Introduction
The function of zooplankton in the ecosystem as an immense available biomass stock that recycles matter and energy in the pelagic ecosystem, as a provider of larval recruits for benthic and nektonic organisms (meroplankton), and with a relatively fast population response to environmental conditions is widely recognized and long-term time series of zooplankton community structure and abundance provide crucial information to better understand marine ecosystem dynamics (Eloire et al., 2010; Lavaniegos et al., 2015). Both seasonal and interannual variations in the pelagic ecosystems are evidenced by changes in zooplankton species abundance, zooplankton biomass, and assemblage composition of taxonomic groups. Time series that monitor abundances among different zooplankton functional groups provide valuable information regarding changes in food-web structures and their response as a function of climate change at multiple spatiotemporal scales (Edwards & Richardson, 2004; Lavaniegos et al., 2015). A detailed analysis of changes in abundance among multiple zooplankton functional groups also provides strong evidence of spatial and seasonal dynamics in the marine ecosystem (Sun et al., 2010) and vertical structure assemblages associated with the oxygen minimum zone in the Eastern Tropical Pacific (Maas et al., 2014).
The seasonal variability of zooplankton abundance is associated with dynamic processes that modify the relative contribution of taxonomic groups (or species), principally hydrodynamic conditions, intraspecific biological processes, and interspecific ecological interactions. Analysis of zooplankton community structure and its relation with changes in hydrological conditions at several spatio-temporal scales can help to characterize the biomass and productivity of marine ecosystems. Seasonal variability of zooplankton communities has been assumed pronounced in polar and temperate ecosystems, moderate in subtropical ecosystems, and low in tropical coastal marine ecosystems (Mackas & Beaugrand, 2010). However, a three-year monthly plankton time-series (1996-1998) in a tropical coastal region south of Cabo Corrientes (19°N, 104°W) showed that marked seasonal environmental changes promote a succession of zooplanktonic species, including ichthyoplankton (Franco-Gordo et al., 2002, 2004), amphipods (Gasca et al., 2012), euphausiids (Ambriz-Arreola et al., 2012), and calanoid copepods (Kozak et al., 2014). These studies showed the temporal variability of the numerically dominant zooplankton species; however, a general perspective on how zooplankton taxonomic groups covary throughout the year in the Eastern Tropical Pacific is currently unknown, contrasting with the relatively well-known seasonal and interannual changes of zooplankton taxonomic and functional groups in the southern region of the California Current System (Lavaniegos et al., 2015). There are few studies that examine the entire zooplankton community structure in bays of continental shell subtropical Gulf of California (De Silva Dávila et al., 2006), the central Mexican Pacific (Siordia-Cermeño et al., 2006; Gómez-Gutiérrez et al., 2014) and Costa Rica Dome (Mass et al., 2014). Siordia-Cermeño et al. (2006) studied the seasonal variability of 16 zooplankton taxonomic groups (sampled during Feb., May., Jul., and Nov. 1996) south of the Cabo Corrientes region. However, seasonal changes in zooplankton relative abundance and frequency were based on comparisons with stomach contents of the numerically dominant fish larvae Bregmaceros bathymaster (prey were mostly ostracods and copepods) making it difficult to visualize the seasonal succession of all zooplankton groups and their association with environmental conditions. Gómez-Gutiérrez et al. (2014) reported the zooplankton abundance around the Islas Marías Archipelago was numerically dominated by tropical coastal species, possibly associated with mesoscale oceanographic processes (eddies and river plumes) originating from the coast of the Cabo Corrientes region (Zamudio et al., 2007; Kurczyn et al., 2013). The vertical structure and distribution of tropical macrozooplankton taxonomic groups were studied during November 2007 and December 2008 (Maas et al., 2014). However, these studies were limited to one or two months; thus seasonal changes of tropical zooplankton taxonomic groups have not been studied in the entire Eastern Tropical Pacific.
The coastal region of the central Mexican Pacific is characterized by the presence of a permanent shallow upper boundary of the oxygen minimum zone (40-120 m depth; <1 mL O2 L-1) and high meso-scale activity, particularly wind-driven upwelling-downwelling events, eddies, coastal fronts, filaments, and continental river runoff (Zamudio et al., 2007; López-Sandoval et al., 2009; Martínez-Flores et al., 2011; Ambriz-Arreola et al., 2012; Cepeda-Morales et al., 2013; Kurczyn et al., 2013). On an interannual scale, the variability in the hydrographic conditions is mainly attributed to the El Niño/La Niña events (Godínez et al., 2010). All these features modulate the hydrographic structure and the primary production rates (López-Sandoval et al., 2009; Cepeda-Morales et al., 2013), and subsequently the seasonal-interannual variability of the inshore zooplankton communities (Suárez-Morales et al., 2000; Franco-Gordo et al., 2004; Gasca & Franco-Gordo, 2008; Gasca, 2009; Gasca et al., 2012; Ambriz-Arreola et al., 2012; Kozak et al., 2014; León-Chávez et al., 2015). Zooplankton ecology research near Cabo Corrientes is considerably less frequent than investigations carried out along the west coast of the Baja California Peninsula, mainly due to the implementation of several long-term monitoring programs like CALCOFI (1949-1980) and IMECOCAL (1997-present) (Lavaniegos et al., 2015) and the relatively less systematic but frequently sampled research cruises carried out in the Gulf of California.
Our work driven-hypothesis is that strong seasonal hydrographic changes occurring in the water column on the central Mexican Pacific (upwelling and downwelling) cause marked seasonality in phytoplankton standing stock (indicated by chlorophyll-a concentration) that result in a pronounced seasonal succession in the community structure of all zooplankton taxonomic groups. We analyzed the monthly zooplankton community structure per taxonomic group at the Bahía de Navidad oceanographic station during an annual cycle (November 2010 to December 2011) with the objective to describe, for the first time, the seasonal succession of the zooplankton communities and its relationships with environmental conditions.
Material and methods
Zooplankton sampling. Zooplankton was collected twice monthly at the station named "Bahía de Navidad" (19°09́03̋N, 104°44 ́50 ̋W), located on the inshore central Mexican Pacific. Because there were three to five extra sampling days per month (albeit with an irregular frequency), we decided to average zooplankton abundances per month. A total of 43 zooplankton samples were collected from November 2010 through December 2011. Zooplankton samples were collected on board a 6-m length fiberglass boat exclusively at night (about 04:30 am, before sunrise) to avoid bias due to daytime net avoidance and diurnal deep vertical distribution that would likely decrease zooplankton abundance and taxonomic group composition. A simple-conic net (0.5 m mouth diameter, black 250 μm mesh, and 1.7 m long) was towed near the sea surface at a constant speed (~4 km h-1) for ten minutes. A digital flowmeter (General Oceanic R2030) was fixed to the mouth of the net to calculate the volume of filtered seawater. All samples were preserved in a 4% formalin-seawater solution saturated with sodium borate (Smith & Richardson, 1977).
Temperature, salinity, and dissolved oxygen concentration profiles were recorded until 90 m depth (seafloor at 100 m) with a Seabird SBE 19 plus CTD and a dissolved oxygen sensor calibrated according to manufacturer specifications (http://www.seabird.com/sites/default/ files/documents/Seasave_7.23.2.pdf). Seawater was also collected at distinct sampling depths with a Niskin bottle (5 L) and the dissolved oxygen concentration was measured using the Micro-Winkler titration method. Occasional simultaneous measurements of dissolved oxygen concentration at the same depth and time using both measurement methods were compared for calibration purposes to correct the oximeter measurements.
Laboratory analyses. Because collected zooplankton biovolume was typically low (10-30 mL), all the zooplankton taxonomic groups were counted from the entire zooplankton samples, except copepods that accounted for >60 % of total abundance, which were counted from aliquots carried out with a Folsom splitter (1/2 to 1/64, counting > 650 individuals per sample). All the organisms were identified to major taxa following Newell and Newell (1966) and Todd et al. (2006). Although small Euphausiacea larval stages (calyptopis) were not counted by the absence of a specialist; therefore this component of total zooplankton abundance was neglected from further statistical analyses. Taxonomic group abundances were standardized to individuals per cubic meter (ind × m-3).
Data analysis. Based on the vertical structure of the seawater column (1996-1998), Ambriz-Arreola et al. (2012) proposed that south of Cabo Corrientes region three seasonal climatic periods prevail: (1) A mixed period from February to May, typified by a well-mixed water column, relatively low temperatures (< 24° C), high salinities (> 34), shallow mixed layer depth (< 30 m), and high zooplankton biovolume driven by intense coastal upwelling events (> 100 CUI m3 s−1 per 100 m coastline). (2) A stratified period spanning July to November, characterized by a strong vertical stratification of the water column, high temperatures (> 25 °C), low salinities (< 34) (due to the regional rainy season), relatively deep mixed layer depth (> 40 m depth), and low zooplankton biovolume (oligotrophic conditions). (3) Two brief transitional semi-mixed periods; one from mixed-to-stratified conditions (June) and another from stratified-to-mixed conditions (December/January). The oceanographic and biological variables time series recorded during 2011 were classified a priori following these three climatic periods.
Differences in median abundance among zooplanktonic taxa grouped per climatic periods were tested using a Kruskal-Wallis test (Zar, 1996). The mixed layer depth was calculated as the depth at which the temperature was 1 °C lower than the temperature recorded at 10 m depth (Ambriz-Arreola et al., 2012) Seawater samples were collected at 10 m using a Niskin bottle (5 L) in order to estimate chlorophyll-a concentrations (Chl-a), filtering 1 L of seawater through GF/F filters (0.47 μm pore) and frozen at -4°C until spectrophotometrical analysis. To extract the Chl-a, the GF/F filters were homogenized (10 mL of 90% acetone, 24 h in dark conditions) then centrifuged (10 min at 3500 rpm). The supernatant was poured into a quartz cuvette and analyzed using a spectrophotometer (Spectronic model Genesys 8) to estimate the in situ Chl-a concentration (mg Chl-a m-3) (Strickland & Parsons, 1972).
The depth of the strata with hypoxic conditions was defined as strata where dissolved oxygen concentration was < 1.5 mL O2 L-1 and the oxygen minimum zone was the strata with oxygen concentrations below 1 mL O2 L-1 (Fernández-Álamo & Färber-Lorda, 2006; Tremblay et al., 2010). The daily coastal upwelling index (CUI; m3 s-1 per 100 m of coastline) (Bakun, 1975) from the location 21° N, -107° W was used to explore temporal variability of coastal upwelling-downwelling conditions on the day of zooplankton sampling and 15 days before each sampling event (http://www.pfel.noaa.gov/products/PFEL/modeled/indices/upwelling/NA/data_download.htlm). The monthly anomaly values of CUI were calculated as follows:
Where Zi is the CUI anomaly of the ith month, Xi is the CUI value in the ith month and in the jth year, and Yi is the mean CUI value in the ith month and in the jth year recorded during the 2000-2010 period.
To select the most robust canonical analysis, detrended canonical correspondence analysis (DCA) was used to obtain the length of the environmental gradient (Ter Braak & Prentice, 2004). The redundancy analysis (RDA) was later selected as a linear response model since the length of the environmental gradient tested was < 2 standard deviations (Ter Braak & Prentice, 2004). We used the RDA to explore the multivariate relationship between abundances of the zooplankton taxonomic groups and the recorded environmental variables (Legendre & Legendre, 2000). Seven environmental variables were used as independent variables: temperature, salinity, Chl-a concentration recorded at 10 m depth, mixed layer depth, upper boundary depth of the hypoxic strata (< 1.5 mL O2 L-1), and CUI value on zooplankton sampling day and 15 days before zooplankton sampling. RDA analysis was carried out using the abundance of the zooplankton taxonomic groups as logtransformed values [log(x+1)] and scaling was focused on the intergroup correlations. Ordination analysis was conducted with forward selection of environmental variables using the CANOCO for Windows software ver. 4.5 (Ter Braak & Šmilauer, 2002).
Results
Hydrographic seasonal variability. The daily and monthly average of CUI (Fig. 1a ), the monthly anomalies of CUI (Fig. 1b ), and hydrographic structure (Fig. 2) recorded during Nov 2010-Dec 2011 at the Bahía de Navidad station showed that coastal upwelling events occurred from February to May and downwelling events prevailed from June to October. During Nov 2010-Dec 2011 the highest CUI values (upwelling events) occurred during the mixed period (mean = 84, range = 6 to 287 m3 s-1 per 100 m coastline), while the lowest CUI values (downwelling events) occurred during the stratified period (mean = -5, range = -325 to 88 m3 s-1 per 100 m of coastline) (Fig. 1 a, b ).

Figures 1a-b: Bakun coastal upwelling index (CUI; m3 s-1 per 100 m coastline) calculated at 21° N, 107° W during November 2010-December 2011. (a) Daily values (black line) and monthly mean (red horizontal lines); (b) CUI historical monthly means (± standard deviation) during the 2000-2010 decade (filled circles) and anomalies recorded during 2010-2011 (bars; secondary y axis -please notice the different scale-). Data from NOAA/NMFS/PFEG (http://www.pfeg.noaa.gov/products/PFEL/modeled/indices/upwelling/).
During mixed and semi-mixed periods (Nov 2010 - May 2011) a shallow mixed layer depth and shoaling of the hypoxic strata (< 25 m deep) prevailed, associated with the cold (< 23 °C) and salty (> 34.2) upwelled seawater near the surface (Fig. 2). In contrast, during the stratified period (June to November 2011) a strong stratification of the water column developed above 45 m depth, with a moderate depth of the mixed layer (< 40 m), halocline (15-45 m), and the upper boundary of the hypoxic stratum (20-80 m) (Figs. 2a-c). Mean Chl-a concentration, as a proxy of phytoplankton standing biomass, was typically low throughout the year (< 1 mg Chl-a m-3); however, significantly higher Chl-a concentrations were detected during the mixed seawater column period (0.20.9 mg Chl-a m-3) than during the stratified period (<0.2 mg Chl-a m-3) (Kruskal-Wallis, p<0.05). Intermediate Chl-a concentrations were measured during the semi-mixed period (transitional period, June and December-January).

Figures 2a-c Vertical profiles of (a) temperature (°C), (b) salinity, and (c) dissolved oxygen (mL O2 L-1) recorded during November 2010 to December 2011 in the oceanographic station Bahía de Navidad, Mexico. Periods: M = mixed S = stratified and SM = semi-mixed, OMZ = upper boundary of the hypoxic strata. During Nov 2010-June 2011 CTD casts were deployed only until 30 m depth, thus no deeper records are available.
Zooplankton seasonal succession. A total of 19 zooplankton taxonomic groups were recorded from November 2010 to December 2011 at Bahía de Navidad (Table 1, Figs. 3a-b). Copepods numerically dominated the zooplankton community during the annual cycle (monthly average = 66%, range = 38-91%). Copepod monthly geometric mean (GM) varied between 14 and 2505 ind × m-3 (annual mean = 734 ind × m-3) with maximum abundance during the mixed period (Table 1, Fig. 3b).
Table 1 Abundance of zooplankton per taxonomic group (ind × m-3) recorded among oceanographic periods at Bahía de Navidad, Jalisco, Mexico between November 2010 and December 2011.
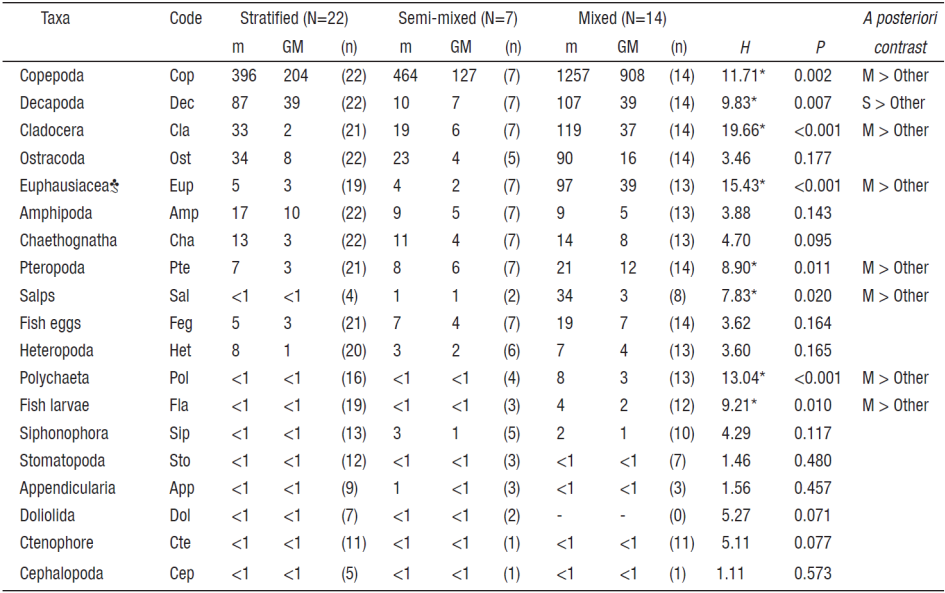
m = Mean, GM = geometric mean, n = number of the samples in which each taxonomic group occurred, N = total number of zooplankton samples analyzed during each oceanographic period, and * = Multiple comparisons among periods with Kruskal-Wallis test (H-one way ANOVA, post-hoc) showing significant differences (α = 0.05, a posteriori contrast). Code corresponds to the identifier used in Fig. 4. ♣ = Euphausiacea abundance does not include the abundance of calyptopis larval stages.
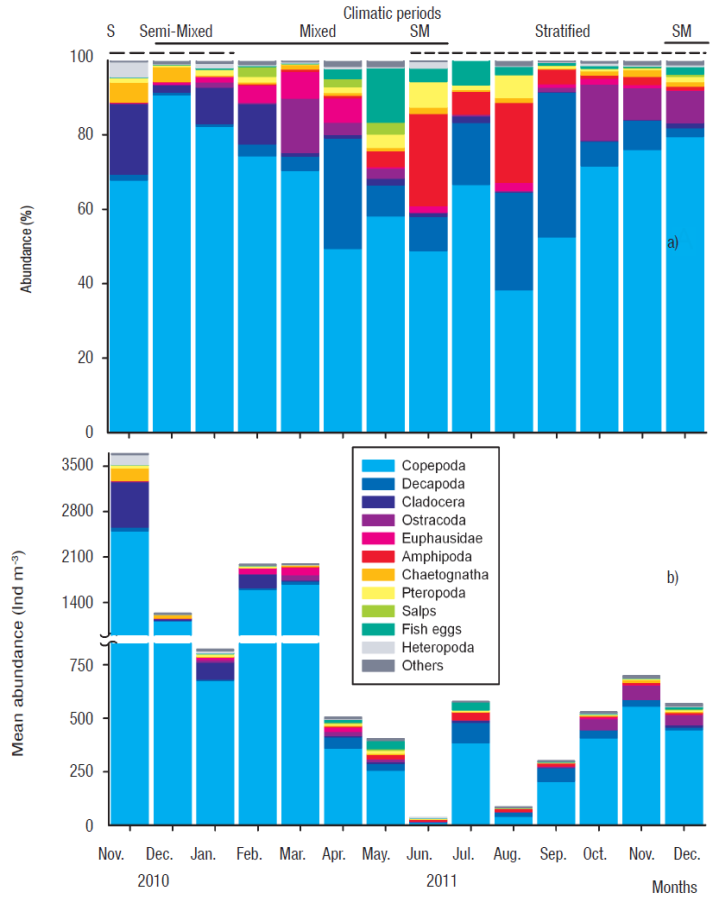
Figures 3a-b Monthly abundance of the dominant zooplankton groups (ind × m-3) at Bahía de Navidad , Mexico, from November 2010 to December 2011. (a) Relative abundance, and (b) stacked geometric means. Others = total abundance of Polychaeta, fish larvae, Siphonofora, Stomatopoda, Appendicularia, Doliolida, Ctenophora, and Cephalopoda.
The next eight most abundant zooplankton taxonomic groups were decapod larvae (11 %), amphipods (5 %), cladocerans (4 %), ostracods (4 %), fish eggs (3 %), euphausiids (2 %; without calyptopis larvae), chaetognaths (1.5 %), and pteropods (1.5 %) that together accounted for about 32% of the total zooplankton abundance recorded throughout the year. The other ten taxonomic groups comprised < 2% of the total zooplankton abundance (Table 1, Fig. 3a). Overall, copepods, cladocerans, euphausiids, pteropods, salps, polychaetes, and fish larvae had a similar seasonal succession pattern, significantly increasing their abundance during the mixed period (Kruskal-Wallis, p < 0.005), while the decapod larvae showed an increasing abundance during the stratified period (Table 1, Figs. 3a-b). Zooplankton abundance increased markedly during the semi-mixed period (June-September 2011), which is the transition from mixed (upwelling) to stratified (downwelling) conditions and the onset of the rainy season (Figs. 3a,b). A clear seasonal succession of the zooplankton taxonomic groups showed high predominance of copepods with a relatively low number of taxonomic groups during the mixed period, and an increase in the number of taxonomic groups during the mixed-stratified transitional period, when relative copepod abundance was the lowest. During the stratified period, abundance of copepods decreased and zooplankton groups showed higher evenness (Figs. 3a-b).
Redundancy analysis (RDA). The eigenvalues for canonical axis 1 (0.321) and axis 2 (0.075) explained 89 % of the total variance estimated of the relationship between zooplankton taxonomic group abundances and the environmental variable gradients (Table 2). Based on the inter-set correlations, axis 1 (72 %) showed high negative correlation values with 10-m temperature and mixed layer depth, and positive correlations with 10 m salinity and CUI on sampling day and 15 days before each sampling day, representing the seasonal changes in the column water structure. 10 m Chl-a concentration was strongly positively correlated with axis 2 (a gradient of food availability for herbivorous and omnivorous zooplankton taxonomic groups) (Table 2, Fig. 4). Positions of variables on the RDA ordination tri-plot clearly showed the zooplankton taxa composition strongly associated with environmental conditions that prevailed in the study area. Axis 1 showed a gradient of three groups: a mixed group was located on the right of the tri-plot, characterized by samples collected mostly during the mixed period with intense upwelling conditions [cold water (< 23 °C) with comparatively high salinity (> 34.4), high Chl-a concentrations (indicative of higher values of CUI), and relatively shallow mixed layer depth and shoaled upper boundary of the hypoxic strata (< 30 m depth)]. Copepods, cladocerans, euphausiids, pteropods, salps, polychaetes, and fish larvae taxa were located (associated) on the right side of the RDA-tri-plot. In contrast, samples located on the left side of the RDA were collected during the stratified period (stratified group) and featured high temperature (> 25 °C), low salinity (< 33.8) in the upper 50 m of the column, a seasonal shallow halocline (< 45 m depth), lower Chl-a concentrations (oligotrophic conditions), relatively deeper mixed layer depth, and deeper upper boundary of the hypoxic strata (> 40 m depth). The vectors of 10 m Chl-a concentration and temperature were not completely opposite, while the vectors MLD and depth of the upper boundary of the hypoxic strata (OMZ) co-varied with an inverse pattern in relation with Chl-a 10 m concentration. Only amphipods were found in the stratified group. The third cluster included sample units collected during the semi-mixed period semi-mixed group -Stomatopoda, Copepoda, Ostracoda, Decapoda, and Chaetognatha) (Fig. 4). These three consecutive zooplankton associations indicated that all taxa were strongly associated with the first environmental axis, in a clear seasonal succession of the zooplankton community.
Table 2: Summary of redundancy analysis (RDA) using seven environmental variables and abundance of 19 taxonomic zooplankton groups collected during 43 zooplankton samples at Bahía de Navidad, Mexico. (November 2010 to December 2011).

* = Significant correlations (α = 0.05) with each environmental variable and the RDA axes.

Figure 4: Redundancy analysis (RDA) ordination tri-plot showing, the zooplankton taxonomic categories (black arrows) in relation to environmental variables (red arrows). Symbols are oceanographic stations classified per climatic periods that prevailed in the south coastal region of Cabo Corrientes, Mexico (sensu Ambriz-Arreola et al., 2012). Taxonomic categories code was defined in Table 1. MLD = mixed layer depth, OMZ = upper boundary of the oxygen minimum zone, 10 m Temp = 10 m depth temperature, 10 m Sal = 10 m salinity, 10 m chl-a = 10 m chlorophyll at concentration, CUI sd = coastal upwelling index on the sampling day, and 15-CUI CUI 15 days before sampling.
Discussion
The total zooplankton abundance recorded during the present study had a clear seasonal pattern, with high densities during mixed water conditions and significantly lower densities during the stratified period. This seasonality was similar to previous observations from the Jalisco coast of zooplankton biomass (Franco-Gordo et al., 2001) and specific taxonomic groups of ichthyoplankton (Franco-Gordo et al., 2008), amphipoda, (Gasca et al., 2012) euphausiacea (Ambriz-Arreola et al., 2012), and calanoid copepods (Kozak et al., 2014) and several zooplankton taxonomic groups: copepoda, ostracoda, euphausiacea, chaetognatha, phylum mollusca and polychaethe (Siordia-Cermeño et al., 2006). These investigations showed that the seasonal variability of calanoid copepod species in Mexican Pacific coastal waters was mostly associated with the periodicity of coastal upwelling process, with maximum abundances during the mixed period and lowest during the stratified period. But not all zooplankton taxonomic groups responded in the same phenological pattern. Analysis of the entire zooplankton community structure provides a broad perspective regarding the complexity of the epipelagic tropic web food, and indirectly productivity at low trophic levels (high carbon content when crustaceans prevail, low carbon content when chaetognaths and jelly zooplankton prevail) (Lavaniegos et al., 2015). Although analysis of the entire community structure at species level provides a more detailed perspective, this requires considerable taxonomic expertise and is a time consuming exercise, making it more practical with relatively fewer samples (Gómez-Gutiérrez et al., 2014; Maas et al., 2014) or for specific taxonomic groups (Franco-Gordo et al., 2008; Gasca et al., 2012; Ambriz-Arreola et al., 2012; Kozak et al., 2014). We sampled at night to prevent visual avoidance of the net, and near surface because this is the time when zooplankton typically migrates upward. This sampling strategy likely resulted in the collection of more zooplankton than could have been obtained during daytime sampling.
Our results showed that the upwelling conditions in Mexican tropical Pacific prevailed for most of 2011 (except Jun-Aug, stratified conditions), which is relatively longer than previously described (1996-1998) (López-Sandoval et al., 2009; Ambriz-Arreola et al., 2012; AmbrizArreola, 2013) (Fig. 1 a-b). This caused relatively colder conditions (< 23 °C; mainly from Nov 2010 to June 2011), characterized by a shallow (< 80 m) mixed layer and a superficial hypoxic layer (<1.5 ml L-1; Fig. 2c) and moderately high surface Chl-a concentrations (<2.5 mg m-3). However, the in situ values of Chl-a recorded during the annual cycle were relatively similar in magnitude as those historically reported in this tropical region (López-Sandoval et al., 2009). The RDA showed that in 2011 temperature and CUI were the main environmental variables influencing temporal zooplankton taxonomic group variability, as was previously recognized in the same region during 1996-1998 (Ambriz-Arreola et al., 2012; Kozak et al., 2014). Jiménez-Pérez et al. (2013) showed considerably high zooplankton abundances (15×103 to 5×106 ind × m-3) in Bahía de Banderas (nearly 230 km north of our area of study) during specific upwelling pulses associated with blooms of harmful algae (February to April, 2011). They reported that copepods accounted for 95 % of the total zooplankton abundance during February 2011. Gómez-Gutiérrez et al. (2014) also found that the crustaceans numerically dominated (92.3 %), with copepods being the most abundant group, during November 2010 in the Islas Marías Archipelago, very similar to our study. But our mean abundance (986 ind × m-3) during the annual cycle was twelve times higher (mean abundance = < 80 ind × m-3) than that reported in the Islas Marías, located between 90-120 km from the coast (Gómez-Gutiérrez et al., 2014), showing the high productivity of the coastal habitat.
Lavaniegos et al. (2012) reported that copepods (83 % and 98.8 % in winter and spring, decreasing their relative abundance in autumn) and cladocerans were the dominant taxonomic groups in Bahía de los Ángeles, Gulf of California, a region with a dynamic hydrologic regime influenced by upwelling, tides, and uneven topography. These authors mentioned that high zooplankton biovolumes were recorded during the cold period (winter and spring) and a high spatial variability was observed, especially during summer.
Recently, Kurczyn et al. (2013) documented that during November 2005, an upwelling event (in combination with an equatorward flow) generated a cyclonic meso-scale eddy that brought up colder, saltier, and hypoxic waters in the upper 100 m off the Cabo Corrientes region. Similarly, several other studies have documented upwelling events during the stratified period (July to November) (Cepeda-Morales et al., 2013; León-Chávez et al., 2010). These observations combined with our results indicate that the Cabo Corrientes region is highly influenced by the occurrence of upwelling events during most of the year, which promotes high biological productivity in this coastal-tropical ecosystem (Pennington et al., 2006; López-Sandoval et al., 2009; León-Chávez et al., 2010, 2015; Ambriz-Arreola et al., 2012; Ambriz-Arreola, 2013). In fact, our multivariate statistical analysis confirmed that the hydroclimatic periods (mixed, stratified, and semi-mixed groups) showed significant differences in sea surface temperature, salinity, mixed layer depth, and upwelling intensity and duration (Fig. 4, Table 2). This seasonal distinction among hydro-climatic periods (even during a relatively cold year) influenced the seasonal variability in zooplankton community structure, suggesting that dynamic upwelling intensity directly influences the relative abundance of zooplanktonic taxonomic groups and succession throughout time. For example, crustaceans (mainly copepods, cladocerans, euphausiids, and ostracods) numerically dominated most of the zooplankton samples, mainly linked with the regional intensity and duration of coastal upwelling process that prevail in the study area (see Fig. 4, Table 1- Table 2) (López-Sandoval et al., 2009; León-Chávez et al., 2010; Ambriz-Arreola et al., 2012; Kurczyn et al., 2013) and have also been observed along the west coast of Baja California (Lavaniegos et al., 2015).
Ambriz-Arreola (2013) documented the first record of juvenile and adult specimens (including ovigerous females) of the subtropical neritic euphausiid Nyctiphanes simplex Hansen 1911 at the monitoring station of Bahía de Navidad during April and May 2011, suggesting this was one of the southernmost temporal extensions of its distribution range in the northern hemisphere (Brinton, 1979; Brinton & Townsend, 1980; Brinton et al., 2000). This uncommon southern distribution was attributed to the intense and unusually long coastal upwelling activity corridor along the Cabo Corrientes region which occurred during 2011, and therefore we propose that future records of N. simplex in this region could be a potential biological indicator of intensification and longer duration of upwelling events in the Cabo Corrientes region.
In conclusion, these results indicate that the upwelling conditions that prevail in the study area are strongly associated with the total zooplankton abundance and their seasonal succession is numerically dominated by crustacean taxa. Finally, it is worth mentioning that this zooplankton time series represents the starting point of an ongoing coast-ocean monitoring program in the southern Cabo Corrientes region.











 nueva página del texto (beta)
nueva página del texto (beta)


