies containing bloom-forming Microcystis (Ramírez-García et al., 2002). Available literature also suggests that copepods are selective grazers and hence probably avoid Microcystis, large cladocerans are usually absent in Microcystis-blooms and, rotifers and protozoans are generally too small to consume colonial Microcystis (Kâ et al., 2012). Therefore, it is not clear if the eventual extinction of large sized (>1000 μm) cladocerans in eutrophic waterbodies is the result of toxin-induction in cyanobacteria through zooplankton grazing or feeding difficulties associated with mechanical manipulation of large colonies. Feeding studies tend to suggest, cladocerans including Daphnia pulex and rotifers including Brachionus calyciflorus are capable of feed on sonicated cyanobacterial cells (Pérez-Morales et al., 2014). Laboratory studies also show that MCs from ingested cyanobacterial cells cause reduction in somatic and population growth rates of both cladocerans and rotifers (Nandini & Rao, 1998). It is not known if the production as well as the quantity of MCs in natural populations of Microcystis is mainly due to the presence of cladocerans or rotifers.
Therefore, the aim of this work was to determine the effect of the presence of the cladoceran Daphnia pulex and the rotifer Brachionus calyciflorus on the production of MCs in the sonicated cells of Microcystis spp.
Materials and methods
Zooplankton species Daphnia pulex (1500 μm) was originally isolated from Espejo de Los Lirios Lake, while Brachionus calyciflorus (175 μm) was from Xochimilco Lake (both Lakes in Mexico City). Clonal cultures were separately established for each zooplankton species starting from a single parthenogenetic female. The zooplankton species were cultured in EPA medium (Weber, 1993) and fed using the laboratory-grown green alga Scenedesmus acutus (Meyen, 1829) for several months before experimentation. EPA medium is moderately hard water; this was prepared by dissolving NaHCO3, CaSO4, MgSO4 and KCl (96, 60, 60 and 4 mg, respectively) in one liter of distilled water. Scenedesmus acutus was batch-cultured using Bold's basal medium supplemented with NaHCO3 (0.25 g/L every third day) (Borowitzka & Borowitzka, 1988).
Colonies of Microcystis spp. were freshly collected from Chapultepec Lake (Mexico City) during summer season. Once in the laboratory, selected colonies were sonicated using an ultrasonicator (ColePalmer Instruments Co., USA) at 20 kHz 50 Watts for 1 min to release unbroken Microcystis cells from mucilage and quantify. Then unicellular Microcystis were maintained at three cell densities (0.5, 1.5 and 4.5 x 106 cells/mL, adding EPA medium to achieve the desired densities) in the presence of D. pulex (25, 5 and 0 (control), individuals) or B. calyciflorus (250, 50 and 0 (control), individuals). Experiments were conducted in 50 mL in transparent jars. Treatments were replicated 3 times and incubated at 25 ± 2 °C. The experiments were finished at day 4. Thus, the experimental design was as follow, for each zooplankton species 27 test jars were used (3 Microcystis cell densities x 3 zooplankton densities (including control) x 3 replicates). Loss of Microcystis cells due to possible feeding by the tested zooplankton was taken into account (Pérez-Morales et al., 2014).
Once finished the experiments, zooplankton species were filtered and, Microcystis cells were frozen at -80 °C for two days and then thawed at room temperature for 1 day. The material was then sonicated in an ultrasonicator (ColePalmer Instruments Co., USA) at 20 kHz 50 Watts for 5 min. The freeze/thaw cycle was repeated 5 times (including sonication) to disrupt the cells and release toxins, following Pietsch et al. (2001). Worth noting that previous test on laboratory shown that 1 min of Microcystis colony sonication release Microcystis cells from mucilage with no lysis of individual cells (Pérez-Morales et al., 2014); in this study it was found that 5 min of sonication disrupt Microcystis cells, then MCs inside the cells may be released.
Samples were filtered to obtain cellular extracts under low vacuum, through nitrocellulose membrane filters (Ø 47 mm and nominal pore size of 0.45 μm; Whatman International®, Kent, UK) to remove broken cells. Cell extracts were stored in darkness and frozen until evaluation. Then quantification of MCs concentration in the cell extracts was performed with ELISA test, based on specifications of the QuantiPlateTM kit for MCs detection (EnvirologixTM). The test quantified total MCs in water samples from which we derived the MCs concentration per cell. The same procedure was followed for controls (sonicated Microcystis cells but without the presence of zooplankton).
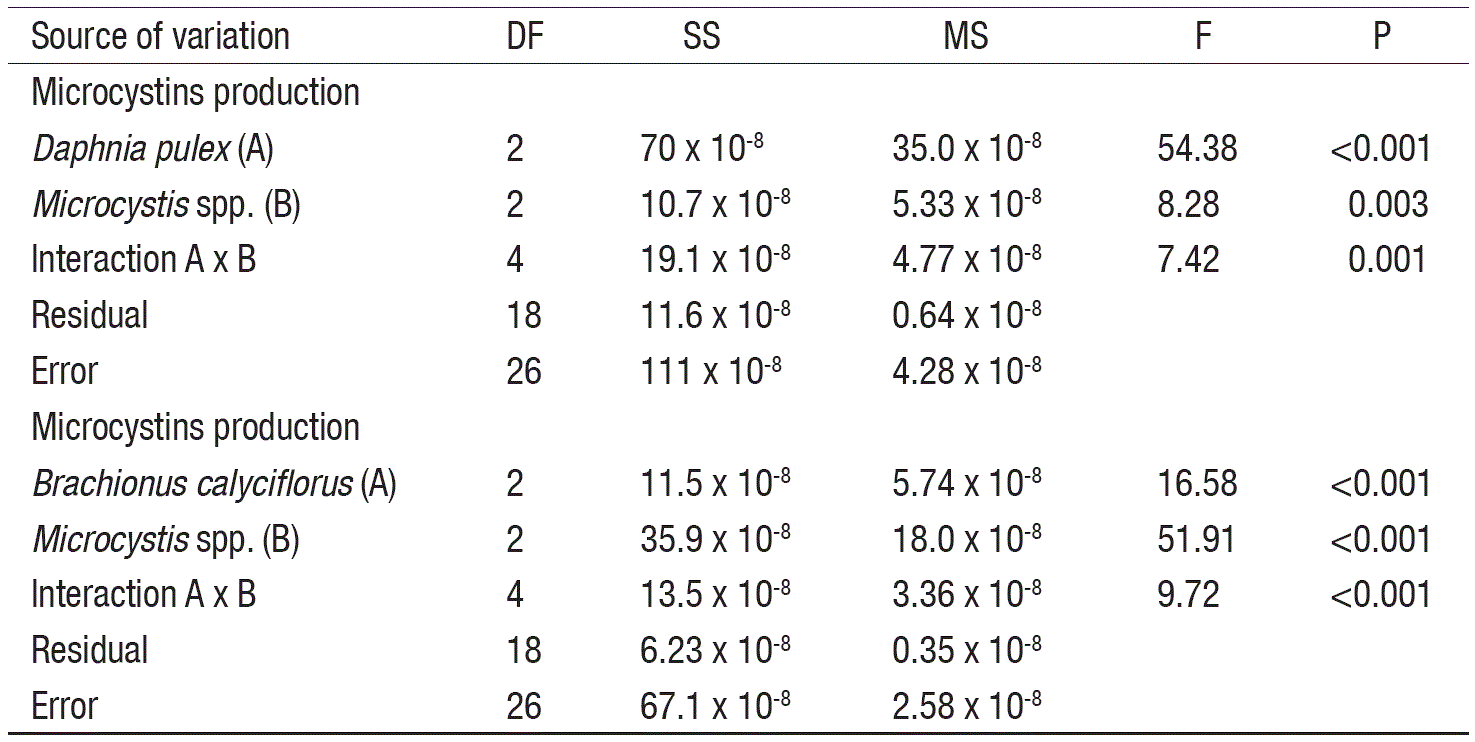
Data on MCs production for each zooplankton species were statistically tested using two-way statistical analysis of variance (ANOVA), and Tukey post-hoc multiple comparisons test. The minimum level of statistic significance was set at p<0.05 (SigmaPlot ver. 11, Systat Software, San Jose, CA).
Results
In controls, the MCs levels decreased (3.98 to 1.32 x 10-4 ng/cell) with the increase of cell density (Table 1). At low cell density and in the presence of high zooplankton density, the MCs concentration was significantly higher as compared to controls (p<0.001, F-test, Table 2). However, under low zooplankton density and at low cell density (0.5 x 106 cell/mL), the MCs levels did not vary significantly. At intermediate cell density (1.5 x 106 cell/mL) MCs content not showed significant differences among zooplankton densities compared to control, excepting the interaction with the higher density of Daphnia, with a MCs content of ~4.71 x 10-4 ng/cell. Moreover, interaction among low zooplankton density and intermediate cell density (1.5 x 106 cell/mL) induce higher MCs production than interaction among low zooplankton density and high cell density (4.5 x 106 cell/mL) for both rotifers and cladocerans; these results showed significant differences. Differences in Microcystis cell densities among treatments (including control) before and after of the experiments were not significant.
Table 1 Microcystins production (ng/cell. • 10-4) in Microcystis spp. induced by zooplankton (Daphnia pulex and Brachionus calyciflorus).
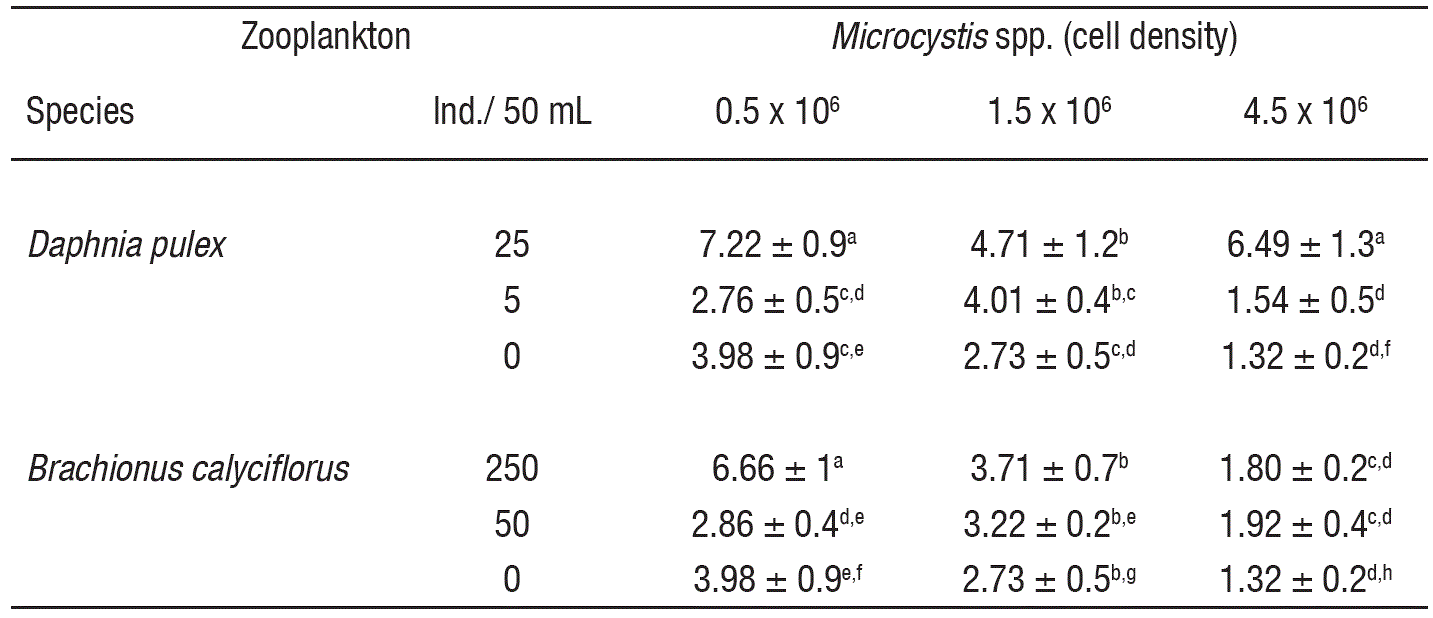
Data show the mean ± standard deviation based on 3 replicates. For each variable, data carrying similar alphabet are not statistically significant (p>0.05, Tukey test).
Discussion
Microcystins produced by cyanobacteria are a global problem because they limit the use of potable water, recreational area, or agricultural irrigation; the maximum permissible value of MCs for drinking water sources is set to 1 ng/mL (WHO, 1999). In Mexican freshwater bodies used for drinking-water or irrigational purposes, the MCs levels in certain months occasionally reach values much higher than the maximum permissible limits (Vasconcelos et al., 2010; Pineda-Mendoza et al., 2012). For example, recently Alillo-Sánchez et al. (2014) have reported higher than 5 ng/mL MCs levels in Valle de Bravo, a drinking water reservoir in Central Mexico.
It is well known that abiotic factors such as light intensity, temperature and nutrients (nitrogen and phosphorus compounds, mainly) are among the main triggers to induce MCs production; otherwise, fewer studies are focused in biotic factor as triggers of MCs in Microcystis, mainly phytoplankton competition and zooplankton grazing (Izydorczyk et al., 2008; Deblois & Juneau, 2012).
Under natural conditions, Microcystis cells from Chapultepec Lake contain certain level of MCs (Arzate-Cárdenas et al., 2010; Vasconcelos et al., 2010), which was corroborated in control test (without the zooplankton presence). Here it was detected an inverse relation between MCs concentration and cell density, which could be generated as a response for resources competition, then may increase their cell density and grow faster may possibly increase at the expense of toxin production (Reynolds, 2006; Hudnell, 2008).
In this work, the results showed a clear effect of zooplankton species on Microcystis, because MCs production in Microcystis was higher in presence of zooplankton as compared with controls; specially, the presence of D. pulex induced significantly higher MCs levels in Microcystis than B. calyciflorus. In this regard, Jang et al. (2003; 2007) have examined the feeding activity of zooplankton (Moina macrocopa (Straus, 1820), Daphnia magna (Straus, 1820) or D. pulex) and quantified the direct (Microcystis cultured with zooplankton) and the indirect (Microcystis cultured using zooplankton-conditioned medium) effects on the production of MCs in four Microcystis aeruginosa ((Kützing) Kützing, 1846) strains. Moreover, they showed that the MCs production was 5 times higher when exposed to direct zooplankton grazing pressure and, indicated that all strains of M. aeruginosa tested increase toxin production when exposed to herbivorous zooplankton; also they found that large zooplankton (D. magna) caused more MCs production in M aeruginosa strains (included one strain previously reported as non-toxic) than small zooplankton (M. macrocopa) over the time exposure. Similar results were reported by Izydorczyk et al. (2008), they found sufficient evidence that shows not only the abiotic factors such as temperature and nutrients but also the presence of herbivorous zooplankton such as large cladocerans (Daphnia cucullata (G.O. Sars, 1862) and D. longispina (O.F. Müller, 1875)) and small cladocerans (Bosmina sp., Alona sp. and Chydorus sp.), may influence strongly in MCs content of Microcystis.
In addition to the direct grazing, mere presence of zooplankton is sufficient to induce defense mechanisms such as colony formation, reduction of growth rate and toxin production in cyanobacteria (Jang et al., 2003). Some authors have documented that the release of infochemicals from zooplankton is an important factor to induce MCs production in cyanobacteria; in turn, it has been documented that each stage of the life cycle of zooplankton may induce different levels of MCs (Izydorczyk et al., 2008; Jang et al., 2008). For example, some studies document that adult zooplankton induce higher levels of MCs in Microcystis rather than the neonates or juveniles. Regarding this, Jang et al. (2008) suggested that adult zooplankton possibly produced more infochemical signals than the equal numbers of juveniles and neonates. Thus, it appears that the MCs production in cyanobacteria may also be influenced by different developmental stages of zooplankton and/ or physiological characteristics such as body size, and feeding habits of herbivores. In this study, was used only adult zooplankton and hence the differences in the MCs production were probably not related to the physiological factors or to the developmental stages of cladocerans and rotifers.
In summary, it was determined that the presence of cladocerans triggered higher MCs levels compared to rotifers in Microcystis spp. from Chapultepec Lake. Worth noting that several colonial and filamentous cyanobacteria inhabit Chapultepec Lake, and at least two Microcystis species (MCs producers confirmed) are present, of which M. aeruginosa is strongly dominant. Besides, some filamentous cyanobacteria species has been associated to Microcystis colonies; however, these do not produce MCs (Arzate-Cárdenas et al., 2010; Pineda-Mendoza et al., 2012).
Lastly, this work confirms that zooplankton may induce MCs production in Microcystis. Whether other zooplankton species (copepods, other cladocerans or rotifers) or zooplankton densities may increase MCs production in Microcystis spp. remains to be tested. Further researches are needed to determine the specific role of zooplankton in the stimulation of cyanobacteria to produce toxins.











 nova página do texto(beta)
nova página do texto(beta)