Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Hidrobiológica
versão impressa ISSN 0188-8897
Hidrobiológica vol.23 no.2 Ciudad de México Mai./Ago. 2013
Zooplankton in a whale shark (Rhincodon typus) feeding area of Bahía de los Angeles (Gulf of California)
Zooplancton en una área de alimentación del tiburón ballena (Rhincodon typus) en la Bahía de los Ángeles (Golfo de California)
María Fernanda Hernández-Nava and Saúl Álvarez-Borrego
Departamento de Ecología Marina, División de Oceanología, CICESE, Carretera Ensenada -Tijuana No. 3918, Zona Playitas, Ensenada, Baja California, 22860. México. e-mail: alvarezb@cicese.mx.
Recibido: 16 de agosto de 2012.
Aceptado: 9 de enero de 2013.
ABSTRACT
Bahía de los Ángeles (BLA) is highly influenced by oceanographic processes that occur in Canal de Ballenas, favoring primary and secondary production inside the bay. Zooplankton is an important item in the diet of the whale shark (Rhincodon typus). The whale shark supports BLA's eco-tourism industry. El Rincón (southern BLA) is a preferential feeding area of the whale shark. Zooplankton surface samples were collected at twelve locations in El Rincón in September, November, and December, 2009. Eleven phyla were identified, belonging to both meroplankton and holoplankton. In September the meroplankton fraction was 62.6% of the total abundance, in November it only accounted for 8.6%, and in December it accounted for 14%. This suggests that the breeding season of fish and invertebrates was prior to the September sampling. Copepods, which are the preferred prey for immature whale sharks, always had the largest fraction of the holoplankton. Copepods had relatively low levels of abundance at all sampled locations (<10,000 ind m-3) in September and December. Their largest abundance was in November (up to >50,000 ind m-3), with an average of 73.8% of total zooplankton abundance. Whale sharks were sighted feeding in November, at sites with large abundance of zooplankton, especially Acartia spp. Whale sharks were not sighted when Acartia was absent. Data in the literature and in this work supports the perception that high abundance of Acartia may be the determining factor for the congregation of whale sharks in El Rincón.
Key words: Acartia, Bahía de los Ángeles, copepods, whale shark, zooplankton.
RESUMEN
La bahía de los Ángeles (BLA) está influenciada por procesos oceanográficos en el Canal de Ballenas que favorecen la producción primaria y secundaria en la bahía. El zooplancton es un elemento importante en la dieta del tiburón ballena (Rhincodon typus). El tiburón ballena apoya la industria eco-turística en BLA. El Rincón (parte sur de BLA) es una área preferencial de alimentación del tiburón ballena. Se colectaron muestras superficiales de zooplancton en 12 sitios de El Rincón en septiembre, noviembre y diciembre, 2009. Se identificaron once fila del meroplancton y el holoplancton. En septiembre la fracción del meroplancton fue 62.6% de la abundancia total del zooplancton, en noviembre y diciembre fue 8.6% y 14%, respectivamente. Esto sugiere que ocurrió un desove de peces e invertebrados antes del muestreo de septiembre. Los copépodos, que son las presas preferidas de tiburones ballena inmaduros, siempre presentaron la fracción más grande del holoplancton. Los copépodos tuvieron abundancias relativamente bajas en todas las localidades muestreadas (<10,000 ind m-3) en septiembre y diciembre, y grandes en noviembre (hasta >50,000 ind m-3), con un promedio de 73.8% de la abundancia total de zooplancton en noviembre. Se observaron tiburones ballena alimentándose en noviembre, en sitios con abundancias grandes de zooplancton, especialmente Acartia spp. El tiburón ballena no se observó cuando Acartia estaba ausente. La información en la literatura y en este trabajo apoya la percepción de que el factor determinante para la congregación del tiburón ballena en El Rincón son las abundancias elevadas de Acartia.
Palabras clave: Acartia, Bahía de los Ángeles, copépodos, tiburón ballena, zooplancton.
INTRODUCTION
Bahía de los Ángeles (BLA) is located in the Gulf of California at the eastern coast of the Baja California peninsula, adjacent to Canal de Ballenas (Fig. 1). The high rate of water exchange between Canal de Ballenas and BLA favors the input of dissolved nutrients and planktonic biomass to the bay (Gilmartin & Revelante, 1978). High primary production of the channel is due to intense mixing processes caused by phenomena associated to tides and winds, producing an ecological effect similar to constant upwelling with nutrients input to surface waters throughout the year (Álvarez-Borrego & Lara-Lara, 1991; Delgadillo-Hinojosa et al., 1997). Water exchange between the channel and BLA increases the abundance of plankton in the latter and makes it a suitable habitat for planktivorous organisms like the whale shark (Rhincodon typus Smith, 1828) (Nelson & Eckert, 2007; Rodríguez-Dowdell et al., 2008). Muñoz-Barbosa etal. (1991) reported that integrated primary production in BLA depends on tidal conditions and indicated that in winter it is greatest with neap tides (up to 0.34 g C m-2 h-1). Winds are the factor that most influences the horizontal transport and vertical mixing regulating plankton biomass in the bay (Amador-Buenrostro et al., 1991).

Zooplankton is the link that converts and transfers the energy from phytoplankton to higher trophic levels, although trophic studies for BLA are scarce and there are few studies on zooplankton for this bay (García-García, 2002; Nelson & Eckert, 2007; Lavaniegos etal., 2012). The herbivorous zooplankton plays a vital role, both as predators and prey. Within the wide variety of the zooplankton groups the most abundant are the copepods, which are between 50 and 80% of total zooplankton abundance in both oceanic and coastal waters (Gasca & Suárez, 1996).
García-García (2002) collected zooplankton samples in BLA in November 2001 and observed a predominance of copepods, especially in areas of whale shark sightings. Nelson & Eckert (2007) sampled BLA in summer and beginning of autumn, and found high densities of copepods (>10,000 ind m-3) in areas where whale sharks were feeding actively. Both studies were based on counts of major zooplankton taxa. Lavaniegos et al. (2012) sampled at three locations (off La Gringa and off Punta Arena, and at El Rincón) (Fig. 1) on three occasions in 2003 (end of May-beginning of June, middle of July, and end of October) and four occasions in 2004 (beginning of March, beginning of June, end of July, and middle of October). In the June and October 2004 surveys an additional location off Punta Roja (Fig. 1) was included. Lavaniegos et al. (2012) described seasonal variability of zooplankton taxa in BLA considering their sampling dates as representative of winter, spring, summer, and autumn, respectively. Copepods were dominant during winter and spring (83-99% of the zooplankton abundance), experiencing a dramatic decrease in autumn 2003 (37-66%) and in summer 2004 (25-45%). Acartia clausi (Giesbrecht, 1889) was the main contributor to the copepods' abundance during spring (median = 28,034 ind m-3); the maximal zooplankton abundance (40,468 ind m-3, 99.5% copepods) was found off Punta Arena in October, where two whale sharks were foraging (Lavaniegos et al., 2012).
The whale shark is one of the main resources for BLA's eco-tourism industry. Whale shark is the biggest fish in the planet and it congregates at BLA from June through November to feed on zooplankton blooms (Rodríguez-Dowdell et al., 2008; Cárdenas-Torres et al., 2007). The preferred feeding area for the whale shark is El Rincón at the southern end of BLA, where there has been the greatest number of sightings and where zooplankton densities are often significantly higher than those of other BLA areas (Nelson & Eckert, 2007). Immature whale sharks prevail in coastal locations worldwide (Eckert & Stewart 2001). Borrell et al. (2012) compared isotopic values of whale sharks (∂15N and ∂13C) with those of other components of the food web to corroborate that the species has a primarily zooplanktivorous diet despite the wide spectrum of prey that it is known to consume. As the size of the whale sharks increases, the contribution to the diet of small fish and/or of zooplankton of larger size and higher trophic level increases (Borrell etal., 2012). The objective of this study was to determine the composition and abundance of zooplankton by major taxonomic groups in El Rincón, during autumn months, with the aim of improving the understanding of whale sharks' behavior.
MATERIALS AND METHODS
Study area. BLA is separated from Canal de Ballenas by ten islands. The four largest islands are Coronado, Ventana, Cabeza de Caballo, and Piojo (Fig. 1). It has a NW-SE layout, with 16 km length, and 6.4 km at the widest part. The lunar semidiurnal (M2) tidal currents are relatively slow (~3 cm s-1). In contrast, currents induced by winds show relatively high magnitudes (up to 25 cm s-1 at the intensification areas) (Amador-Buenrostro et al., 1991). During winter the prevailing southward and southeastward winds generate surface southward currents, with a main entry through the northern channel, between La Gringa point and Ventana island, with a SW longshore flux that is extended to El Rincón, and it has one exit out from the bay through the southern channel, between Punta Roja and Cabeza de Caballo Island. In summer, water flow is reversed with the prevailing westward and northwestward winds, with the water coming into the bay through the southern channel. Once in the bay, the flow splits, and part turns to the west, while the other part to the south, surrounding the whole bay. From the western branch there is a lot of water that goes out along the channel located between the islands, and the second branch unites with the first one to go out along the northern channel. Spring and autumn are transitional seasons with variable wind direction (Amador-Buenrostro et al., 1991).
Annual average surface water temperature is 22.7 ± 1.4 oC (mean ± standard error). The coldest months are January and February, with 15 to 17 oC, and July and August are the warmest ones with temperatures between 28.5 and 29.8 oC. Surface salinity is ~35.14 and ~35.6 in winter and in summer, respectively (Barnard & Grady, 1968; Blanco-Betancourt et al., 2004).
Sample collection. Zooplankton surface samples were taken at twelve sites in El Rincón, at the southern end of the bay (Fig. 2), on 26 September, 10 November, and 9 December, 2009. Geographic positioning was done with a GPS (Garmin eTrex model). These sites were chosen because of the frequent sightings of whale sharks in this bay area. Surface temperature and salinity were measured using a hydrographic multiparameter sensor (CTD, Idronaut Sr1). Horizontal surface plankton tows were taken with a 150 urn mesh and 50 cm diameter mouth conical net, tied to a rope. Tows were taken from a 7 m long boat with an out-of-board motor. Ten meters of rope were released out as the boat was moving slowly, and then manually recovered as quickly as possible. As a first approximation, filtered water volume (~2 m3) was calculated based on net mouth area multiplied by towed distance (this is an overestimation because filtering is not 100% efficient). Plankton samples were placed in glass jars, and they were preserved in 4% formaldehyde solution, with sodium borate saturated solution as a buffer.

In order to perform the zooplankton counting, each sample was filtered and rinsed with distilled water to remove formaldehyde. The sample volume was adjusted to 200 ml, it was homogenized and an aliquot was taken with a 12.5 ml Stempel pipette. The aliquots were placed in Petri dishes and analyzed by microscopy. Counts were performed using Yamaji's (1976) artwork on main zooplankton groups. Only copepods were identified at the genus and/or species level. Palomares-García et al's (1998) identification key for copepods in Mexico's Pacific coast was used for this purpose. Abundances of each taxa are expressed in number of individuals per cubic meter (N m-3). These abundances are underestimations because of the overestimation of filtered water by the sampling net, as mentioned above (this affected the accuracy but not the precision of the abundance estimates).
Kruskal-Wallis tests were performed to compare the abundances of zooplankton groups between the sampling months; also the abundances of copepods genera (or species) were compared between the sampling months. When there was a significant difference, Mann-Whitney tests were performed for pairs of months in order to locate exactly where the difference was (De Veaux et. al., 2005).
RESULTS
Average sea surface temperatures (SST) were 28.29 ± 0.05 °C, 23.17 ± 0.04 °C, and 19.63 ± 0.01 °C for the September, November, and December samplings, respectively, with a mean decrease between each month of 5 and 3.5 °C. In general, the space distribution of temperature during each sampling was very homogeneous. Surface salinity ranged from 35.6 in September to 34.8 in November, with intermediate values in December.
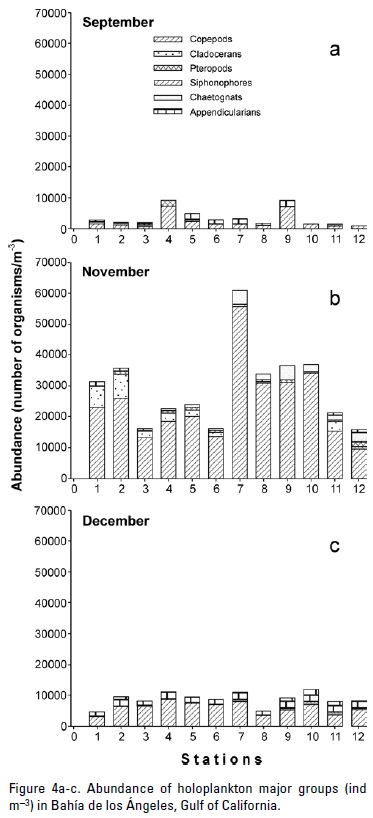
Eleven zooplankton phyla were identified: Cnidaria, Ctenophora, Annelida, Mollusca, Arthropoda, Bryozoaria, Phoronida, Chaetognatha, Nemertea, Echinodermata, and Chordata. They belong to both meroplankton (zooplankters that spend only part of their life as plankton, like fish larvae), and holoplankton (zooplankters spending all their life as plankton, like copepods). In September the meroplankton contributed the largest fraction of the total abundance, with 62.6%. The fractions were reversed in the other two months. In November, meroplankton accounted for 8.6% of the total abundance only, and it accounted for 14% (Fig. 3) in December. Copepods always had the largest fraction of the holoplankton. Copepods had relatively low levels of abundance at all sampled sites (<10,000 ind m-3) in September and December. In November copepod abundance was >10,000 ind m-3 at all sampled sites, with the highest abundance at location seven (>50,000 ind m-3) (Figure 4). Contribution of copepods to the zooplankton abundance was 21.3% in September, increasing significantly to73.8% in November, and 59% in December (Table 1). Similar to copepods, cladocerans had a larger percent abundance in November (6.9%) than in the other two months (0.3% in September and 2.8% in December, Table 1).
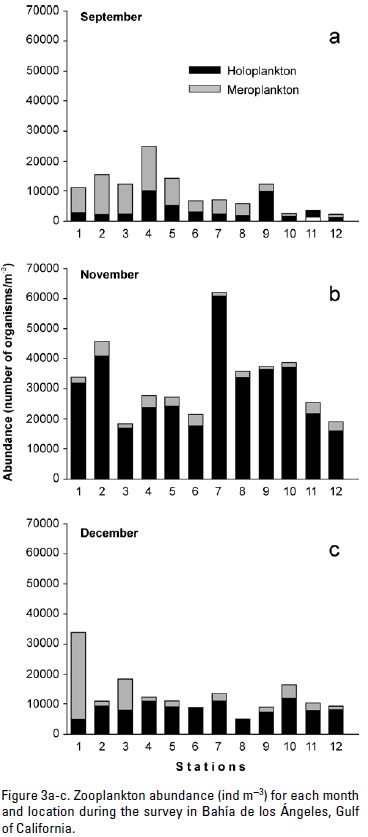
The largest meroplankton absolute abundance occurred in September, and the lowest in December. In September, coastal stations (1-8) had the largest meroplankton abundance, while in November meroplankton abundance was relatively homogeneous throughout El Rincón (Fig. 5). There were no statistically significant differences between the abundance monthly means of pteropods, ostracods, barnacle larvae and crustacean nauplii (Kruskal-Wallis test, Table 1). Nemertineans and echinoderms larvae were the meroplanktonic groups that showed the largest significant differences between months and with maxima in September (15.7 and 9.5%, respectively), while their contributions were relatively low in November and December (<3%). Crustacean nauplii and the unidentified invertebrate larvae had a similar behavior with maxima also in September (8.1% and 16.5%, respectively) (Tables 1 and 2). On the other hand, decapods larvae showed lowest abundance in September (0.1%) and increased significantly throughout December (0.8%) (Table 1). Fish larvae abundance increased significantly from November to December, but it was only 0.1% for the latter month. Bryozoan larvae and fish eggs decreased from September through December, unlike the fish larvae (Tables 1 and 2).

Four orders of copepods were identified, with 21 families and 27 genera. Only 24 species of copepods were identified. In November, 23 copepod genera were recorded. Only 18 genera were recorded in September, and 21 for December. Calanoida showed the highest number of identified genera (16). In this order, Acartia (Dana, 1846) was the most abundant in September (79.4%, 1.6 x 103 ind m-3), and November (50.9%, 12.3 x 103 ind m-3), but it greatly decreased in December (3.6%, 207 ind m-3). Differences between these abundances were statistically significant. Despite having contributed a lower percentage in November than in September, the absolute abundance was highest in November (Tables 3 and 4). Paracalanidae belongs to this order, and its highest abundance was also in November (19.6%, 4.8 x 103 ind m-3), with very few individuals in September (9 ind m-3), and intermediate abundance in December (24.3%, 1.4 x 103 ind m-3). It was the dominant family of Calanoida in December (Fig. 6). The rest of Calanoida genera had a small contribution to the overall abundance of copepods in this month. Poecilostomatoida was represented by five genera (Oncaea Philippi, 1843; Conaea Geisbrecht, 1891; Saphirella Wolfenden, 1906; Farranula Wilson 1936; and Corycaeus Dana, 1846). Oncaea had the highest contribution to overall abundance. Its greatest absolute abundance occurred in November (1.6 x 103 ind m-3) (Fig. 6, Tables 3-4). Cyclopoida was represented by one-genus only. In September, Oithona's (Baird, 1843) abundance was very low, and it increased significantly in subsequent months (Fig. 6, Tables 3 and 4). Harpacticoida was represented by five genera (Clytemnestra Dana, 1848; Microsetella Brady and Robertson, 1873; Macrosetella Scott, 1909; and Euterpina Norman, 1903). These genera contributed little to overall copepod abundance. Euterpina acutifrons (Dana, 1847) was the most abundant Harpacticoida species. Maximum abundance of E. acutifrons occurred in December (306 ind m-3) (Table 4). The copepodites (copepods larvae) had very low abundance in September (102 ind m-3), maximum abundance in November (2.2 x 103 ind m-3), and relatively high abundance in December (1.2 x 103 ind m-3) (Fig. 6, Tables 3 and 4).

DISCUSSION
Throughout 2009 the prevailing winds were from the north-northeast. This was because of La Niña event (http://www.noaa.gov) that caused "winter" conditions during our sampling months. Amador-Buenrostro et al. (1991) predicted that water exchange between the bay and Canal de Ballenas under these conditions causes the input of nutrients to the bay, with the effect of higher primary production. Phytoplankton analysis was not included in our study, but samples were taken and it was possible to visually assess that highest phytoplankton abundance occurred in December, possibly because of lowest zooplankton abundance and therefore low grazing pressure. Water circulation in El Rincón favors nutrient retention and stability of the water column, increasing primary production (Muñoz-Barbosa et al., 1991; Delgadillo-Hinojosa etal., 1997; Millán-Núñez & Yentsch, 2000), and subsequently raising the abundance of phytophagous organisms.
In general, the temporal pattern of zooplankton abundance found for El Rincón conforms to those described for other bays in the Gulf, like Bahía de La Paz and Guaymas (Palomares-García, 1996; González-Navarro & Saldierna-Martínez, 1997; Manrique, 1977), and to those described by Brinton et al. (1986) for some areas of the Gulf of California. September samples presented the greatest abundance of meroplankton within our sampling period. This suggests that the breeding season of fish and invertebrate species was prior to September. The copepods and copepodites abundance increase from September to November was possibly because of a larger availability of food (bacterioplankton and nan-noplankton) in the latter month. In Canal de Ballenas there is a semiannual variation of chlorophyll a concentration, with a maximum centered in October-November (Fig. 2a of Santamaría-Del-Ángel et al., 1994), with a larger abundance of food for grazers during this period. The subsequent copepod abundance decline in December could be related to the lower temperature, as described by Landry (1978) and Uye (1982). Certain copepods, such as Acartia, Paracalanus (Boeck, 1864), and Microsetella prefer predation over herbivory (Turner, 2004). Regardless of their development stage, these copepods feed on copepodites and small larvae. September 2009 mean absolute copepod abundance is similar to Lavaniegos et al. (2012) for October 2003 and 2004, but they are only about one third of the value reported by Nelson and Eckert (2007) for summer and autumn 1999. Possibly, the underestimation was caused by the capture of fewer small copepods by the slightly larger mesh used by Lavaniegos et al. (2012), and also because of the assumption of 100% efficiency in water filtering by the sampling net used (without a flow-meter) by Lavaniegos et al. (2012) and in this study.
Appendicularians large abundance in December was caused by increased phytoplankton abundance. Appendicularians show a high grazing activity (Alldredge, 1981). Appendicularians' mucosal structures are a food source for fish larvae, which abundance also increased in December. Possibly, the remaining zooplankton groups responded more to the temperature decrease than to other physical and biological factors. Grazers had two different temporal patterns: crustaceans (cladocerans and copepods) were most abundant in November; while tunicates (appendicularians and doliolids) were most abundant in December.
In addition to some of the zooplankton taxa reported here for El Rincón, Nelson and Eckert (2007) reported two more, euphausids and stomatopods. García-García (2002) also recorded stomatopods for BLA. Euphausids, which have a more oceanic distribution, were reported for BLA locations near the islands. The list presented here for meroplankton is up to now the most complete record for the bay. Nelson and Eckert (2006), García-García (2002), and Lavaniegos et al. (2012) also reported copepods as the most abundant zooplanktonic group in the bay. The four orders of the subclass Copepoda that were found in this work were reported by Lavaniegos et al. (2012) for five different locations throughout BLA.
Analyses based on stomach contents, fecal samples, behavioral observations, and plankton tows, indicate that whale sharks primarily feed on a variety of planktonic organisms. These include euphausids, copepods, chaetognaths, crab larvae, molluscs, siphonophores, salps, sergestids, isopods, amphipods, stomatopods, coral spawn, and fish eggs. In addition, they also feed on small squid and fish. Aggregations occur in response to plankton blooms or mass spawning events (Motta etal., 2010 and works cited therein). Clark and Nelson (1997) found a high correlation between whale shark sightings and large abundances of the temperate-subtropical species Acartia clausi (Giesbrecht 1889), in Bahía de La Paz. Also, Lavaniegos et al. (2012) reported high abundances of A. clausi in a BLA location where two whale sharks were observed actively feeding on plankton.
Within BLA, the highest abundances of Acartia have been reported for El Rincón, and in November of most sampled years: 1984, 2001, 2002 (Palomares-García, 1996; Hacohen-Domené, 2004), and 2009 (this study). But, Lavaniegos et al. (2012) reported no significant differences in abundance between their sampling sites for any taxon. The contribution of Acartia to total copepod abundance was remarkable in BLA, and even greater than some entire families in September and November, 2009. Two Acartia species were identified in 2009 [Acartia tonsa Dana, 1849 and A. clausi Giesbrecht, 1889) but counting was performed at the genus level because of the difficulty in distinguishing between the two species. There is a co-dominance of both species in the study area. The persistence of winds from the north-northeast in 2009, as mentioned above, favored the development of high abundances of Acartia in September and November. Both, Paracalanidae and Acartia, were described by Brinton et al. (1986) as abundant coastal organisms in the Gulf of California, and in December 2009 Paracalanidae was relatively abundant when Acartia had very low abundance in El Rincón.
Juvenile whale sharks were observed feeding on dense copepod swarms in Bahía de La Paz. Adults occurred in oceanic waters and fed on patches of euphausids (Ketchum et al., 2012). Although whale sharks ingest different zooplankton taxa, high proportion of copepods in BLA indicates a preference for these crustaceans (Lavaniegos et al., 2012). In November 2009, whale sharks were sighted feeding in El Rincón at our sampling locations with the highest Acartia abundance, and this agrees with other reports of sightings at BLA (García-García, 2002; Ávila-Moreno, 2005; Nelson & Eckert, 2007) and Bahía de La Paz (Hacohen-Domené, 2004). Within this study's sampling period, November 2009 was the month with highest Acartia's abundance. Members of this genus are capable of generating large local aggregations, making it an easy and abundant source of food for the whale shark (Uye, 1982). Thus, data in the literature and in this study strongly support the perception that the presence of whale sharks in BLA is mainly because of the presence of this food source. Possibly, the high availability of this copepod at El Rincón is a factor contributing to the congregation of whale sharks in this part of BLA. Zooplankton studies carried on for BLA, and studies of its relation to the presence of feeding whale sharks should be considered as preliminary, therefore a proper monitoring program has to be implemented. As indicated by Lavaniegos et al. (2012), plankton productivity should be addressed in conservation measures for protected areas and ecological reserves.
ACKNOWLEDGMENTS
M. F. Hernández-Nava was granted a scholarship by CONACYT. We thank Abraham Vázquez-Haikin and the Whale Shark Monitoring Group at Bahía de los Ángeles, CONANP, for their help during sampling. Oscar Sosa-Nishisaki and Sharon Herska helped with the project design, and Bertha Lavaniegos-Espejo allowed M. F. Hernández-Nava to use her laboratory and helped her with the identification of organisms. J. M. Domínguez and F. Ponce helped with the graphs.
REFERENCES
Alldredge, A. L. 1981. The impact of appendicularian grazing on natural food concentrations in situ. Limnology and Oceanography 26: 247-257. [ Links ]
Álvarez-Borrego, S. & J. R. Lara-Lara. 1991. The physical environment and primary productivity of the Gulf of California. In: Dauphin, J. P. & B. R. Simoneit (Eds.) The Gulf and Peninsular Province of the Californias. American Association of Petroleum Geologists. Memoir 47, Tulsa. pp. 555-567. [ Links ]
Amador-Buenrostro, A., S. J. Serrano-Güzmán & M. L. Argote-Espinoza. 1991. Numerical model of the circulation induced by the wind at Bahía de los Ángeles, B. C., Mexico. Ciencias Marinas 17: 39-57. [ Links ]
Ávila-Moreno, B. 2005. Una contribución al conocimiento de la biología, comportamiento y hábitat de las congregaciones de tiburón ballena Rhincodon typus (Smith, 1828) de Bahía de los Ángeles, Baja California, México. Tesis de Licenciatura. Facultad de Ciencias Marinas, UABC, Ensenada. 58 p. [ Links ]
Barnard, J. L. & J. R. Grady. 1968. A biological survey of Bahía de los Ángeles, Gulf of California. Mexico I. General account. Transactions of the San Diego Society of Natural History 15: 51-66. [ Links ]
Blanco-Betancourt, R., I. Pacheco-Ruiz, J. M. Guzmán-Calderón, J. A. Zertuche-González, A. Che-Barragán, A. Martínez-Díaz-De-León, A. Gálvez-Téllez & M. López-Vivas. 2004. Base de datos de la temperatura del agua de mar de seis bahías de la costa noroccidental del Golfo de California, México. Reporte técnico. Instituto de Investigaciones Oceanológicas, UABC, Ensenada. 35 p. [ Links ]
Borrell, A., A. Aguilar, M. Gazo, R. P. Kumarran & L. Cardona. 2011. Stable isotope profiles in whale shark (Rhincodon typus) suggest segregation and dissimilarities in the diet depending on sex and size. Environmental Biology of Fishes 92: 559-567. [ Links ]
Brinton, E., A. Fleminger & D. Siegel-Causey. 1986. The temperate and tropical planktonic biotas of the Gulf of California. California Cooperative Fisheries Investigations Reports 27: 228-266. [ Links ]
Cárdenas-Torres, N., R. Enríquez-Andrade & N. Rodriguez-Dowdell. 2007. Community-based management through ecotourism in Bahía de Los Ángeles, Mexico. Fisheries Research 84: 114-118. [ Links ]
Clark, E. & D. R. Nelson. 1997. Young whale sharks, Rhincodon typus, feeding on copepod bloom near La Paz, Mexico. Environmental Biology of Fishes 50: 63-73. [ Links ]
De Veaux, R. D., P. F. Velleman & D. E. Bock. 2005. Stats, Data and Models. Pearson Education, New York. 743 p. [ Links ]
Delgadillo-Hinojosa, F., G. Gaxiola-Castro, J. A. Segovia-Zavala, A. Muñoz-Barbosa & M. V. Orozco-Borbón. 1997. The effect of vertical mixing on primary production in a bay of the Gulf of California. Estuarine, Coastal and Shelf Science 45:135-148. [ Links ]
Eckert, S. A. & B. S. Stewart. 2001. Telemetry and satellite tracking of whale sharks, Rhincodon typus, in the Sea of Cortez, Mexico, and the North Pacific Ocean. Environmental Biology of Fishes 60: 299-308. [ Links ]
García-García, B. 2002. Relación entre la biomasa zooplanctónica y los avistamientos de Tiburón Ballena (Rhincodon typus; Smith 1828) en Bahía de los Ángeles, B. C. México. Tesis de licenciatura. Facultad de Ciencias Marinas, UABC, Ensenada. 50 p. [ Links ]
Gasca, R. & E. Suárez-Morales. 1996. Introducción al estudio del zooplancton marino. ECOSUR-CONACYT, México. 711 p. [ Links ]
Gilmartin, M. & R. Revelante. 1978. The phytoplankton characteristics of the barrier islands lagoons of the Gulf of California. Estuarine and Costal Marine Science 7: 29-47. [ Links ]
Saldierna-Martínez. 1997. Zooplancton de la Bahía de La Paz, B. C. S. (1990-1991). In: Urbán, R. J. & R. M. Ramírez (Eds.). La Bahía de La Paz, Investigación y Conservación. UABC-CICIMAR-SCRIPPS. pp. 315-345. [ Links ]
Hacohen-Domené, A. 2004. Abundancia y riqueza especifica de presas preferenciales del tiburón ballena (Rhincodon typus, Smith 1828), en Bahía de la Paz, B. C. S. Tesis de licenciatura. Universidad Autónoma de Baja California Sur, La Paz. 57 p. [ Links ]
National Oceanic and Atmospheric Administration of the United States of America (NOAA). Available on line at: http://www.noaa.gov. (downloaded february 27, 2012). [ Links ]
Ketchum, J. T., F. Galván-Magaña & A. P. Klimley. 2012. Segregation and foraging ecology of whale sharks, Rhincodon typus, in the southwestern Gulf of California. Environmental Biology of Fishes 96 (6): 779-795. [ Links ]
Landry, M. 1978. Population dynamics and production of a planktonic marine copepod, Acartia clausii, in a small temperate lagoon on San Juan Island, Washington. Internationale Revue der Gesamten Hydrobiologie und Hydrographie 63: 77-120. [ Links ]
Lavaniegos, B. E., G. Heckel & P. Ladrón-De-Guevara. 2012. Seasonal variability of copepods and cladocerans in Bahía de los Ángeles (Gulf of California) and importance of Acartia clausi as food for whale sharks. Ciencias Marinas 38: 11-30. [ Links ]
Manrique, F. A. 1977. Seasonal variation of zooplankton in the Gulf of California. In: Dona, P. (Ed.). Proceedings of the Symposium on Warm Water Zooplankton, UNESCO/NIO Special Publication, Goa, India. pp. 242-249. [ Links ]
Millán-Núñez, E. & C. M. Yentsch. 2000. El Canal de Ballenas, Baja California, como ambiente favorable para el desarrollo del fitoplancton. Hidrobiológica 10: 91-100. [ Links ]
Motta, P. J., M. Maslankab, R. E. Hueterc, R. L. Davis, R, De-La-Parra, S. L. Mulvanya, M. L. Habeggera, J. A. Strothere, K. R. Maraa, J. M. Gardinera, J. P. Tyminskic & L. D. Zeigler. 2010. Feeding anatomy, filter-feeding rate, and diet of whale sharks Rhincodon typus during surface ram filter feeding off the Yucatan Peninsula, Mexico. Zoology 113: 199-212. [ Links ]
Muñoz-Barbosa, a., G. Gaxiola-Castro & J. A. Segovia-Zavala. 1991. Temporal variability of primary productivity, chlorophyll and seston in Bahía de los Ángeles, Gulf of California. Ciencias Marinas 17: 47-68. [ Links ]
Nelson, J. D. & S. A. Eckert. 2007. Foraging ecology of whale sharks (Rhincodon typus) within Bahía de los Ángeles, Baja California, México. Fisheries Research 8: 47-64. [ Links ]
Palomares-García, J. R. 1996. Estructura espacial y variación estacional de los copépodos en la Ensenada de La Paz. Oceánides 11: 29-43. [ Links ]
Palomares-García, J. R., E. Suárez-Morales & S. Hernández-Trüjillo. 1998. Catálogo de los copépodos (Crustacea) pelágicos del Pacifico Mexicano. ECOSUR, México D. F., 352 p. [ Links ]
Rodríguez-Dowdell, N., R. Enríqüez-Andrade & N. Cárdenas-Torres. 2008. Tiburón Ballena. In: Danemann, G. D. & E. Ezcurra (Eds.) Bahía de los Ángeles: recursos naturales y comunidad. PRONATURA Noroeste/ INE/SEMARNAT /SDNHM, México D. F. pp. 363-383. [ Links ]
Santamaría-Del-Ángel, E., S. Álvarez-Borrego & F. E. Muller-Karger. 1994. Gulf of California biogeographic regions based on coastal zone color scanner imagery. Journal of Geophysical Research 99: 7411-7421. [ Links ]
Turner, J. T. 2004. The importance of small planktonic copepods and their roles in pelagic marine food webs. Zoological Studies 43: 255-266. [ Links ]
Uye, S. 1982. Population dynamics and production of Acartia clausi Giesbrecht (Copepoda: Calanoida) in inlet waters. Journal of Experimental Marine Biology and Ecology 57: 55-83. [ Links ]
Yamaji, I. 1976. Illustrations of the marine plankton of Japan. Hoikusha Publ., Osaka, 369 p. [ Links ]














