Introduction
Seagrass meadows are prominent components of the littoral zone of tropical and temperate seas, provide habitat and food for organisms, and modulate sedimentary and biogeochemical processes (Duarte & Chiscano, 1999). In general, seagrasses are assigned to two families, Potamogetonaceae and Hydrocharitaceae, encompassing 12 genera of angiosperms containing about 50 species (Hemminga & Duarte, 2000). In addition to their local importance, seagrasses are significant contributors to the primary production of the global ocean (Duarte & Chiscano, 1999), which suggests that they could be analyzed further as a potential human-food source (Montaño et al., 1999). Seagrasses have been used as human food especially by coastal populations (Hemminga & Duarte, 2000), and for a variety of remedial purposes in folk medicine (e.g. treatment of fever and skin diseases) (De la Torre-Castro & Ronnback, 2004). In some countries, seagrasses are used as medicine, food, fertilizer, and livestock feed (Rengasamy et al., 2013).
The nutrient concentration of aquatic resources has been relatively well documented (Duarte, 1992). There are a numerous reports regarding the nutritional content of seaweeds (Rhodophyta, Phaeophyta, and Chlorophyta) as a potential food source (Plaza et al., 2008; Gupta & Abu-Ghannam, 2011; Mohamed et al., 2012). Yet, there is still a dearth of studies on the nutritional composition of seagrasses and their possible utilization as a source of human food, and information regarding the Gulf of Mexico in particular is limited to the reports by Dawes (1986, 1990) that showed high levels of ash and protein in three seagrass species.
The aim of this paper involved examining the chemical nutritional content in three seagrass species from Laguna de Términos, Campeche, Mexico: Thalassia testudinum König, Halodule wrightii Ascherson, and Syringodium filiforme Küitzing during the rainy season of 2004.
Knowledge of the chemical composition of seagrass is important both for the assessment of the nutritional value of marine invertebrate or vertebrate herbivores and for the evaluation of potential sources of protein, carbohydrates, and lipids for commercial use or for possible human consumption. We note that, like most flora, the nutrient content of seagrass is affected by external factors such as geographic location, environmental conditions, seasons, and sampling conditions (Renaud & Luong-Van, 2006).
Materials and methods
Laguna de Términos (Fig. 1) is the largest coastal lagoon in Mexico (Carvalho et al., 2009). Situated at the southern extreme of the Gulf of Mexico, between latitudes N18° 27' 37'' and N 18° 47' 36'' and longitudes W 91° 14' 44'' and W 91° 53' 55'', the lagoon has a total surface area is 2,500 km2 and a mean depth of 3.5 m. It is connected to the Gulf of Mexico by two openings. Three rivers provide most of the freshwater input to the lagoon: Palizada, Chumpán, and Candelaria. The climate is determined by three seasons: dry, rainy, and north winds. The dry season commonly lasts from March to May and rains are heaviest from June to October. The north-winds season lasts from November to February (David & Kjerfve, 1998). In this system, seagrass are important components of coastal ecosystems and include three species: Thalassia testudinum, Halodule wrightii, and Syringodium filiforme. T. testudinum, commonly known as turtle grass, is a major component of the seagrass community, providing food for urchins, sea turtles, and fishes, and habitat for a diverse population of epiphytes (Pirog, 2011). H. wrightii, also known as shoal grass, is found in the intertidal zones of shallow waters with sandy or muddy substrates at depths of 0 to 2 m. It is well established from the southeastern United States to South America; it occupies the shallowest waters in the Gulf of Mexico and is often exposed during low tides (McGovern & Blankenhorn, 2007). S. filiforme commonly known as Manatee grass, is an important component of seagrass beds in shallow warm waters. It typically grows at depths ranging from 1 to 3 m and is found in the sublittoral zone of marine waters with sandy or muddy substrate (Duarte et al., 2007).
Using a hand shovel, we collected triplicate samples of each seagrass species during the 2004 rainy season (October) in the north region of the lagoon (Fig. 1). They were placed into plastic bags, stored on ice, and transported to the laboratory, where they were washed with distilled water to remove sand and surface debris, and holdfasts and epiphytes were removed. Rhizomes, thoroughly cleaned, were weighed (Sartorius Analytical Balance with 0.01 mg resolution) and then dried at 115° C for 5 h until a constant dry wet was obtained.
Proximate analysis was carried out on samples of the three species following the methods of analysis described by the Association of Official Analytical Chemists (AOAC, 2000). Analysis included the determination of crude protein, crude lipids, crude fiber, dry matter, nitrogen-free extract, and ash.
The Kjeldahl method was employed to determine the nitrogen-free extract and the crude protein (x 6.25). Crude lipids were extracted by continuous heat extraction of all soluble substance in petroleum ether. The organic matter and ash contents were determined based on methods outlined in AOAC by combustion at 550° C during 5 hours; the final ash was considered to be the mineral portion of the sample. The replicates of each sample were used for statistical analysis and the values were reported as mean ±1 standard error (SE).
Results
Table 1 shows the results of the proximate analysis (mean ±SE) of the three species of seagrass.
Table 1 Proximate analysis values (means ±1 standard error (SE)) of three seagrass species from Laguna de Términos, Campeche, Mexico, during the rainy season of 2004.
The crude protein value (Fig. 2a) was higher for S. filiforme at 10.43%, followed by T. testudinum with 8.47%, and H. wrightii with 8.10%. In contrast, the crude-lipid content was significantly different (p <0.001), with low content in all samples, i.e., 2.13, 2.33 and 0.83% for S. filiforme, H. wrightii and T. testudinum, respectively (Fig. 2b). The crude fiber content (Fig. 3a) was higher for S. filiforme with 19.43%, followed by H. wrightii with 19.03%, and T. testudinum with 15.70%. The dry matter content (Fig. 3b) was higher for T. testudinum with 12.27%, followed by S. filiforme with 11.50%, and H. wrightii at 9.47%. The difference observed for these two groups was p=0.008.
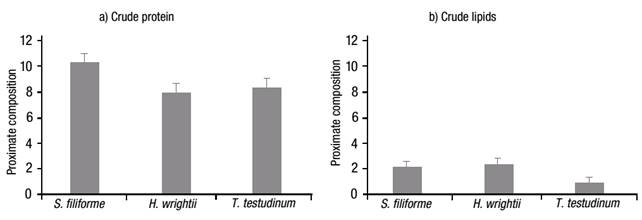
Figures 2a-b Proximate composition of three selected seagrass species from Laguna de Términos, Campeche, Mexico, during the rainy season of 2004: a) Crude protein, and b) Crude lipids. Values represent means ±1 standard error (SE).
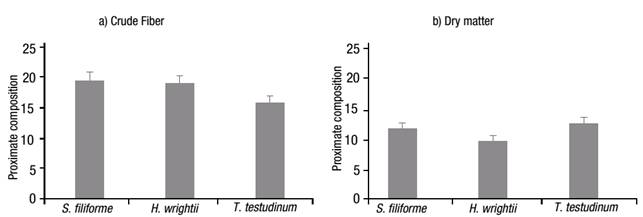
Figures 3a-b Proximate composition of the three selected seagrass species from Laguna de Términos, Campeche, Mexico, during the rainy season of 2004: a) Crude fiber, and b) Dry matter. Values represent means ±1 standard error (SE).
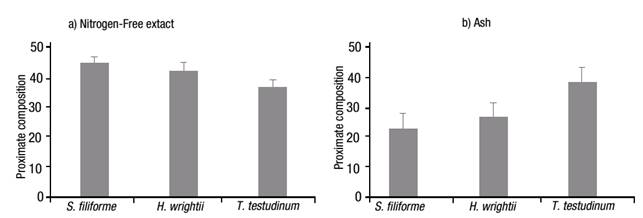
The nitrogen-free extract content (Fig. 4a) was higher for S. filiforme with 45.37%, followed by H. wrightii with 42.87%, and T. testudinum with 37.27%; ash content (Fig. 4b), an indicator of total mineral or inorganic content of the sample, was high for the three species: 23.43% for S. filiforme, 27.23% for H. wrightii and 38.77% for T. testudinum. The difference for these two groups was p=0.081.
Discussion
Seagrass productivity can surpass that of wheat, corn, and sugar beets (Rollon & Fortes, 1990). The high productivity of seagrass suggests that it should be further explored as a potential food source for humans (Montaño et al., 1999).
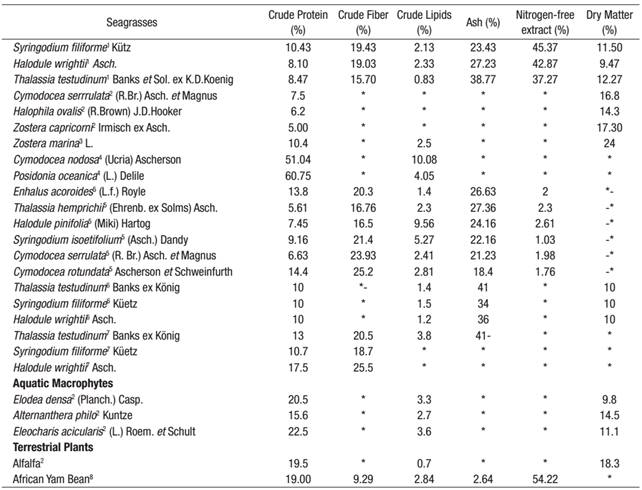
In this study, protein content ranged from 8.47 to 10.43%, and S. filiforme was found to have the most. These concentrations were similar to other published data for the seagrass Enhalus acoroides Linnaeus f. royle, which is traditionally consumed in some countries (Montaño et al., 1999). The values observed in this study were comparable to those of Dawes (1986, 1990), who reported crude protein values for S. filiforme (~10%) on the West Coast of Florida (Table 2). Recently, similar concentrations of protein (~14%) were observed for the seagrass Cymodocea rotundata Ehrenberg (Table 2) in the Gulf of Mannar, India (Rengasamy et al., 2013). As suggested by Athiperumalsami et al. (2008), high protein levels in seagrasses point to an interesting possibility for exploiting natural marine resources in processed form to alleviate problems of protein deficiency in developing countries. In comparison with most industrially-exploited seaweeds (Rhodophyta, Phaeophyta, and Chlorophyta), the protein fraction of brown seaweed is low (~15%) compared with that of green or red seaweed (~47%) (Arasaki & Arasaki, 1983). Except for Undaria pinnatifida Harvey, which has a protein level between 11 and 24%, most industrially-exploited brown seaweed (Laminaria digitata Hudson, Ascophyllum nodosum Linnaeus, Fucus vesiculosus Linnaeus, and Himanthalia elongate Linnaeus), have a protein content ~15%, similar to that of the seagrass S. filiforme studied here. Recently, the crude protein level for the African Yam Bean (Sphenostylis stenocarpa Hochst. ex A. Rich) has been reported at ~19% (Table 2) (Ndidi et al., 2014), which is similar to S. filiforme.
Table2 Comparative proximate composition values for different species of the 1present study; 2Aketa & Kawamura, 2001; 3Dawes & Guiry, 1992; 4Shams et al., 2013; 5Rengasamy et al., 2013; 6Dawes, 1986; 7Dawes, 1990; 8Ndidi et al., 2014.
*Not available data.
Significant variations in the lipid content of seagrasses species have been attributed to age, stage of growth, and ecological variations. The crude-lipid-content value presented in this study for S. filiforme (2.13%) was consistent with those reported for E. acoroides (1.40%) by Rengasamy et al. (2013). By contrast, the lipid content reported by Pradheeba et al. (2011) for E. acoroides (3.2%) was slightly higher than the values found by this study. In comparison, the lipid content in seaweeds is generally low, ranging from 0.12% for Jania rubens Linnaeus to 6.73% for Laurencia papillosa C.Agardh (Polat & Ozogul, 2009). Low lipid levels are also reported for Codium fragile Suringar, Gracilaria chilensis C. J. Bird, Macrocystis pyrifera Linnaeus (0.7-1.5%), Eucheuma cottonii Weber-van Bosse, Caulerpa lentillifera J. Agardh and Sargassum polycystum C. Agardh (0.29-1.11%) (Matanjun et al., 2009; Ortiz et al., 2009), of the same order, particularly for S. filiforme (2.13%), as the values found herein.
In this study, the fiber content was higher for S. filiforme (19.43%). The high proportion of fiber in this seagrass is in line with that reported for the seagrass C. rotundata (Rengasamy et al., 2013). Many components of the dietetic fiber show antioxidant and immunological activity (Suzuki et al., 2004). The World Health Organization (WHO) recommends a fiber intake of 22-23 g for each 1000 kcal of food (Kanwar et al., 1997). Dietary fiber is necessary for digestion, elimination of wastes, and contraction of the muscle walls of the digestive tract (Rengasamy et al., 2013). Recently, a dietary pattern containing low lipids and high fiber products (as observed for S. filiforme in this study) was associated with a lower risk of breast cancer (Kushi et al., 2012).
Nitrogen-free extract (NFE) consists of carbohydrates, sugars, starches, and a major portion of materials classified as hemicellulose in feeds (AOAC, 2000). Castro-González et al. (1991) indicated that the seaweed Macrocystis pyrifera contains ~46% of nitrogen-free extract; results presented here concur, but were considerably higher than the 23% reported by El-Deek and Brikaa (2009) for seaweed used in poultry diets.
Seagrass mineral content is higher than that of land plants and animal products (Ortega-Calvo et al., 1993). Sweet corn has a lower content (2.6%), while spinach has an exceptionally high mineral content (20.4%) for a land plant (Rupérez, 2002). The ash content in this study was higher for T. testudinum (38.77%) and lowest for S. filiforme (23.43%). Similar ash content was reported for E. acoroides (24.6%) and T. hemprichii Ehrenb. ex Solms (22.6%) by Yamamuro & Chirapart (2005). Highest ash content (~50%) has been reported in Halophila ovalis R. Brown in Australia (Yamamuro et al., 2001). The ash values obtained in the three species analyzed herein fell well within the wide ranges reported (8 to 40%) for some seaweeds (Rupérez, 2002).The proximate composition values in this study, in particular the high protein, high ash and low lipid content for S. filiforme, were comparable to those reported in terrestrial plants. Table 2 shows values reported for some seagrasses as well as macrophytes and terrestrial plants. Except for the seagrass Posidonia oceanica Linnaeus, the crude protein values reported herein agree with other species (both aquatic and terrestrial). For example, reported African Yam Bean values (Table 2) are of the same order as the seagrasses analyzed in this study, suggesting that the latter can be an important food supplement to help meet the recommended daily adult intake of some minerals and trace elements. The proximate composition of seagrasses has been correlated to seasonal periods, with low values in the winter months and high values in the spring and summer months. This cyclic response corresponds to a winter dieback or slowdown of growth and a summer growth peak due to a continued buildup of photosynthesis (Dawes, 1986). Thus, we expect a temporal variation in seagrasses from Laguna de Términos, given the wide climate variation in the area.
In summary, the chemical contents reported in this study, in particular the high protein, high ash and low lipid content would appear to make S. filiforme a good dietary supplement and source of nutrients for human consumption. Given these high nutritional levels, this seagrass could also be used as food for farmed fish. For example, protein requirements for the optimal growth of Nile tilapia (Oreochromis niloticus) depend on the source of the protein, the size of the fish, its age, and the energy content of its diet, reported to vary from as high as 45-50% for first feeding larvae, 35-40% for fry and fingerlings, and 25-35% percent for juveniles. The best protein digestibility occurs at 25 °C, and the optimum dietary protein to energy ratio was estimated around 110 to 120 mg per kcal digestible energy for fry and fingerling, respectively (Stickney, 2006). Tilapia require about 40-45% protein for optimum reproduction, spawning efficiency, and larval growth and survival.
The lipid nutrition of farmed tilapia has been studied by Ng and Chong (2004). The minimum requirement of dietary lipids in tilapia diets is 5%, but improved growth and protein utilization efficiency has been reported for diets with 10-15% of lipids.
The daily protein requirement of common carp is about 1 g/kg body weight for maintenance and 12 g/kg body weight for maximum protein retention. The efficiency of nitrogen utilization for growth is highest with a protein intake of 7 to 8 g/kg body weight/day. Crude protein levels ranging from 20% to 38% appear to satisfy the fish optimally. This level has been determined by using semi-purified diets containing a single high-quality protein source. When the diet contains sufficient digestible energy, the optimal protein level can be effectively kept at 15-30% (Watanabe, 1982). As an omnivorous fish, common carp (Cyprinus carpio) can effectively utilize both lipids and carbohydrates as dietary energy sources. The enrichment of the digestible energy content from 13 to 15 MJ/kg diet by addition of lipid at levels of 5-15% to diets did not result in higher growth rates or improved net protein utilization (Takeuchi et al., 1979). Increasing dietary lipid seems to increase its body deposition.
In Mexico, as in other countries, new possibilities for using seagrasses could exist in developing functional foods for human nutrition, particularly the protein-rich species. In addition, high ash and nitrogen-free extract content were a common feature in the three species studied. Based on our results, these seagrasses, used as a food supplement, could help fulfill the recommended daily adult requirements of some macro-minerals and trace elements. Lipid content of all seagrass was generally low. Yet, like any other ingredient, seagrasses have to meet the industrial specifications and official consumer safety regulations dealing with microbiological quality and heavy metal contents. More research is needed to evaluate the nutritional value of seagrass, especially in the fields of biochemical analysis. The use of seagrass with high protein levels as a pellet binder in the production of foods for aquaculture fish could be another application of this marine plant resource.
Proteins are the primary components of living things. The presence of high protein levels in seagrasses indicates that they may have increased value as a food or that a protein base bioactive compound may be isolated in the future.











 nueva página del texto (beta)
nueva página del texto (beta)