Introduction
The indirect effects of carnivores on primary producers, as mediated by herbivores, can control community dynamics in a number of ways (Terborgh et al., 2001; Shurin et al., 2002; Trussell et al., 2004). Traditionally, it has been suggested that the consumption of herbivores by carnivores translates into a lower impact on primary producers (e.g. Rosenzweig, 1973; Oksanen et al., 1981), a situation referred to as a density-mediated indirect interaction (DMII) (Abrams, 1995; Werner & Peacor, 2003). More recently, it has been suggested that predators can induce changes in the phenotypic traits of their prey that reduce or eliminate the risk of predation. Evading carnivores could diminish the foraging activities of herbivores and therefore reduce their impact on primary producers (Abrams, 1995; Lima, 1998; Sih et al., 2000; Lima, 2002), an effect known as a trait-mediated indirect interaction (TMII) (Abrams, 1995; Werner & Peacor, 2003).
In the marine environment, the morphological and behavioral plasticity of prey in response to predators has been widely reported (Trussell & Smith, 2000; Trussell & Nicklin, 2002; Dill et al., 2003; Trussell et al., 2004). Within the marine environment, rocky intertidal shores can be used to evaluate the relative importance of non-lethal effects due to variations in predator density and prey recruitment, variations that lead to uncertainty in predation risk between sites (Navarrete & Manzur, 2008; Wieters et al., 2008).
The sun-star Heliaster helianthus (Lamarck, 1816) is the most notable rocky intertidal predator along the central-northern coasts of Chile and southern coasts of Peru (Castilla & Paine, 1987; Tokeshi, 1989). In central Chile, H. helianthus is a generalist predator that increases its consumption of limpets when the availability of its primary prey, the mussel Perumytilus purpuratus (Lamarck, 1819), is diminished (Paine et al., 1985; Navarrete & Manzur, 2008; Barahona & Navarrete, 2010). Limpets (Fissurella spp.) play a key role in the community structure of the mid-intertidal zone through the consumption of the dominant corticated algae Mazzaella, and this can entirely modify the intertidal landscape, especially when there is a high abundance of large Fissurella limpets (Oliva & Castilla, 1986; Moreno & Jaramillo, 1983; Jara & Moreno, 1984; Aguilera, 2011).
In general, the activity patterns, foraging modes, and habitat use of intertidal limpets are altered by predation risk from diverse taxa (Branch, 1981; Phillips & Castori, 1982; Hahn & Denny, 1989; Sorensen & Lindberg, 1991; Iwasaki, 1993). Studies concerning the behavioral ecology of Fissurella spp. highlight predation risk as a determinant for higher activity levels during nocturnal low tide (Franz, 1990; Pino et al., 1994; Serra et al., 2001). More recently, Escobar & Navarrete (2011) evaluated the escape responses of Fissurella crassa (Lamarck, 1822) and Fissurella limbata (Sowerby, 1835) when faced with changes in predation risk and considering contact history with the predator H. helianthus. However, this study presented major weaknesses in its evaluations; one being that the resources available to prey were not considered and the other being that the physiological significance of the escape response on F. crassa trait variations was not considered.
The aim of the present research was to evaluate the body condition and escape response of the intertidal herbivore Fissurella crassa in natural habitats differing in predation risk and in resource availability, as estimated through the abundance of the predator H. helianthus and algae, respectively. It was hypothesized that higher algae abundance and body conditions would be found in zones with a greater H. helianthus predation risk.
Materials and methods
Study sites. In the autumn of 2009, two sites (El Litre and Playa Chica) located at Quintay, Valparaíso, Chile (33° 11´ S; 71° 1´ W) were selected for observations. Both sites are characterized as upwelling areas (Pulgar et al., 2011), in addition to belonging to a Management and Exploitation Area for Benthic Resources where the catch of certain invertebrate species, such as Fissurella spp., is regulated. El Litre and Playa Chica were selected because prior evidence indicates that El Litre has a higher density of predators as compared to Playa Chica (Molina et al., 2014), with a distance of 3.8 km between sites. However, both sites have a high abundance of F. crassa. For site evaluations, sectors were chosen with moderately sloped (< 45°) rocky substrates and with semi-exposure to waves. In each of these sectors, the abundance of the predator H. helianthus, the mussel P. purpuratus, and of the limpet F. crassa were determined along with the richness and abundance of algae and the body condition and escape response of F. crassa.
Determining predator (H. helianthus), prey (P. purpuratus and F. crassa), and algae abundance. At each site, the abundances of H. helianthus, the mussel P. purpuratus, and the limpet F. crassa were determined by using two horizontal transects located in the low- and mid-intertidal zones of the coast. A minimum of 16 and a maximum of 40 quadrats of 0.25 m2 were located at intervals of 2-3 meters along each transect. In each quadrat, the number of H. helianthus and the intertidal limpets F. crassa were recorded along with the coverage percentage of P. purpuratus. The coverage percentage of mussels was determined with the help of a grid containing 25 squares of 10x10 cm, with each square accounting for 4% coverage. Likewise, the coverage percentage and number of algae species per site were estimated using a minimum of 10 and maximum of 50 quadrats along each of the two transects. The taxonomic identification of algae, at least to the genus level, was performed using conventional techniques (Womersley, 1984) according to Ramírez and Santelices (1991) and Hoffmann and Santelices (1997).
Physiological state of F. crassa. Specimens of F. crassa were manually collected from the intertidal zone at each study site (El Litre n= 271, Playa Chica n= 205). All specimens were deposited in plastic bags, labeled, and transported to the laboratory where they were frozen (-20°C). In the laboratory, the maximum shell length was measured, and the intestine and muscular foot of each limpet were isolated and weighed. Additionally, the body condition was estimated from the residuals of the relationship between soft tissue biomass and maximum shell length (Jakob et al., 1996).
Escape response of F. crassa. The characterization and quantification of the escape response of F. crassa was performed at both sites during low tide, at similar hours of the day, and on limpets located on moderately sloped (< 45°) rocky substrates with similar textures and humidity and without algal cover or seawater flushing. Specimens of F. crassa were identified according to the morphological traits described by Oliva and Castilla (1992).
In predator trials, the sun-star H. helianthus was manually deposited, while moist, on the apical orifice of the limpet's shell (El Litre n= 57 and Playa Chica n= 29) (modified from Espoz & Castilla, 2000). To validate the ability of the limpet to detect and escape from H. helianthus, control trials were performed via the direct stimulation of the apical orifice of F. crassa with a plastic tube (n= 15 individuals per site) (Escobar & Navarrete, 2011). The sun-stars were collected in situ before the trials and were changed every three assays.
The time, in seconds, was recorded from the moment of contact until the manifestation of behaviors associated with escape or permanence in place (movement or inaction, respectively) using a digital chronometer (CASIO Frogman, DW 9900). All trials continued until the entire limpet's body was out of stimulus range or, in the case of inaction, until 15 minutes (approximately double the response time recorded by Espoz & Castilla, 2000). The assays were performed only one time per individual limpet. In order to assess the relative speed of the limpet in exhibiting distinct behavioral responses, the percentage of total time taken by the limpet to express each behavior was calculated.
Data analyses. A two-way analysis of variance (ANOVA) (General Lineal Models) test was used to compare the abundances of the predator H. helianthus, the mussel P. purpuratus, the limpet F. crassa, and of algae between sites (El Litre and Playa Chica) and between intertidal zones (mid and low). The site was considered a random factor, and the intertidal zone was a fixed factor (Mixed Model). The site was considered a random factor given the study's aim of understanding how variations in predator abundance and resource availability for the prey influence the behavior and physiological state of the prey. The intertidal zone was considered a fixed factor because tidal zones have a physical basis for definition (see Bennington & Thayne, 1994). As a response variable, the abundance of each taxon was considered, and, in the case of algae, the coverage of dominant functional groups was also taken into account.
Given that gut biomass, body condition, and muscular foot biomass are affected by a limpet's body size, the residual of these relationships were compared between study sites using one-way ANOVA (General Lineal Models). The total response time and the percentage of total time used by F. crassa in expressing any response were evaluated between sites by one-way ANOVA (General Lineal Models). Finally, repeated measures ANOVA was used to evaluate the temporal sequence of the limpets' behavioral responses by considering the sites and the lapsed time between behaviors (Sokal & Rohlf, 1981; Zar, 1996).
Results
Predator and prey abundance and algae richness and abundance. The predator abundance of the sun-star H. helianthus was greater at El Litre than at Playa Chica (ANOVA, F 1,126 = 8.84, p = 0.003; Fig. 1a), without differences between intertidal zones. In contrast, the abundance of its principal prey, the mussel P. purpuratus, was lower at El Litre than at Playa Chica and greater in the mid-intertidal zone than in the low-intertidal zone (ANOVA, site: F1,72 = 32.15, p < 0.001; intertidal zone: F1,72 = 5.33, P = 0.024; Fig. 1b). Additionally, the abundance of Fissurella spp. did not show significant differences between sites, and at both sites, abundance was higher in the low-intertidal zone (ANOVA, F1,126 = 10.65, p = 0.001; Fig. 1c).
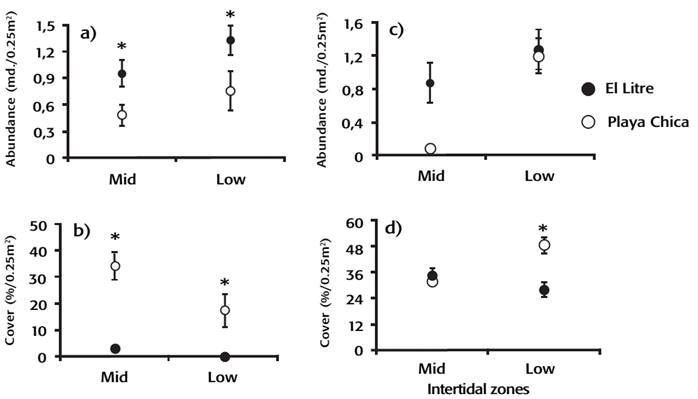
Figures 1a-d: Average abundance of predator, prey, and algae at the different study sites and intertidal zones. (a) Sun-star H. helianthus. (b) Mussels P. purpuratus. (c) Limpets Fissurella spp. (d) Algae indicate ± standard error. * indicates significant differences between sites.
A total of 18 algae species were recorded, of which 11 were found at El Litre and 16 at Playa Chica (Table 1). The low-intertidal zone of Playa Chica showed a higher total coverage of algae than the mid-intertidal zone and as compared to the two intertidal zones of El Litre. (ANOVA, F1,273 = 15.11, p < 0.001, Fig. 1 d, Tukey a-posteriori test, p < 0.05). Analysis of the coverage percentage of common algae species indicated that some species varied between sites depending on the intertidal zone, with Ulva sp. specifically showing greater coverage in the mid-intertidal zone at El Litre as compared to Playa Chica (Fig. 2 a). In contrast, in the low-intertidal zone, Ulva sp., Porphyra sp., Polysiphonia sp., and Mazzaella sp showed a higher coverage at Playa Chica than at El Litre (ANOVA, F7,728 = 15.87, p < 0.001,Tukey, a-posteriori test p < 0.05; Fig. 2a and b). Evaluation of the dominant functional groups indicated that ephemeral algae were more abundant at El Litre in the mid-intertidal zone, while at Playa Chica these were more abundant in the low-intertidal zone (ANOVA, F1,467 = 10.89, p = 0.001; Fig. 2 a and b).
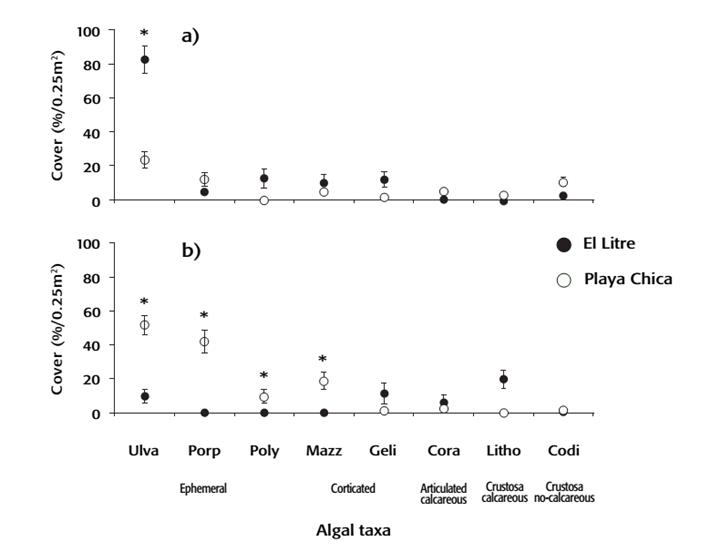
Figures 2a-b Average coverage of algal taxa at the different study sites and intertidal zones. (a) Mid-intertidal zone. (b) Low-intertidal zone. Bars indicate ± standard error. Ulva sp., Porp= Porphyra ps., Poly= Polysiphonia sp., Mazz= Mazzaella sp., Geli= Gelidium spp., Cora= Corallina officianalis var. chilensis, Liho= Lithothamnion sp., and Codi= Codium dimorphum. Eph: Ephimeral, Cort: Corticated, Artic calc: Articulated calcerous, Crust calc: Crustosa calcareous, and Crust no-calc: Custosa no-calcareous.
Physiological state of F. crassa . The gut biomass, body condition, and muscular foot biomass of F. crassa were at least two times greater at El Litre than at Playa Chica (ANOVA, gut biomass: F1,430 = 36.54, p < 0.001; body condition: F1,469 = 13.25, p < 0.001; muscular foot biomass: F1,435 = 11.12, p < 0.001; Fig. 3a-c).

Figures 3a-c: Residuals from the regression between (a) gut biomass, (b) soft biomass, and (c) muscular foot biomass of Fissurella crassa individuals at El Litre and Playa Chica. El Litre; Playa Chica. Bars indicate ± standard error.
Escape response of F. crassa. In the trials, 29 H. helianthus individuals of 15 cm in diameter were used; 19 of which were from El Litre and 10 of which were from Playa Chica. Limpets with a shell length of 6.59 ± 2.08 cm (mean ± SD) for El Litre and of 6.24 ± 0.99 cm for Playa Chica were obtained with no significant differences between sites (ANOVA, F1,114 = 0.586; p = 0.448). All F. crassa in the control trials responded by firmly fixing to the substrate (Fig. 4a). On the contrary, limpets subjected to contact with the sun-star H. helianthus showed at least one or all of the following behaviors associated with escape in a temporal sequence of occurrence: display of the mantle and pallial tentacles, projection of cephalic tentacles, and/or active displacement of individuals away from the point of contact (Repeated measures ANOVA, F2,128 = 17.53, p< 0.001; Figs. 4b and 5). The total time used for these successive behaviors did not show significant differences between sites (ANOVA, F1,84 = 0.74, p = 0.393; Fig. 5), as was also found for the percentage of time spent on the first two responses (ANOVA, display of the mantle and pallial tentacles: F1,79 = 0.73, p = 0.394; cephalic tentacles projection: F1,71 = 0.21, p = 0.650; Fig. 6). However, the proportion of time used by F. crassa to start displacement was lower at El Litre, the site with the highest density of predators and, in general, lower algal resources (ANOVA, F1,74 = 8.03, p = 0.006; Figs. 1d and 6).

Figures 4a-b: Percentage of limpets showing the following behaviors: display of the mantle and pallial tentacles, projection of cephalic tentacles, active displacement, and fixation to the substrate; during (a) the control trials and (b) the predator trials.

Figure 5: Time used by F. crassa to initiate the following behaviors recorded in response to the presence of H. helianthus: display of the mantle and pallial tentacles, projection of cephalic tentacles, and active displacement. Bars indicate ± standard error.
Discussion
The obtained results showed a higher abundance of H. helianthus and lower abundance of P. purpuratus at El Litre, which suggest that probability of predator encounters could be greater for the limpet population at El Litre. Moreover, F. crassa at this site showed an intense escape response to the presence of a predator and higher body conditions than at Playa Chica. Considering this, the lower algae coverage at El Litre could be related to higher algae consumption at this site as compared to Playa Chica, with differences between sites at the genus-level in association with algae important to the diet of F. crassa. In this regard, some studies suggest that the per capita predation pressure of H. helianthus on limpets is negatively associated with the environmental availability of the mussel P. purpuratus (Navarrete & Manzur, 2008; Barahona & Navarrete, 2010). Thus, the probability of a predator encounter could be more important for the limpet population at El Litre, especially when considering that the recognition of and escape from predators are characteristics retained by limpets that would be favored by a contact history between predator and prey (Escobar & Navarrete, 2011).
On the other hand, the total level of resources available to F. crassa showed differences between sites only in the low-intertidal zone, where the greatest abundance of limpets was found (Fig. 1c). In general, the total algae coverage was lower at El Litre than at Playa Chica (Fig. 1 d), and a genus-level assessment showed that the differences between sites were associated with the importance of each algae in the F. crassa diet. Identified algae included ephemeral forms such as Ulva sp., Porphyra sp. and Polysiphonia sp. and corticated forms such as Mazzaella sp. and Gelidium sp. (Moreno & Jaramillo, 1983; Santelices et al., 1986; Osorio et al., 1988; Aguilera, 2011). Most of these taxa were less abundant at El Litre compared to Playa Chica (Fig. 2 b), with the exception of Ulva sp. that presented a higher abundance in the mid-intertidal zone, where the abundance of F. crassa was low (Fig. 2a). Thus, El Litre was characterized by low food availability for limpets and a greater probability of encountering predators.
According to evidence that incorporates the trade-off between mortality risk and resource acquisition (Lima & Dill, 1990; McNamara & Houston, 1994; Werner & Anholt, 1993; Anholt & Werner, 1995), a lower abundance of important algae for the Fissurella spp. diet at the site with higher predator density (Fig. 1 a) could be expected to lead to less foraging activity by limpets. In this sense, the greater gut biomass of limpets at El Litre (Fig. 3a) suggests more foraging activity. Moreover, the body condition and muscular foot biomass recorded for limpets at this site suggest a better nutritional state (Fig. 3b-c).
Additionally, similar densities of F. crassa between El Litre and Playa Chica (Fig. 1 c) suggest that the differential abundance of H. helianthus between sites did not affect limpet mortality. In this sense, reducing the probability of predator encounters or reducing the probability of prey death in predator encounters through escape responses are survival strategies (Sih, 1984; Miner et al., 2005). The present data indicated that there was greater foraging activity and a better nutritional state for F. crassa at El Litre (Fig. 3), therefore suggesting a more developed escape response of F. crassa at El Litre than at Playa Chica (Fig. 6). This is consistent with other studies at these research sites (Pulgar et al., 2012a; Pulgar et al., 2012b). The effectiveness of escape responses in reducing predation rates by H. helianthus is supported by field observations along the coast of Chile (Castilla, 1981; Gaymer & Himmelman, 2008; Escobar & Navarrete, 2011). The present results indicated that at both sites H. helianthus induced behavioral responses in F. crassa associated with escape from predation (Fig. 4a), including the following three sequential behaviors: display of the mantle and pallial tentacles, cephalic tentacle projection, and, finally, displacement (Fig. 5). However, limpets at the site with higher predator density and fewer algae resources (Fig. 1, El Litre) were quicker to displace and escape (Fig. 6). The absence of these behaviors during control trials, where the limpets stayed firmly fixed to the substrate (Fig. 4 a), suggests that F. crassa is able to discriminate between different stimuli. Variations in the escape response could result from contact history between prey and predator at a given site (Sih, 1992; Schmitz et al., 2004).
In general, all of the processes involved in the capture and allocation of energy when in the presence of a predator have been related to behavioral changes (Lima & Bednekoff, 1999; Werner & Peacor, 2003; Schmitz et al., 2004). More specifically, it has been suggested that physiological restrictions could be related to temporal and spatial activity patterns of Fissurella spp. (Franz, 1990; Serra et al., 2001). In this sense, the escape response of F. crassa to a predator at the site with higher H. helianthus density (Figs. 1, 4 , and 5) would require a better body condition and greater muscular foot development that could be obtained by increased foraging (Fig. 3). In this regard, the development of the muscular foot in F. crassa is an important attribute against the risks of dislodgement and predation (Serra et al., 2001). Thus, the increase in foraging activity at the site with higher predator density could be associated with increased energy demands imposed by the escape response (e.g. faster displacement), with subsequent effects on food abundance (e.g. algae coverage) (Figs. 1d and 6).
Studies on the processes that structure communities have typically focused on density-dependent perspectives, such as lethal effects (Begon et al., 2006), and have not considered behavioral changes and the consequences of these for community structure (Espoz & Castilla, 2000; Werner & Peacor, 2003; Trussell et al., 2004; Schmitz et al., 2004). In the present study, the predator appears to affect the behavioral traits of the prey, which in turn would impact the algae consumption needed to meet the energy demands associated with these behaviors. The current results emphasize the need to consider a physiological perspective when studying indirect, trait-mediated effects, which could help elucidate how environmental variation is expressed through physiological characteristics and ecological processes (Dahloff et al., 2002; Menge et al., 2002; Pulgar et al., 2011; Pulgar et al., 2012a; Pulgar et al., 2012b).











 nueva página del texto (beta)
nueva página del texto (beta)




