Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Hidrobiológica
versión impresa ISSN 0188-8897
Hidrobiológica vol.23 no.2 Ciudad de México may./ago. 2013
Feeding of the scyphomedusa Stomolophus meleagris in the coastal lagoon Las Guásimas, northwest Mexico
Alimentación de la escifomedusa Stomolophus meleagris en la laguna costera Las Guásimas, noroeste de México
Jesús Guadalupe Padilla-Serrato,1 Juana López-Martínez,1 Alejandro Acevedo-Cervantes,2 Edgar Alcántara-Razo1 and Carlos Hiram Rábago-Quiroz1
1 Centro de Investigaciones Biológicas del Noroeste, S.C. (CIBNOR), Unidad Sonora, Campus Guaymas. Apdo. Postal 349. Guaymas, Sonora. 85454. México.
2 Instituto Tecnológico de Guaymas (ITG), Km 4 carretera al Varadero Nacional S/N, sector Las Playitas, Guaymas, Sonora. 85480. México. e-mail: jlopez04@cibnor.mx.
Recibido: 27 de Julio de 2012.
Aceptado: 14 de enero de 2013.
ABSTRACT
The cannonball jellyfish (S. meleagris) has reached production levels that has led it to become an important fishery resource in Las Guásimas, Sonora, a coastal lagoon in northwestern Mexico; however, its ecological importance and role in the ecosystem remain unstudied. This contribution describes the diet composition of this species in order to reveal its trophic importance in this coastal lagoon. Up to 17 jellyfish were captured in each of three surveys (March 2008, February and April 2009), their stomachs were extracted and analyzed to determine their diet composition. The quantitative methods: frequency of occurrence (F), numeric (N), gravimetric (W) and the index of relative importance, were used to measure the diet components, and Levin´s index to measure the diet amplitude. Thirteen preys were identified; all belonging to the zooplankton community; the most important prey were the anchovy eggs. Our results show that S. meleagris is a specialist predator (with a marked preference for certain prey), classified in the third trophic level (3.2), as a secondary consumer.
Key words: Medusae, trophic levels, trophic preferences, zooplankton.
RESUMEN
La medusa "bola de cañón" (S. meleagris), ha alcanzado altos niveles de producción en la laguna costera de Las Guásimas, Sonora, en el noroeste de México, lo que la ha llevado a ser un recurso pesquero importante; no obstante, se desconoce su importancia y papel ecológico en el ecosistema. Este trabajo describe la alimentación de esta especie, con el fin de conocer su importancia trófica en esta laguna costera. Se capturaron 17 organismos en cada una de tres campañas realizadas (marzo, 2008, febrero y abril, 2009); se les extrajeron los estómagos y se analizaron para determinar la composición de su dieta. Se utilizaron los métodos cuantitativos de frecuencia de ocurrencia (F), numérico (N), gravimétrico (P) y el índice de importancia relativa (IIR) para determinar la composición de la dieta. Para estimar la amplitud de la dieta, se usó el índice de Levin. Se identificaron 13 tipos de presas, todas pertenecientes a la comunidad del zooplancton; los huevos de anchoa fueron el tipo de presa más importante. Los resultados mostraron que S. meleagris es un depredador especialista (con una preferencia marcada por un tipo de presa), con un nivel trófico terciario (3.2), lo que permite clasificarla como un consumidor secundario.
Palabras clave: Medusa, niveles tróficos, preferencias tróficas, zooplancton.
INTRODUCTION
Currently, the state of Sonora in northwestern Mexico is one of the countries' regions where the highest production of jellyfish -as a fishery resource-, is obtained. Particularly, in the coastal lagoon of Las Guásimas, the jellyfish Stomolophus meleagris (Agassiz, 1862) is captured since 2003, with volumes up 17,000 t per year (López-Martínez & Álvarez-Tello, 2008), mostly intended for the international market, mainly Asian (Hsieh et al., 2001; Omori & Nakano, 2001).
There have been several efforts focused on the development of processing technologies, and also studies aimed to evaluate the potential use of this fisheries resource, including aspects such as growth, mortality, recruitment, abundance, and the use of predictive models (López-Martínez & Álvarez-Tello, 2008). Despite these research efforts, no previous data are related to feeding and quantitative analyses of S. meleagris diet in this region. Such basic studies are necessary to determine the trophic role of this species in the ecosystem.
Jellyfish are generally considered carnivorous organisms and have a great importance as planktonic predators. Some include in their diet first and second order carnivores (Ramírez & Zamponi, 1981), other species prefer small crustaceans, fish larvae and eggs (Alvariño, 1985); these preferences appear to be highly related to the prey size (Purcell & Arai, 2001). Jellyfish also play an important role in the ecosystem because they are part the diet of other pelagic organisms, including siphonophores, ctenophores, chaetognaths (Alvariño, 1985), other jellyfish (Larson, 1987), fish (Runge et al., 1987), and turtles (Johnson-Diaz et al., 1993; Márquez, 1996).
Some aspects related to the feeding of S. meleagris were described by Larson (1991) based on a survey of populations from the Florida region. Puente-Tapia (2009) studied this species in Mandinga lagoon and in the Carmen-Pajonal-Machona lagoon system, both in the southern Gulf of Mexico. Both studies emphasized that their results are representative of the conditions prevailing in their regions. This paper aimed to perform a quantitative analysis of S. meleagris diet and to determine the trophic role of this jellyfish in the coastal lagoon of Las Guásimas, Sonora, NW Mexico.
MATERIALS AND METHODS
The study area is located in NW Mexico, in the coastal plain of the state of Sonora (27 °49´-27 °55´N and 110 °29´-110 °40´W) (Fig. 1). The coastal lagoon of Las Guásimas has an area of 51 km2, it has two estuaries (Bachoco and Mapoli) and two sandy barriers at both ends of the system's mouth, one in the north and one in the south. The lagoon has a channel of 3.25 km width, through which it maintains a continuous communication with the Gulf of California (Chávez-López & Álvarez-Arellano, 2006).

The highest abundance of S. meleagris has been observed to occur from February to April in this area (López-Martínez & Álvarez-Tello, 2008); this is why jellyfish were sampled within this time period during three sampling surveys: March 2008 and February and April 2009. Seventeen jellyfish were captured in each survey, using a ring or spoon net (1 cm mesh aperture). Specimens were fixed in 10% formalin -buffered with borax-for subsequent analysis. In the laboratory the total weight (g) and the total length (mm) of each jellyfish were recorded, and dissections were performed to extract their stomachs.
Stomach contents were removed and analyzed with the aid of a stereoscopic microscope. The organisms found in each stomach were identified to the lowest taxon possible, based on specialized keys for zooplankton (Snyder & Fleminger, 1965; Shanks, 2001). After the identification of prey items, the number and wet mass of each prey group was registered.
Cumulative prey curves derived from plotting the cumulative number of stomachs analyzed against the cumulative number of prey taxa (or prey categories), were performed. This allowed assessment of the sufficiency of sample sizes for an accurate characterization of the diet (Ferry & Cailliet, 1996; Wetherbee & Cortés, 2004). The cumulative curves were generated using the method proposed by Jiménez-Valverde and Hortal (2003) and following Clench's equation:
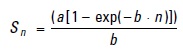
where:
Sn is the number of species observed in each sample,
a is the increase rate of new species and
b is the parameter related to the shape of the curve. These curves reach an asymptote as the sample size becomes sufficient to describe the entire range of the diet. The asymptote of curve -total number of species predicted-is calculated as a/b.
To assess the reliability of analysis, two criteria were taken into consideration: 1) when the proportion of preys is greater than 70% that allows estimation of species richness (asymptote) is stabilized; and 2) values <0.1 on the asymptote of the curve indicate that new preys are less frequent. Even when the species richness was considered incomplete, if it meets the second criterion, it was considered reliable.
The relationship between predator size (TL) and prey abundance was determined through the Pearson's correlation (Ríus-Díaz et al., 1999); the correlation between the variables was estimated through direct permutations method (Zar, 1996).
The quantitative analysis of the food components found in the stomachs was performed through diverse methods proposed for Hyslop (1980):

where:
No is the number of occurrences of category i and
Ns is the total number of stomachs analyzed.

where:
Ni is the number of organisms in category i and
Nt is the total number of organisms found in all categories.
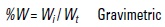
where:
Wi is the weight of category i and
Wt is the total weight of all the categories found.

The IRI incorporates the three first methods (Pinkas et al., 1971), and was used to calculate the relative contribution of each food category to the diet (Cortés, 1999). Dietary specialization was calculated via Levin's measure, using the technique proposed by Labropoulou and Eleftheriou (1997). Levin´s standardized equation is as follows:

where:
Bi is the measure of Levin´s niche breadth,
pij is the proportion of each prey category i in the diet and
n is the total number of prey categories in the diet.
The standardized Levin´s index was used on a scale of 0 to 1 where values <0.6 indicate a narrow niche breadth (specialist predator) and ≥0.6 indicate a broad niche breadth (generalist predator) (Labropoulou & Eleftheriou, 1997).
The trophic level was estimated to establish the position of S. meleagris within the food web following Cortés (1999) as:

where:
TLk is the trophic level for each species
TLj is the trophic level of each prey category j,
Pj is the proportion of each prey category j (using the%IRI) in the diet of S. meleagris, and n is the total number of prey categories.
RESULTS
Fifty one specimens of S. meleagris ranging from 40 to 140 mm of TL (Fig. 2) and representing a wet biomass between 23.3 to 815.6 g were analyzed during the three surveys in Las Guásimas coastal lagoon. These size ranges suggest that the specimens analyzed include young and adult stages. A total of 51 stomachs were examined, of which 44 contained at least one prey item; only 7 stomachs were empty.
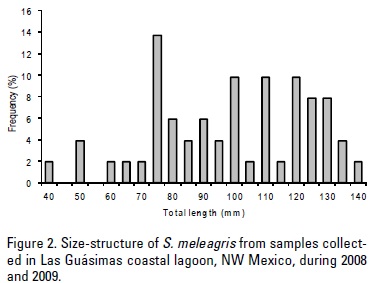
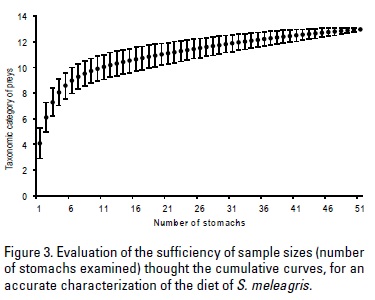
The cumulative curves reached an asymptote (values of the asymptote 0.012) and the preys proportions (>70% and up 99%) indicated that the stomachs analyzed (51), were sufficiently reliable for describing S. meleagris' diet. The trophic spectrum of S. meleagris diet was composed of 13 prey types; the most important dietary component during the surveyed period was the anchovy eggs. Ten prey types were identified in the March 2008 survey, 6 in February 2009 (survey with the lower abundance of prey types) and 10 in April 2009 survey (Fig. 4).
Other prey groups like amphipods, cladocerans, foraminiferans, and eggs of Opisthonema libertate (Gunther, 1867) were of low importance in terms of occurrence. They were occasional prey in S. meleagris' diet.
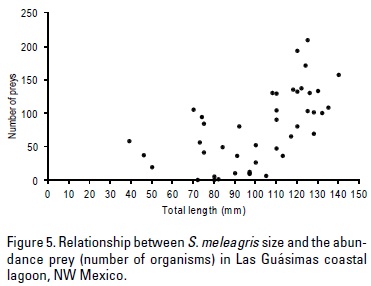
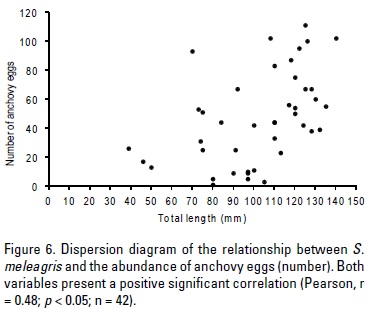
The relationship between predator size and prey abundance showed that the largest sizes of S. meleagris have a higher rate of prey ingestion (Fig. 5). A positive correlation between the predator size (jellyfish) and the abundance of eggs of anchovy (r = 0.48; p < 0.05; n = 42) was observed (Fig. 6).
Results of the quantitative stomach contents analysis of S. meleagris captured during the study period are presented in figure 7. According to the numeric methods used, the quantitative composition of S. meleagris diet was composed of 3,334 preys and the gravimetric method showed 1,465.75 mg of prey weight. Fish, mollusks, and crustaceans were identified as the most important groups by both methods. The frequency of occurrence method diverged as it marked the crustaceans as the most frequent group followed by fish and mollusks. At the species prey level, anchovy eggs were the most important prey according to the three methods (Fig. 7).
Overall, the IRI showed that fish are the principal diet component of S. meleagris, which revealed that they are prey at the early stages, followed by mollusks (larval and young stages), and crustaceans. The most representative preys were anchovy eggs and bivalves mollusks (Fig. 7). Levin´s index showed that S. meleagris is a specialist predator (Bi = 0.12), indicating a diet based in few preys. According to Cortés' (1999) criteria, S. meleagris was classified at the third trophic level (3.2), as a secondary consumer.
DISCUSSION
In general, jellyfish are considered carnivore and important plankton predators, so they are deemed as first and second order carnivores. The prey preference has relation with the size of the jellyfish (Purcell & Arai, 2001; Hansson & Kiørboe, 2006). The feeding spectrum of S. meleagris from Las Guásimas coastal lagoon includes a reduced species number (13), in comparison with previous studies about the feeding of the same species in other geographical areas. In the northwestern sector of the Gulf of Mexico (Florida, U.S.A.), Larson (1991) reported a total of 24 taxa and Puente-Tapia (2009) reported 20 taxa in coastal systems of the southern Gulf of Mexico. The difference between the numbers of prey components can be attributed to the higher salinity conditions inside Las Guásimas (36-41 ups; Arreola-Lizárraga, 2003) in comparison with those in the adjacent oceanic waters. Local evaporation rates are ten times higher than pluvial precipitation over the lagoon (antiestuarine lagoon) (García, 2004; Arreola- Lizárraga, 2003), which causes the variation on abundance and a lower diversity of prey.
Considering the relationship between predator size and prey abundance, it was observed that largest jellyfish individuals consumed larger preys. A similar behavior was observed in the freshwater medusa Craspedacusta sowerbyi (Lankester, 1880) (Spadinger & Maier, 1999). The planktonic medusoid stage of S. meleagris has a span of 3-6 months; during this period its growth rate is higher than that of its benthic stage (López-Martínez et al., 2007; López-Martínez & Álvarez-Tello, 2008). At this time the species starts its reproductive period; as a result, feeding activities increase to store energy in order to improve gonadic development and subsequent growth (Carvalho-Saucedo et al., 2010).
In relation to the preference of S. meleagris for a specific type of prey, Purcell (1992) and Purcell et al. (1994), mentioned that fish eggs are an important component of jellyfish diet. In the present study the anchovy eggs were the principal feeding component for S. meleagris; this behavior could result from the high availability of this prey in the system, something that should be confirmed by the prey availability analysis. A different behavior was observed by Larson (1991), who determined that mollusk larval stages (veligers) are the principal prey. Puente-Tapia (2009) determined copepods as the most important prey group for this species.
The results obtained about the prey size preferred by S. meleagris were similar to those reported by Larson (1991), differing only on their abundances, which suggest that jellyfish captures a similar size range of prey because the feeding structures are size-selective. The channels formed by the scapulets (folds at the bases of the lobes of the manubrium) of S. meleagris can be very narrow and it is where food is handled. Selectivity could also result from structural differences of nematocysts that favor the capture of a particular kind of prey (Purcell & Mills, 1988; Carr & Pitt, 2008; Regula et al., 2009). In comparison with other jellyfish, this species lacks tentacles that would help capturing a wider range of prey (Costello & Colin, 1995). It has been suggested that the mucus production in this species can play an important role in prey capture; nevertheless, this behavior appears to result from stress caused by manipulation (Larson, 1991). Shanks and Graham (1988) found that mucus secretion is a response to predation.
Anchovy eggs have no mobility, thus explaining the major importance of this item on S. meleagris diet (Fancett, 1988; Costello & Colin, 1994). In jellyfish, the different mechanisms determining predation rates and prey selection are not well defined yet (Costello & Colin, 1994; Colin & Costello, 2007). However, some prey species have visual and sensory mechanisms to detect and escape before contact with the jellyfish. In addition, very small prey can escape through the scapulets' filters (Larson, 1991). In general Cnidarians are effective predators of fish eggs and other zooplankton that have a relatively low mobility (Bunn et al., 2000).
Larson (1991) suggested that S. meleagris is a planktivorous polytrophic (but feeds in major quantity from a few species), a behavior that was also found during the present study, where the anchovy eggs represented 83.3% of their diet. Furthermore, Levin´s index showed the same trend, the diet is largely based in a few species, thus indicating that S. meleagris is a specialist predator at Las Guásimas coastal lagoon.
The low relative abundances of other prey could be caused by the lack of knowledge of the circadian rhythm and digestion (Nogueira-Júnior & Haddad, 2008). However, Fancett and Jenkins (1988) mentioned that the efficiency of jellyfish feeding is similar during the day and night. For this reason we suggest that future studies should include specimens captured both at night and during daytime in order to determine if prey selection varies during the cycle. The lower prey abundance observed in February 2009 is attributed to the fact that organisms were not fixed immediately after collection, which allowed a longer digestion time, thus causing a lower prey number; the same was observed by Larson (1976).
Sommer et al. (2002) considered jellyfish as the end of the trophic chain in the pelagic environment because of their low nutritional value; they represent a relatively low value as food for vertebrates. Heeger et al. (1992) did not consider them important in the food chain. According to our results the jellyfish S. meleagris can be situated in the trophic level three (3.2) as a zooplankton consumer and it can be deemed as a secondary consumer too.
Based on the results of this study, we can conclude that S. meleagris is an important zooplankton predator in the surveyed area, feeding preferably on anchovy eggs, thus agreeing with the observations by Purcell et al. (1994) and Kremer (2005). This fact has ecological implications, because the periods of massive jellyfish aggregations (characteristic of this species according to Alvariño, 1985 and Loman-Ramos et al., 2007), can be affected negatively by the abundance of prey species, while increasing natural mortality due to predation of S. meleagris. It has been documented that in other important fisheries regions, high jellyfish populations have contributed to the collapse of fisheries because of the high rates of zooplankton consumption that disrupt the zooplankton community (Kingsford et al., 2000; Hiromi et al., 2005; Coll et al., 2006; Tremblay, 2010).
The fact that S. meleagris is a specialist predator is most relevant because Las Guásimas (like other coastal systems) is an important area for breeding and reproduction of many organisms including commercially valuable species, such as Sardinops sagax (Jenyns, 1842), O. libertate, the blue shrimp Litopenaeus stylirostris (Stimpson, 1874) and the crab Callinectes spp, plus some mollusks. Hence, the high abundance of jellyfish in the area could become a factor affecting the volumes of these important resources both for fishing activities and management and for the regional economy. This is an issue that requires a deeper analysis.
ACKNOWLEDGEMENTS
Funding was provided by the basic science grant CONACYT CB2008-01-000000000106787. Thanks to Eloísa Herrera-Valdivia and Rufino Morales-Azpeitia from the Fisheries Laboratory of CIBNOR (Unidad Guaymas), where samples were analyzed; Karina de la Rosa-Meza, two referees, and Eduardo Suarez for their comments to improve this manuscript.
REFERENCES
Alvariño, A. 1985. Predation in the plankton realm; mainly with reference to fish larvae. Investigaciones Marinas CICIMAR 2: 1-122. [ Links ]
Arreola-Lizárraga, J. A. 2003. Bases de manejo costero: Patrones ecológicos en la laguna costera de Las Guásimas, Territorio Yaqui, México. Tesis de Doctorado, Centro de Investigaciones Biológicas del Noroeste S.C. La Paz B.C.S, México. 61 p. [ Links ]
Bunn, N. A., C. J. Fox & T. Webb. 2000. A literature review of studies on fish egg mortality: Implications for the estimation of spawning stock biomass by the annual egg production method. Scientific Series, Technical Report, CEFAS, Lowestoft (111), 37 p. [ Links ]
Carr, E. F. & K. A. Pitt. 2008. Behavioral responses of zooplankton to the presence of predatory jellyfish. Journal of Experimental Marine Biology and Ecology 354: 101-110. [ Links ]
Carvalho-Saucedo, L., F. García-Domínguez, C. Rodríguez-Jaramillo & J. López-Martínez. 2010. Variación lipídica en los ovocitos de la medusa Stomolophus meleagris (Scyphozoa. Rhizostomeae), durante el desarrollo gonádico, en la laguna Las Guásimas, Sonora, México. Revista de Biología Tropical 58: 119-130. [ Links ]
Chávez-López, S. & A. D. Álvarez-Arellano. 2006. Batimetría, sedimentos y ambientes de depósito en la laguna costera de Guásimas Sonora, México. Investigaciones Geográficas 60: 7-21. [ Links ]
Colin, S. P. & J. H. Costello. 2007. Functional characteristics of nematocysts found on the scyphomedusa Cyanea capillata. Journal of Experimental Marine Biology and Ecology 351: 114-120. [ Links ]
Coll, M., L. J. Shannon, C. L. Moloney, I. Palomera & S. Tudela. 2006. Comparing trophic flows and fishing impacts of a NW Mediterranean ecosystem with coastal upwelling systems by means of standardized models and indicators. Ecological Modelling 198: 53-70. [ Links ]
Cortés, E. 1999. Standardized diet compositions and trophic levels of sharks. ICES Journal of Marine Science 56: 707-717. [ Links ]
Costello, J. H. & S. P. Colin. 1994. Morphology, fluid motion and predation by the scyphomedusae Aurelia aurita. Marine Biology 121: 327-334. [ Links ]
Costello, J. H. & S. P. Colin. 1995. Flow and feeding by swimming scyphomedusae. Marine Biology 124: 399-406. [ Links ]
Fancett, M. S. 1988. Diet and prey selection of scyphomedusae from Port Phillip Bay, Australia. Marine Biology 98: 503-509. [ Links ]
Fancett, M. S. & G. P. Jenkins. 1988. Predatory impact of scyphomedusae on ichthyoplankton and other zooplankton in Port Phillip Bay. Journal of Experimental Marine Biology and Ecology 116: 63-77. [ Links ]
Ferry, L. & G. M. Calliet. 1996. Sample size and data analysis: are we characterizing and comparing diet properly? In: MacKinlay, D. & K. Shearer (Eds.). Feeding ecology and nutrition in fish: Proceeding of the Symposium of the feeding ecology and nutrition in Fish. International Congress on the Biology of fishes. American Fisheries Society, pp. 71-80. [ Links ]
García, E. 2004. Modificaciones al sistema de clasificación climática de Köppen para adaptarlo a las condiciones de la República Mexicana. 5ª. Ed. Instituto de Geografía, Universidad Autónoma de México (UNAM), México, D.F. 90 p. [ Links ]
Hansson, L. J. & T. Kiørboe. 2006. Prey-specific encounter rates and handling efficiencies as causes of prey selectivity in ambush-feeding hydromedusae. Limnology and Oceanography 51: 1849-1858. [ Links ]
Heeger, T., U. Piatkowski & H. Möller. 1992. Predation on jellyfish by the cephalopod Argonauta argo. Marine Ecology Progress Series 88: 293-296. [ Links ]
Hiromi, J., T. Kasuya & H. Ishii. 2005. Impacts of massive occurrence of jellyfish on pelagic ecosystem. Bulletin of the Plankton Society of Japan 52: 82-90. [ Links ]
Hsieh, Y. H., L. Fuiming & J. Rudloe. 2001. Jellyfish as food. Hydrobiologia 451: 11-17. [ Links ]
Hyslop, J. F. 1980. Stomach contents analysis. A review of methods and their application. Journal of Fish Biology 17: 411-429. [ Links ]
Jiménez-Valverde, A. & J. Hortal. 2003. Las curvas de acumulación de especies y la necesidad de evaluar la calidad de los inventarios biológicos. Revista Ibérica de Aracnología 8: 151-161. [ Links ]
Johnson-Díaz, K., J. L. Sierra-Cabral & A. I. Erosa-Solana. 1993. Un tesoro de la Naturaleza: Las Tortugas Marinas. Ed. EDAMEX. México, D.F. 177 p. [ Links ]
Kingsford, M. J., K. A. Pitt & B. M. Gillanders. 2000. Management of jellyfish fisheries, with special reference to the order Rhizostomeae. Oceanography and Marine Biology: An Annual Review 38: 85-156. [ Links ]
Kremer, P. 2005. Ingestion and elemental budget for Linuche unguiculata, a scyphomedusa with zooxanthellae. Journal of the Marine Biological Association of the United Kingdom 85: 613-625. [ Links ]
Labropoulou, M. & A. Eleftheriou. 1997. The foraging ecology of the two pair of congeneric demersal fish species: importance of morphological characteristics in prey selection. Journal of Fish Biology 50: 324-340. [ Links ]
Larson, R. J. 1976. Cubomedusa: feeding-functional morphology, behavior and phylogenetic Position. In: Mackie, G. O. (Ed.). Coelenterate ecology and behavior. New York, U.S.A., pp. 237-245. [ Links ]
Larson, R. J. 1987. Trophic ecology of planktonic gelatinous predators in Saanich Inlet, British Columbia: diets and prey selection. Journal of Plankton Research 9: 811-820. [ Links ]
Larson, r. J. 1991. Diet, prey selection and daily ration of Stomolophus meleagris, a filter feeding Scyphomedusa from the NE Gulf of Mexico. Estuarine, Coastal and Shelf Science 32: 511-525. [ Links ]
Loman-Ramos, L., U. Ordóñez-López & L. Segura-Puertas. 2007. Variación espacial de la comunidad de medusas (Cnidaria) del sur del Golfo de México, durante el otoño de 1999. Hidrobiológica 17: 203-212. [ Links ]
López-Martínez, J., R. Morales-Azpeitia, G. Padilla Arredondo, E. Herrera-Valdivia, C. Rodríguez & E. Alcántara-Razo. 2007. Estimaciones de abundancia de la medusa "Bola de Cañón" (Stomolophus meleagris) al sur de Sonora, para el establecimiento de una pesquería sustentable. Informe Final de Actividades. Guaymas, Sonora, México. 146 p. [ Links ]
López-Martínez, J. & J. Álvarez-Tello. 2008. Medusa bola de cañón: recurso de exportación. Ciencia y Desarrollo 34: 8-15. [ Links ]
Márquez, R. 1996. Las Tortugas marinas y nuestro tiempo. Fondo de la Cultura Económica. México, D.F. 197 p. [ Links ]
Nogueira-Júnior, M. N. & M. A. Haddad. 2008. The diet of Cubomedusae (Cnidaria, Cubozoa) in southern Brazil. Brazilian Journal of Oceanography 56: 157-164. [ Links ]
Omori, M. & E. Nakano. 2001. Jellyfish Fisheries in Southeast Asia. Hydrobiology 451: 19-26. [ Links ]
Pinkas, L., M. S. Oliphant & I. L. Iverson. 1971. Food habits of albacore, bluefin tuna and bonito in California water. Fishery Bulletin 152: 105. [ Links ]
Puente-Tapia, F. A. 2009. Distribución en México de Stomolophus meleagris L. Agassiz, 1862 (Cnidaria: Scyphozoa: Rhizostomeae) y aspectos poblacionales en algunos sistemas estuarino-lagunares. Tesis de Licenciatura. UNAM. México D.F. 95 p. [ Links ]
Purcell, J. E. 1992. Effects of predation by the scyphomedusae Chrysaora quinquecirrha on zooplankton population in Chesapeake Bay, U.S.A. Marine Ecology Progress Series 87: 65-76. [ Links ]
Purcell, E. J. & M. N. Arai. 2001. Interactions of pelagic cnidarians and ctenophores with fish: a review. Hydrobiologia 451: 27-44. [ Links ]
Purcell, J. E. & C. E. Mills. 1988. The correlation of nematocyst types to diets in pelagic Hydrozoa. In: Hessinger, D. A. & H. M. Lenhoff (Eds.). The Biology of Nematocysts. San Diego, California, U.S.A. pp. 463-485. [ Links ]
Purcell, J. E., D. A. Nemazie, S. E. Dorsey, E. D. Houde & J. C. Gamble. 1994. Predation mortality of bay anchovy Anchoa mitchilli eggs and larvae due scyphomedusae and ctenophores in Chesapeake Bay. Marine Ecology Progress Series 129: 63-70. [ Links ]
Ramírez, F. C. & M. O. Zamponi. 1981. Hydromedusae. In: Boltovskoy, D. (Ed.). Atlas del zooplancton del Atlántico Sudoccidental y métodos de trabajo con el zooplancton marino. Publicación especial del Instituto Nacional de Investigación y Desarrollo Pesquero. Argentina (2). pp. 443-469. [ Links ]
Regula, C., S. P. Colin, J. H. Costello & H. Kordula. 2009. Prey selection mechanism of ambush-foraging hydromedusae. Marine Ecology Progress Series 374: 135-144. [ Links ]
Ríus-Díaz, F., F. J. Barón-López, E. Sánchez-FonT & L. Parras-Guijosa. 1999. Bioestadística: Métodos y aplicaciones. Ed. SPICUM, 3ra Edición. Málaga, España. 220 p. [ Links ]
Runge, J. A., P. Pepin & W. Sluert. 1987. Feeding behavior of the Atlantic mackerel Scomber scombrus on hydromedusa Aglantha digitale. Marine Biology 94: 329-333. [ Links ]
Shanks, A. L. 2001. An identification guide to the larval marine invertebrates of the Pacific Northwest. Waldo Hall. Oregon State University. U.S.A. 304 p. [ Links ]
Shanks, A. L. & W. M. Graham. 1988. Chemical defense in a scyphomedusa. Marine Ecology Progress Series 45: 81-86. [ Links ]
Snyder, H. G. & A. Fleminger. 1965. A catalogue of zooplankton samples in the marine invertebrate collections of Scripps Institution of Oceanography. La Jolla, University of California, San Diego, Scripps Institution of Oceanography. U.S.A. 170 p. [ Links ]
Sommer, U., H. Stibor, A. Katechakis, F. Sommer & T. Hansen. 2002. Pelagic food web configurations at different levels of nutrient richness and their implications for the ratio fish production: primary production. Hydrobiologia 484: 209-211. [ Links ]
Spadinger, R. & G. Maier. 1999. Prey selection and diel feeding of freshwater jellyfish, Craspedacusta sowerbyi. Freshwater Biology 41: 567-573. [ Links ]
Tremblay, L. 2010. Effects of global fisheries on the biomass of marine ecosystems: a trophic-level-based approach. Master Thesis. The University Of British Columbia, Vancouver, Canada. 72 p. [ Links ]
Wetherbee, B. & E. Cortés. 2004. Food consumption and feedings habits. In: Carrier, J., Musick, J., & Heithaus, M. (Eds.). Biology of sharks and their relatives. U.S.A. pp. 225-246. [ Links ]
Zar, J. H. 1996. Biostatistical Analysis. Prentice-Hall, New York, U.S.A. 662 p. [ Links ]














