Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Hidrobiológica
versão impressa ISSN 0188-8897
Hidrobiológica vol.24 no.1 Ciudad de México Abr. 2014
Artículos
Effects of hydrographic conditions on the transport of neon flying squid Ommastrephes bartramii larvae in the North Pacific Ocean
Efecto de las condiciones hidrográficas en el transporte de las larvas de calamar rojo Ommastrephes bartramii en el Océano Pacífico Norte
Yoshiki Kato1, Mitsuo Sakai1, Masachika Mmasujima2, Makoto Okazaki2, Hiromichi Igarashi3, Shuhei Masuda3 and Toshiyuki Awaji4
1 Tohoku National Fisheries Research Institute, 25-259 Aza, Shimomekurakubo, Hachinohe, Aomori 031-0841, Japan.
2 National Research Institute of Fisheries Science, 2-12-4 Fuku-ura, Kanazawa-ku, Yokohama, Kanagawa 236-8648, Japan.
3 Japan Agency of Marine-Earth Science and Technology, Showa-cho, Kanazawa-ku, Yokohama 236-0001, Japan.
4 Graduate School of Science, Kyoto University, Kitashirakawa Oiwake-cho, Kyoto 606-8502, Japan e-mail: kyoshiki@affrc.go.jp
Recibido: 2 de febrero de 2012.
Aceptado: 9 de septiembre de 2013.
ABSTRACT
The neon flying squid, Ommastrephes bartramii, is widespread in subtropical and temperate regions. In the North Pacific Ocean, the species is comprised of two spawning cohorts; an autumn cohort and a winter-spring cohort. Interestingly, despite their apparently contiguous hatching periods, there is a marked disparity in the mantle length of both cohorts. We hypothesized that differences in the ambient temperature during larval development were responsible for the observed disparity in mantle size. Numerical simulations of ambient temperature revealed that water temperatures were approximately 1 °C higher in areas inhabited by the autumn cohort than they were in areas inhabited by the winter-spring cohort. The findings imply that differences in ambient water temperature and nutrient condition may be responsible for the observed differences in the growth of the autumn and winter-spring cohorts.
Key words: Ambient temperature, neon flying squid, spawning cohort, 4D-VAR.
RESUMEN
El calamar neón, Ommastrephes bartramii, está muy extendido en las regiones subtropicales y templadas. En el Pacífico Norte, la especie se compone de dos cohortes de desove, la de otoño y la de invierno y primavera. Curiosamente, a pesar de sus períodos de incubación contiguos, al parecer hay una clara diferencia en la longitud del manto de ambas cohortes. La hipótesis es que son las diferencias en la temperatura ambiental durante el desarrollo larval fueron las responsables de las diferencias observadas en el crecimiento del manto. Las simulaciones numéricas de la temperatura ambiente en la cohorte de otoño, revelaron que la temperatura del agua fue aproximadamente 1 °C más alta que en la de invierno y primavera, lo que implica que las diferencias en la temperatura del agua es la responsable de las diferencias observadas en el crecimiento durante el otoño y las cohortes de invierno y primavera de O. bartramii.
Palabras clave: Calamar rojo, cohorte desovante, temperatura ambiente, 4D-VAR.
INTRODUCTION
The neon flying squid, Ommastrephes bartramii (Lesueur, 1821), is widespread in subtropical and temperate regions (Roper et al., 1984). This economically important oceanic squid species has been harvested commercially by Japan since 1974, and subsequently by Korea and China. The north Pacific population is comprised of two spawning cohorts; an autumn cohort and a winter-spring cohort (Yatsu et al., 1997; 1998). Interestingly, despite their apparently contiguous hatching periods, there is a marked difference in the mantle length of both cohorts. Stock levels of the autumn-spawning cohort, which is important in the fishery economy because of its large size, were low when large-scale driftnet fishing was widely practiced (1979-1992). After an international moratorium on all large-scale pelagic drift net fishing at the end of 1992, squid stocks increased rapidly (Yatsu et al., 1998; Ichii et al., 2009).
Field observations have shown that both cohorts have a lifespan of one year (O'Dor 1998), and that they migrate between their spawning grounds in subtropical waters (30-35 °N) to their feeding grounds in subarctic waters (40-45 °N) (Ichii et al., 2011; Yatsu et al., 1997, 1998; Chen & Chiu, 2003). Although the migration routes of juvenile neon flying squid have been inferred based on ambient sea temperatures and fisheries data obtained for both cohorts (Murata et al., 1985; Yatsu et al., 1998; Ichii et al., 2006), relatively little is known about the migration events undertaken before the juvenile stage. Thus, to elucidate the effects of hydrographic conditions on the migration and distribution of neon flying squid larvae in the North Pacific Ocean, numerical experiments were conducted using a Lagrangian particle-tracking model.
Numerical modeling has recently been employed to clarify the larval transport mechanisms of a variety of fish species in the open ocean (Kimura et al., 1999; Kim et al., 2007; Kitagawa et al., 2010). These simulations of larval-juvenile dispersal have shown that oversimplification of the biological characteristics and ecological phenomena by models can markedly reduce their applicability to real systems.
In this study, we examined the effect of the ambient water temperature during larval period on differences in the size of the two cohorts using cutting-edge physical simulation data sets.
MATERIALS AND METHODS
Circulation model. Since Lagrangian particle-tracking models require hydrographic data, such as current velocity and water temperature, we employed a four-dimensional variational (4D-VAR) data assimilation system to more accurately define the mean seasonal state of the North Pacific Ocean. The 4D-VAR system is a synthesis of observational records and a sophisticated general circulation model that produces dynamically consistent time-varying data. The system is capable of realistically representing global ocean circulation patterns and requires no artificial temperature sources or sinks, or salinity fields. The resulting dataset enabled us to clarify both water mass formation and movement processes at a horizontal resolution of one degree with 27 vertical levels at monthly intervals (Masuda et al., 2006).
Particle tracking. To estimate the extent of larval transport to temperate waters, simulated larva particles were released in the paralarval distribution range and tracked as passive tracers for a 30-day period. It was assumed that the swimming ability of paralarvae and juveniles is relatively poor. For the particle tracking model, the following equations were applied discretely to individual particles at each time step, starting from an initial condition:
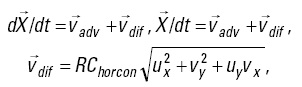
where  is the ocean current velocity obtained from the ocean general circulation model, 4D-VAR,
is the ocean current velocity obtained from the ocean general circulation model, 4D-VAR, 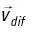 is a random velocity associated with horizontal oceanic diffusion and expressed by the Smagorinsky scheme, R is a random number between -1 and 1 generated at each time step for each individual particle, and Chorcon is the Smagorinsky constant and is assumed to have a value of 0.05.
is a random velocity associated with horizontal oceanic diffusion and expressed by the Smagorinsky scheme, R is a random number between -1 and 1 generated at each time step for each individual particle, and Chorcon is the Smagorinsky constant and is assumed to have a value of 0.05.
RESULTS
In this study, the particles were released in two domains within the areas shown in Figure 1. These areas were selected based on the seasonal distributions of neon flying squid paralarvae that were estimated previously by observations conducted from 1993 to 2001 (Ichii et al., 2004; Mori et al. 1999). Based on observations of the vertical distribution of paralarvae and juveniles in the wild (Okutani, 1968; Young & Hirota, 1990; Saito & Kubodera, 1993), particles were released at depths of 4 m in a horizontal velocity field with no-diffusion. The vertical distribution of the particles was fixed to a depth of 4 m for the duration of the simulation because the distribution of neon flying squid larvae appears to be restricted to near the ocean surface (Saito & Kubodera, 1993; Bower, 1996). Particles simulating the autumn cohort were released on October 1 and November 1 from 1993 to 2001, while particles simulating the winter-spring cohort were released on February 1 and May 1 from 1993 to 2001.
Figure 2 shows the distribution of particles, which remained very close to their initial positions and exhibited very little year-on-year variation. The particles of the autumn cohort moved eastward for approximately 120 nautical miles (220 kilometers), while the particles of the winter-spring cohort moved northward for approximately 120 nautical miles. The track followed by the particles released in November, varied only slightly between years, and the particles that were released in November were were typically present at low densities. Thus, horizontal diffusion of the particles was higher in November than in other months.
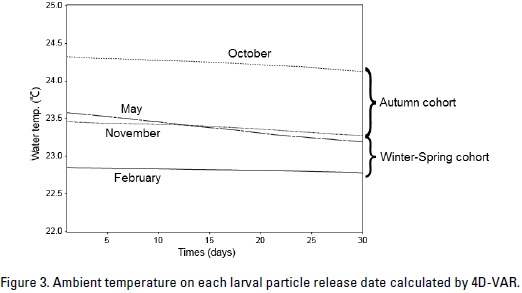
These results closely corroborate the changes observed in larval distribution at ambient water temperatures. Figure 3 the variation in the ambient water temperature over time. The acumulated water temperature for a 30-day period can be calculated using the following equation:
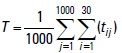
where tij is the ambient water temperature (°C) of particle. j indicated particle identification number. One thousand particles were released at one experiment. i represented days after particles released. T is the accumulated water temperature ( °C-day). There were large difference in theacumulated water temperature between the particles that were released in October and those that were released on February (Fig. 4). The value of difference was 42.5 °Cday.
DISCUSSION
The projections of the Lagrangian particle-tracking model employed in this study showed that larvae are not transported long distance during the larval period. In addition, the ambient water temperature during the larval period of the autumn cohort was estimated to be 1 to 1.5 °C higher than it was during the larval period of the winter-spring cohort. Since individuals belonging to the autumn cohort were typically larger than those of the winter-spring cohort (Yatsu et al. 1997, 1998), it appears likely that the difference in ambient water temperature, especially in the accumulated water temperature, affects larval growth. Semmens and Moltschaniwskyj (2000) concluded that the larger muscle blocks of loliginid squid Sepioteuthis lessoniana Lesson, 1830 affected both body size and individual growth rates. And larval growth in S. lessoniana was also influenced by environmental factors, population density, and, to some extent, genetic background. The presence of a continuous environmental parameter is also considered important for understanding growth dynamics in cephalopod. Statolith analysis of wild-caught individuals revealed that growth was dependent on ambient temperature (Sakai et al., 2004). Growth simulations by Forsythe (1993) revealed that even a 1 °C increase in average temperature during the 90-day exponential growth phase of larvae was capable of producing a two-fold increase in larval weight, while a 2 °C increase would result in a five-fold increase in larval weight over time. Grist and Clers (1999) reported that the body size of individual squid increases rapidly during the first stage of growth and that growth rate is temperature dependent. Andre et al. (2009) employed the model to investigate growth patterns occurring at different temperatures for 2 octopus species, Octopus ocellatus Gray, 1849 and O. pallidus Hoyle, 1885. The model projections were consistent with laboratory data and suggest that increases in temperature as small as 1 °C could have a significant influence on cephalopod growth, affecting the threshold body mass by up to 15.5% and the body mass at 100 d by up to 62.6%. From the above, difference of ambient water temperatures could be caused the difference of mantle length between autumn cohorts and winter-spring cohorts.
However the growth rate was also considered to be affected by nutrient conditions (Ichii et al., 2004; Forsythe, 1993). Ichii et al. (2004) reported that the spawning grounds of the autumn cohort of O. bartramiiare located within the subtropical frontal zone, which is characterized by enhanced productivity in winter due to its proximity to the transition zone chlorophyll front (TZCF); a zone of surface convergence where cool surface waters with high chlorophyll a concentrations from the north sink beneath warm oligotrophic waters from the south (Polovina et al., 2001). Conversely, the spawning grounds of the winter-spring cohort are located in the subtropical domain, which is less productive (Ichii et al., 2009). Unlike their study, satellite chlorophyll data was not employed in this study because of the large spatiotemporal differences in the data resolution between the physical data of the model used in this study and that of satellite data. Future research will focus on the development of an ecosystem model capable of using highresolution physical data to clarify the effect of ambient nutrient conditions on larval growth in O. bartramii in greater detail.
ACKNOWLEDGEMENTS
We thank Dr. T. Ichii and Dr. T. Wakabayashi of the Fisheries Research Agency for their valuable comments. This work was supported by the Research Program on Climate Change Adaptation (RECCA) of the Ministry of Education, Culture, Sports, Science and Technology.
REFERENCES
Andre, J., E. P. M. Grist, J. M. Semmens, G. T. Pecl & S. Segawa. 2009. Effects of temperature on energetics and the growth pattern of benthic octopuses. Marine Ecology-Progress Series 374: 167-179. [ Links ]
Bower, J. R. 1996. Estimated paralarval drift and inferred hatching sites for Ommastrephes bartramii (Cephalopoda: Ommastrephidae) near the Hawaiian Archipelago. Fishery Bulletin 94 (3): 398-411. [ Links ]
Chen, C. S. & T. S. Chiu. 2003. Variations of life history parameters in two geographical groups of the neon flying squid, Ommastrephes bartramii, from the North Pacific. Fisheries Research 63 (3): 349-366. [ Links ]
Forsythe, J. W. 1993. A working hypothesis of how seasonal temperature change may impact the field growth of young cephalopods. In-: O'Dor, R. K., T. Okutani & T. Kubodera (Eds.). Recent advances in Cephalopod fisheries and biology. Tokai University Press, Tokyo. pp. 133-143. [ Links ]
Grist, E. P. M. & S. D. Clers. 1999. Seasonal and genotypic influences on life cycle synchronisation: further insights from annual squid. Ecological Modelling 115 (2-3): 149-163. [ Links ]
Ichii, T., K. Mahapatra, M. Sakai, D. Inagake & Y. Okada. 2004. Differing body size between the autumn and the winter-spring cohorts of neon flying squid (Ommastrephes bartramii) related to the oceanographic regime in the North Pacific: a hypothesis. Fisheries Oceanography 13 (5): 295-309. [ Links ]
Ichii, T., K. Mahapatra, H. Okamura & Y. Okada. 2006. Stock assessment of the autumn cohort of neon flying squid (Ommastrephes bartramii) in the North Pacific based on past large-scale high seas driftnet fishery data. Fisheries Research 78: 286-297. [ Links ]
Ichii, T., K. Mahapatra, M. Sakai & Y. Okada. 2009. Life history of the neon flying squid: effect of the oceanographic regime in the North Pacific Ocean. Marine Ecology Progress Series 378: 1-11. [ Links ]
Ichii, T., K. Mahapatra, M. Sakai, T. Wakabayashi, H. Okamura, H. Igarashi, D. Inagake & Y. Okada. 2011. Changes in abundance of the neon flying squid Ommastrephes bartramii in relation to climate change in the central North Pacific Ocean. Marine Ecology Progress Series 441: 151-164. [ Links ]
Kim, H., S. Kimura, A. Shinoda, T. Kitagawa, Y. Sasai & H. Sasaki. 2007. Effect of El Nino on migration and larval transport of the Japanese eel (Anguilla japonica). ICES Journal of Marine Science 64 (7): 1387-1395. [ Links ]
Kimura, S., K. Doos & A. C. Coward. 1999. Numerical simulation to resolve the issue of downstream migration of the Japanese eel. Marine Ecology Progress Series 186: 303-306. [ Links ]
Kitagawa, T., Y. Kato, M.J. Miller, Y. Sasai, H. Sasaki & S. Kimura. 2010. The restricted spawning area and season of Pacific bluefin tuna facilitate use of nursery areas: A modeling approach to larval and juvenile dispersal processes. Journal of Experimental Marine Biology and Ecology 393 (1-2): 23-31. [ Links ]
Masuda, S., T. Awaji, N. Sugiura, T. Toyoda, Y. Ishikawa & K. Horiuchi. 2006. Interannual variability of temperature inversions in the subarctic North Pacific. Geophysical Research Letters 33 (24): L24610. [ Links ]
Mori, J., M. Okazaki, H. Tanaka & A. Yatsu. 1999. Spawning Ground Surveys of Ommastrephes bartramii in the Subtropical North Pacific Ocean in Autumn, 1997 and 1998. Report of 1998 Annual Meeting on Resources and Fisheries on Squids, Hokkaido National Research Institute of Fisheries, Hokkaido, pp. 85-86. [ Links ]
Murata, M., M. Ishii, Y. Nakamura & C. Shingu. 1985 Distribution and population structure of Ommastrephes bartramii in the North Pacific. Report of the 1984 Annual Meeting on Resources and Fisheries of Squid. Hokkaido National Research Institute of Fisheries, Hokkaido, pp. 76-85. [ Links ]
O'Dor, R. K. 1998. Can understanding squid life-history strategies and recruitment improve management? the South African Journal of Marine Science 20: 193-206. [ Links ]
Okutani, T. 1968. Studies of early life history of decapoden Mollusca III. Bulletin of Tokai Regional Fisheries Research Laboratory 41: 23-29. [ Links ]
Polovina, J. J., E. Howell, D. R. Kobayashi & M. P. Seki. 2001. The transition zone chlorophyll front, a dynamic global feature defining migration and forage habitat for marine resources. Progress in Oceanography 49: 469-483. [ Links ]
Roper, C. F. E., M. J. Sweeney & C. E. Nauen. 1984. FAO Species catalogue. Vol.3: Cephalopods of the world. an annotated and illustrated catalogue of species of interest to fisheries. FAO Fisheries Synopses 125: 1-277. [ Links ]
Saito, H. & T. Kubodera. 1993. Distribution of ommastrephid rhynchoteuthion paralarvae (Mollusca, Cephalopoda) in the Kuroshio region. In-: O'Dor, R. K., T. Okutani & T. Kubodera. (Eds.). Recent Advances in Cephalopod Fisheries and Biology. Tokai University Press, Tokyo. pp. 133-143. [ Links ]
Sakai, M., H. Okamura & T. Ichii. 2004. Mortality of Ommastrephes bartramii paralarvae of autumn cohort in northern waters of Hawaiian Islands, Report of 2003 Annual Meeting on Resources and Fisheries on Squids, Hokkaido: Hokkaido National Research Institute of Fisheries, pp. 35-48. [ Links ]
Semmens, J. M. & N. A. Moltschaniwskyj. 2000. An examination of variable growth in the loliginid squid Sepioteuthis lessoniana: a whole animal and reductionist approach. Marine Ecology Progress Series 193: 135-141. [ Links ]
Yatsu, A., S. Midorikawa, T. Shimada & Y. Uozumi. 1997. Age and growth of the neon flying squid, Ommastrephes bartramii, in the North Pacific Ocean. Fisheries Research 29 (3): 257-270. [ Links ]
Yatsu, A., H. Tanaka & J. Mori. 1998. Population structure of the neon flying squid, Ommastrephes bartramii, in the North Pacific Ocean. In-: Okutani T. (Ed.). International symposium on large pelagic squids. Japan Marine Fishery Resources Research Center, Tokyo, pp. 31-48. [ Links ]
Young, R. E. & J. Hirota. 1990. Description of Ommastrephes bartramii (Cephalopoda: Ommastrephidae) paralarvae with evidence for spawning in Hawaiian waters. Pacific Science 44 (1): 71-80. [ Links ]














