Introduction
The study of reproduction in species subject to exploitation is essential for fishery resource management. Protecting organisms will help assure resources for sustainable fisheries. Size at maturity has been used as one management parameter to allow individuals in the populations to reproduce at least once before being caught (Aragón-Noriega, 2005).
The Cortes geoduck Panopea globosa (Dall, 1898), predominant in the Gulf of California, has been fished since 2002 in Baja California, Mexico. Landings increased from 49 MT in 2002 to 3241 MT in 2013. The value of this fishery is as much as US$30 million annually. Leyva-Valencia et al. (2012) (based on morphometric and taxonomic analyses) and Suárez-Moo et al. (2013) (based on genetic evidence) revealed that P. globosa is also present in Bahía Magdalena located on the southern Pacific coast of the Baja California peninsula (Fig. 1). The Gulf of California population supports more than 80% of the whole geoduck fishery in Mexico, ~2,600 MT yr-1 (Aragón-Noriega et al., 2012).
In severe contrast to the wide knowledge of Panopea generosa (Gould, 1850) from Canada and the United States, (see Straus et al. (2008) for a review), the biological status of P. globosa in the Gulf of California is scarce (Aragón-Noriega et al., 2012). Scientific information on the populations of P. globosa has only begun to be produced. Only three publications are available on the reproductive aspects of P. globosa in Mexico (Aragón-Noriega et al., 2007; Arambula-Pujol et al., 2008; Calderón-Aguilera et al., 2010). The first two were undertaken at the Bahía de Guaymas-Empalme, Sonora (Central Gulf of California), and the third was done at the Upper Gulf of California. The first study addresses the morphometric variation and reproductive biology of samples collected from 2004 to 2005, featuring a predominance of large clams, and documents peak spawning from January to February (winter) during a time of low (18° C) sea surface temperature (Aragón-Noriega et al., 2007). The second study focuses on the same sample collections but compares them to the reproductive cycles of P. generosa from Canada and P. zelandica (Quoy & Gaimard, 1835) from New Zealand (Arambula-Pujol et al., 2008). The third paper deals with the apparent synchrony between larval development and the peak in primary productivity in the Upper Gulf of California, as predicted by Cushing's match-mismatch hypothesis (Cushing, 1982). Through time-series analysis, the author has shown that gametogenic development of P. Globosa is triggered by a steep decrease (4° C) in temperature (Calderón Aguilera et al., 2010). None of these papers examined the issue of size at maturity or its implications for regulatory purposes.
Mexican fishery authorities established a quota of 1% (DOF, 2012) of the estimated biomass of clams with >130 mm of shell length (SL). One possible reason for this regulation is that it represents maturity or some other biological reference point. If the 130 mm SL regulation was based on maturity or some other growth parameter, it is important to have specific knowledge of the area because quotas can be different among regions and among sites within regions.
The aim of this study in two zones of the Gulf of California was to investigate the size at maturity of P. globosa and explore the importance of the 130 mm legal size.
Materials and methods
Study site and sample collection
Samples were only collected in January 2013 because the month of January was documented as the time for species maturation and peak spawning (Aragón-Noriega et al., 2007; Calderon-Aguilera et al., 2010). Samples were collected in the sub-tidal zone from 10 to 25 m in depth in Guaymas, Sonora, Central Gulf of California (27°35' N, 110º06' W), and San Felipe, Baja California (31°09" N, 114º53" W) Western Upper Gulf of California (Fig. 1). Divers used a low-pressure compressor and water jet to loosen the clams from the substrate and harvested them one at a time. Once extracted from the sediment, the clams were taken in coolers to the laboratory to be processed immediately upon arrival. Individual shell length (SL, the straight line distance from the anterior to posterior margins of the shell), was measured to the nearest millimeter using vernier calipers. Individual total wet weight was obtained after draining water from the body cavity; tissue wet weight and gonad weight were measured by separating the shell from the tissue and blotting both dry to remove excess water. All weight data were determined to the nearest 0.1 g using an electronic balance (UVD 500).
Reproductive condition
Histological procedures followed the methods employed in previous studies (Aragón-Noriega et al., 2007; Calderon-Aguilera et al., 2010). Once the whole tissue weight was determined for each individual, the gonad and associated viscera were removed; a portion of the tissue was taken from a standard position in the middle area of the gonad and fixed in Davison's solution for at least 24 h. Samples were dehydrated in a series of ethanol treatments of increasing concentration, cleared in xylene, and embedded in Paraplast at 56° C and sectioned at 5-µm. All sections were stained with hematoxylin and eosin technique and individually mounted on glass slides. The slides were examined under an optical microscope at x4, x10, and x40 magnification. Gonads were placed into five qualitative stages following Campbell and Ming (2003): early active, late active, ripe, partially spawned, and spent/reabsorbing. The gonadal state of each clam was described as one of the five stages based on the most dominant stage present in 10 randomly selected follicles from each sample. Clams were deemed sexually mature if gametes were present and connective tissue had well developed primary cells evident on follicle walls or when oocyte or spermatocyte development was evident. Immature individuals had no differentiation in gonadal tissue and loose vesicular connective tissue in the gonad.
Size at maturity.It is important to note that L50 is a population size at maturity and not one based on an individual. The size at first maturity is the size at which the individuals mature. The size at population maturity was estimated by fitting the following logistic model to the observed data:
where:
Pi is the proportion of mature individuals
r is the slope
L50 is the length which corresponds to 50% mature individuals, and
L is total length in mm.
The logistic curve was fitted, minimizing the negative value of the log-likelihood using a binomial function (Brouwer & Griffiths, 2005):
where:
Pi is the proportion mature individuals in size class i
n is the number of individuals in size class i and
m is the number of mature geoducks in class size i.
Confidence interval. The 95% confidence interval of size at maturity parameters (θ) were estimated after Venzon and Moolgavkor (1988) using the likelihood profile method. These estimations were based on chi square distribution with d degrees of freedom. The confidence interval was defined as all values of θ that satisfy the inequality.
Where:
is the negative log-likelihood of the fitted value of θ and
are the values of the chi square distribution with d = 1 (3.84).
A method of comparing the two curves (San Felipe vs Guaymas) was done following Haddon (2001), which is called analysis of residual sum of squared (ARSS). ARSS is a total comparison, which means that it does not compare the parameters separately but simply tests when two or more curves are statistically different (Haddon, 2001)
It is necessary to mention that the Brouwer & Griffiths (2005) approach was followed and not the traditional jackknife method. What the second method provides is the mean size (or age) of mature animals in the population as the 50% point on the cumulative ogive (all of the animals in the sample must be 100% mature), but not all mature individuals are in reproductive conditions at the same time. For these reasons, the proportions had to be adjusted to represent the number of sexually-mature individuals expressed as a proportion of the reproductive populations in each size class, which is the advantage of the Brouwer and Griffiths (2005) approach over the traditional jackknife method.
Results
A total of 422 geoduck specimens were analyzed during this study (Table 1). Three reproductive stages (undifferentiated, partially spawned, and spent/reabsorbing) were categorized during the month when samples were collected. The sizes of mature females ranged from 90-155 mm SL in both locations, San Felipe and Guaymas, with a mode size of 125 mm SL in both locations (Table 1), but the average size was 126.91 mm SL in San Felipe and 123.24 mm SL in Guaymas.
Table 1 Number of Cortes geoduck Panopea globosa from two locations of the Gulf of California. Total and mature size class.

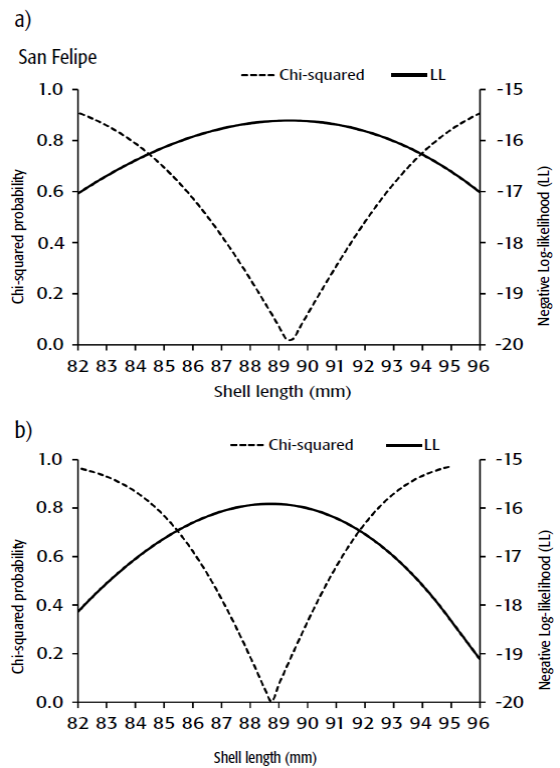
Size at maturity was 88.75 mm SL in Guaymas, and 89.37 mm SL in San Felipe (Fig. 2). The ARSS showed both curves were not significantly different (ANOVA: F = 0.543, p < 0.5881). The likelihood profile of the size at maturity is shown in Figure 3, where the values ranged from 85.25 to 92 mm SL in Guaymas and from 84.5 to 94 in San Felipe.

Figures 2a-b Size at maturity curves for Cortes geoduck Panopea glo bosa collected in the Gulf of California. Circles represent observed data and the line is the adjusted curve. a) San Felipe. b) Guaymas.
Discussion
No organisms smaller than 90 mm SL were collected in this study, which implies that population size at maturity might be shorter than estimated here. The study was conducted only in January because this month is the peak of spawning in this species's very restricted spawning season (Aragón-Noriega et al., 2007; Arambula-Pujol et al., 2008; Calderon-Aguilera et al., 2010). P. globosa have a very short reproductive period of only 3 to 5 months (Aragón-Noriega et al., 2007; Arambula-Pujol et al., 2008) and an extended resting period during which clams could not be sexed. In contrast, P. zelandica have a resting phase of only 1-2 months (Gribben et al., 2004); reproduction of P. generosa from Washington state and British Columbia is continuous with no resting period at all (Andersen, 1971; Sloan & Robinson, 1984). Population size at maturity was found to be higher than previously determined in congeners P. zelandica (55-57 mm SL Gribben & Creese, 2003) and P. generosa (58.3-60.5 mm SL; Campbell & Ming, 2003).
The size at maturity found in our study (88.75-89.37 mm SL) is higher than previously reported for P. generosa (Campbell & Ming, 2003). These authors used the Gauss-Newton least squared method, while Gribben and Creese (2003) determined the constants of the population size at maturity equation, using maximum likelihood methods for P. zelandica. Cortez-Lucero (2013) discussed three methods (linear, least squared, and likelihood), and found the shortest L50 for linear methods. His finding emphasized that the result depends on the method used to fit the equation constants. It is noteworthy that in this study, the L50 was estimated from an expected curve, and it was lower than the sizes observed, which means that L50 was likely underestimated. It is necessary to recall that the Brouwer and Griffiths (2005) approach was followed and not the traditional jackknife method, since the latter uses only 100% mature animals. However, not all mature individuals are in reproductive conditions at the same time, so it could produce an overestimated L50, which is why using this method has implications for management of exploited populations.
Using the least squared method, Campbell and Ming (2003) found the mean size at 50% maturity of 58.3 mm SL in Gabriola Island and 60.5 mm SL in Yellow Bank (Canada). Campbell and Ming (2003) and the present study were conducted in the subtidal zone while Andersen (1971) found 50% maturity at 75 mm SL, but in the intertidal zone in Hood Canal (Washington, USA). Thus the 130 mm SL legal size for P. globosa is well above the size at maturity for P. generosa from intertidal and subtidal populations. A similar pattern was found in P. zelandica (Gribben & Creese, 2003) as geoducks were estimated to be 55 and 57 mm SL at 50% sexual maturity in Kennedy Bay and Shelly Bay, New Zealand, respectively.
At 50% population maturity the size was found to be 89.37 and 88.75 mm SL in San Felipe and Guaymas in the Gulf of California, respectively (Fig. 3). Campbell and Ming (2003) discussed the possible reason for differences in size at maturity within bivalve populations at different locations, which may involve environmental variations (water temperature, current patterns, substrate type, and depth). Although the present study was not designed to explain differences, variation in primary productivity between the regions was described. Kahru et al. (2004) used satellite data to obtain time series of surface chlorophyll concentration and phytoplankton net primary production for 12 sub-areas within the Gulf of California. These time series showed a dominant annual cycle in all sub-areas. The San Felipe area exhibited higher primary production per unit area than Guaymas (Kahru et al., 2004). If we compare the two areas, differences in size at maturity would be expected, but no differences in size at maturity were found between San Felipe and Guaymas. The explanation for size at maturity at individual and population levels cannot be ascribed to one cause. Gribben and Creese (2003) emphasized that sample size could be important in determining maturity rates within P. zelandica populations at different locations. The above discussion may be applied for comparing growth rates between these two regions. Two previous studies (Cortez-Lucero et al., 2011; Cruz-Vásquez et al., 2012) of age and growth in P. globosa have reported an asymptotic length of 122 mm SL in the central Gulf of California (CGC, Guaymas region). One study (Aragón-Noriega et al., 2015) reported the growth rates in two locations of the upper Gulf of California (UGC, San Felipe region). The asymptotic lengths found for P. globosa from both locations in the UGC were 165 and 163.4 mm SL, which showed clearly that Cortes geoducks in the UGC exhibit more rapid growth than those in the CGC. Such growth studies suggest that size or age is not an important parameter for maturation. Once again, environmental variability can be used to explain growth rate differences in P. globosa at the two locations being compared; because noticeable differences in growth rates were found, differences were clearly expected between size at maturity between the two zones compared herein.
It would also be very useful if Mexican fishery authorities would indicate why the establishment of minimum legal size is a good measure for managing bivalve populations. Most bivalves mature at a smaller size than typically demanded by the market, so there is no pressure to harvest very small ones. If management is setting the allocation at 1% of the standing stock above 130 mm, it is being very conservative. I am sure management does not have a good estimate of the population parameters (standing stock, recruitment, and size-specific natural mortality), so there is a desire on their part to be relatively conservative. Actually, the size that currently pays the highest market price is roughly 100 mm SL. In addition, if the harvest size were to be reduced to the 50% level and the population were heavily exploited so that most clams did not get beyond that level, over time the strong selection process would reduce the size at maturity.
One caveat of this study is that no organisms smaller than 90 mm SL were collected. Therefore, the following steps for research must involve performing a histological study to determinate the recruitment size at an individual level.
Finally, this study has reported size at maturity of P. globosa in the Gulf of California and found there is no reason to establishing a legal size of 130 mm SL for P. globosa fishery. This management strategy should be modified according to scientific findings.











 nueva página del texto (beta)
nueva página del texto (beta)