Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Hidrobiológica
versión impresa ISSN 0188-8897
Hidrobiológica vol.23 no.1 Ciudad de México ene./abr. 2013
Artículos
Calcification of the filamentous cyanobacterium Blennothrix ganeshii in calcareous tropical streams of central Mexico region
Calcificación de la cianobacteria filamentosa Blennothrix ganeshii en ríos calcáreos tropicales de la región central de México
Yenny Beltrán-Magos,1 Javier Carmona,2 Gloria Vilaclara3 and Miriam Bojorge-García2
1 Posgrado en Ciencias Biológicas, Universidad Nacional Autónoma de México, Coyoacán, Ciudad de México, D.F., 04510. México.
2 Facultad de Ciencias, Departamento de Ecología y Recursos Naturales, Universidad Nacional Autónoma de México, Circuito exterior, Ciudad Universitaria, Coyoacán, D.F., 04510. México.
3 FES-Iztacala, Universidad Nacional Autónoma de México, Estado de México, 54000. México e-mail: jcj@fciencias.unam.mx
Recibido: 22 de abril del 2012.
Aceptado: 4 de octubre del 2012.
ABSTRACT
Geochemical, mineralogical and microbiological data from four freshwater streams in central region of Mexico indicate the importance of Blennothrix ganeshii mats (Cyanobacteria, Oscillatoriales) in promoting the formation of calcium carbonate crystals. The streams were characterized by alkaline waters and relative physicochemical stability during three seasons (cold dry, warm dry and warm rainy). Calcification took the form of a thick, dense layer of calcium carbonate crystals surrounding the extracellular polymeric substances produced by B. ganeshii filaments, giving an appearance of micritic tubes (structures formed by crystallization in the spaces between filaments) along the sheath surfaces. The precipitate was analyzed using X-ray diffraction and energy dispersal X-ray spectrometry, and the calcite crystal habit was determined. The photosynthetic activity of cyanobacterial growth and the presence of abundant extracellular polymeric substances and epiphytic species promote the absorption of ions and mineral nucleation on the surface of the sediment and contribute to the formation of travertine in tropical regions.
Key words: Blennothrix ganeshii, calcification, cyanobacteria, micritic tubes, tropical streams.
RESUMEN
Los datos geoquímicos, mineralógicos y microbiológicos en cuatro ríos de agua dulce de la región central de México, ponen de manifiesto la importancia de las matas de Blennothrix ganeshii (Cianobacteria, Oscillatoriales) como promotoras de la formación de cristales de carbonato de calcio. Los ríos se caracterizaron por presentar aguas alcalinas y una relativa estabilidad fisicoquímica durante tres estaciones del año (seca fría, seca templada y lluviosa templada). La calcificación estuvo caracterizada como una densa y gruesa capa de cristales de carbonato de calcio que rodean el mucílago extracelular producido por los filamentos de B. ganeshii, dando la apariencia de tubos micríticos (estructuras formadas por un material cristalizado en las hendiduras existentes entre filamentos) a lo largo de la superficie de la vaina. El precipitado fue identificado como calcita por su hábito cristalino típico y por análisis de difracción de rayos-X y espectrometría de dispersión de energía de rayos X. La actividad fotosintética de los crecimientos de la cianobacteria y la presencia de abundantes sustancias poliméricas extracelulares y especies epífitas promueven la absorción de iones y nucleación de minerales en la superficie del sedimento y contribuyen a la construcción de travertino en corrientes de regiones tropicales.
Palabras clave: Blennothrix ganeshii, calcificación, cianobacteria, ríos tropicales, tubos micríticos.
INTRODUCTION
The deposition of calcium salts, generally called calcification, is a common phenomenon associated with many freshwater bacteria (particularly cyanobacteria), small algae, fungi and bryophytes that contribute to the growth of microbial biofilms and mats (Riding, 2000; Pentecost, 2005; Turner & Jones, 2005). Microbial carbonates are most common in the geologic record in seas and lakes but at present they are also important in spring, cave and soil environments. Minerals originated through biologically induced mineralization (BIM) generally nucleate and grow both extracellularly and intercellularly as a result of various metabolic processes, such as photosynthetic uptake of CO2 and/or HCO3 - by cyanobacteria, and ammonification, denitrification and sulfate reduction by other bacteria (Riding, 2000; Couradeau et al., 2012). Extracellular polymeric substances (EPS), widely produced by microbes, are important in providing nucleation sites and facilitating sediment trapping (Riding, 2000). These exopolymers have diverse compositions, depending on the biology of the individual organisms and environmental conditions, but are commonly dominated by negatively charged polysaccharides (Decho, 1990; Turner & Jones, 2005). Although many cyanobacteria have metabolic processes that stimulate calcium carbonate formation, favorable environmental conditions generally appear to be necessary for precipitation to occur (Merz-Preiß & Riding, 1999). This facultative and environmentally dependent calcification reflects the saturation state of the ambient water as well as the metabolic activities of the cyanobacteria.
Blennothrix ganeshii Kützing ex Anagnostidis et Komárek is often a major component of attached lotic communities in submerged (littoral and benthic) habitats and occurs in calcareous regions of Mexico (Montejano et al., 2000; Beltrán-Magos et al., 2005). This perennial cyanobacterium grows in large mats and is an important biotic component in its microhabitat (Carmona et al., 2005). Studies of B. ganeshii have concerned taxonomic and ecological information (Anagnostidis & Komárek, 1988; Valadez-Cruz et al., 1996; Komárek, 1998; Watanabe & Komárek, 1989; Montejano et al., 2000; Cantoral & Aboal, 2001; Beltrán-Magos et al., 2005; Carmona et al., 2005) but studies of calcification processes are sparse. The purpose of this study is to evaluate the mechanisms as well as environmental and biological factors involved in B. ganeshii calcification in four tropical streams in central Mexico.
MATERIALS AND METHODS
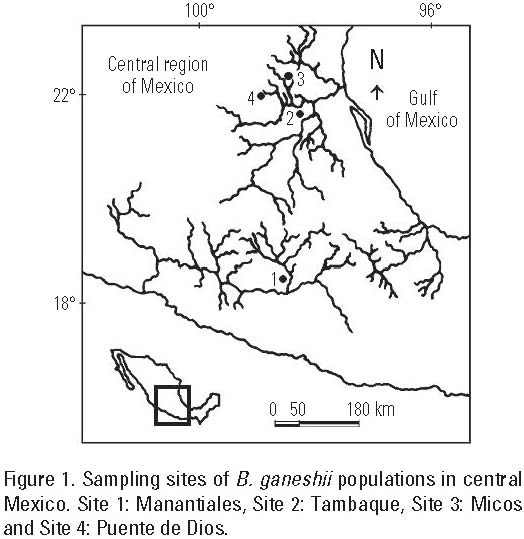
Differences in water chemistry of four freshwater streams with B. ganeshii populations (Manantiales, Tambaque, Micos and Puente de Dios) were evaluated by sampling three times over a period of ten months, from January to November 2004 (Fig. 1). The climatic conditions include an intense summer rainy season (García, 2004). The study period covers three main seasons during the year in Mexico for regions below 800 m.a.s.l. at latitudes of 18-22° N: the cold dry (February), warm dry (May) and warm rainy season (November). Various physical and chemical parameters were recorded in situ at each site. Water temperature, pH and specific conductivity (standardized to 25 °C, K25) were measured with a PC-18 conductivity meter (Conductronic, Puebla, Mexico); dissolved oxygen was measured with an oxygen meter YSI-85 (YSI Incorporated, Ohio, USA); and current velocity and photosynthetically active radiation (PAR) were measured as close as possible to the algal growth using a 2100 current velocity meter (Swoffer Instruments, Washington, USA) and a LI-1000 quantum meter (LI-COR Biosciences, Nebraska, USA) with a flat subaquatic PAR sensor. Depth and type of substrate were also recorded for each site. Oxygen saturation percentage was calculated from dissolved oxygen data, taking into account altitude and water temperature (Wetzel & Likens, 1991).
Water samples for nutrient determination were collected in duplicate. Each sample replicate was filtered in situ with 0.22 µm pore size membranes (Millipore, Massachusetts, USA), preserved with a few drops of chloroform and frozen for subsequent laboratory analysis with a San plus segmented-flow analyzer (Skalar Inc., Georgia, USA), following standard titration. Soluble reactive phosphorous (theoretically, the majority being orthophosphates, P-PO43-), N-NO2 -, N-NO3 - and N-NH4+ were analyzed following the techniques described by AStm (1989) and APHA (1995). Water samples for determination of anions (HCO3 -, CO32-, Cl-, SO42-), total dissolved solids (TDS) and pH were frozen (-20 °C) and preserved in dark conditions, whereas samples for cations (Ca2+, Mg2+, Na+, K+) were preserved with 40% nitric acid (down to pH 2-3). Determination of carbonates was carried out using the titration method, chlorides using the selective electrode method, sulfates using the turbidimetric method and Na+ and K+ using the spectrophotometric atomic absorption method (APHA, 1995).
Saturation of stream water with respect to calcite was calculated according to the saturation index (SI), SI = pH - pHs, where pH is the registered pH and pHs is the calculated pH in equilibrium with CaCO3 at the existing concentrations of Ca2+ and HCO3 - (AStm, 1989). Populations of B. ganeshii were collected for calcification analysis. Maximum differences in carbonate precipitates on cyanobacteria were observed and analyzed at the end of the dry season (May) and at the end of the rainy season (November). Carbonate content of samples was evaluated using the loss-on-ignition method (550 °C for 4 h; Heiri et al., 2001; Boyle, 2004). Calcification morphology and mineral composition was determined using a BX51 light microscope (Olympus Corporation, Tokyo, Japan), a JSM-6380LV scanning electron microscope (SEM; Jeol, Tokio, Japan) and by INCAx-sight energy-dispersive X-ray spectroscopy (EDXS; Oxford instruments, Oxfordshire, UK). Mineralogy was confirmed by X-ray diffraction (XRD) using a powder diffraction meter Broker D8-advance (CuK radiation, graphite monochromator; Bruker AXS, Wisconsin, USA). Samples were oven dried (105 °C for 24 h; Heiri et al., 2001; Boyle, 2004) and ground to a fine powder; analysis was based on the Diffplus Bs software and the International Centre for Diffraction Data (ICDD) database. Significant differences (p < 0.05) in chemical composition between sites and seasons were assessed using one-way analysis of variance (ANOVA), followed by a Scheffé test. Relationships between calcification and physicochemical parameters were evaluated with redundancy analysis (RDA). Statistical analyses were performed with the programs SPSS ver.12 (Levesque, 2006) and XLSTAT ver. 7.5 (XLSTAT, 2004).
RESULTS
Water conditions. Blennothrix ganeshii populations from central Mexico are present under particular chemical and physical conditions: alkaline waters (2.7-4.8 meql-1) dominated by SO42-/HCO3 - and Ca2+/Mg2+, warm temperature (23-29 °C), shallow depth (3-28 cm), moderate current velocity (13-27 cm s-1), high percentage of oxygen saturation (96-107%) and different types of substrata (lime, sand, clay, gravel, boulder or rock). Stream segments were shaded or partly shaded (11-85 µmol photons m-2 s -1; Table 1 and Table 2).
Seasonal variations in temperature, pH, total alkalinities and concentration of Ca2+ are shown in Figs. 2A-D. The highest temperatures were recorded at site 1 throughout the sampling period. The pH of stream water varied over a small range of values (7.0-7.9), generally with a slight increase during the warm dry season, except for site 1. Total alkalinities showed no differences during the seasons at site 1. At sites 2, 3 and 4, lower values were detected during the warm season. Variations in Ca2+ and ionic concentration between sampling dates were noted at sites 2, 3 and 4, with higher concentrations during dry seasons and lower during rainy seasons, showing a seasonal dilution process. Virtually no variation in Ca2+ ions was detected at site 1 during the sampling period.
ANOVA showed significant differences in chemical composition between sites (F = 5.8, p = 0.001-0.02). Scheffé test results indicated the presence of two groups (p < 0.05): one containing sites 1, 2 and 4, and the other containing site 3. No significant differences (p < 0.05) were found in chemical composition among seasons from different sampling years at all sites.
Saturation of stream water with respect to calcite. Seasonal changes in the saturation index of calcite (SI) are presented in Fig. 2E. Stream water was generally supersaturated with respect to calcite (SI > 0). SI values at site 3 were negative in the warm rainy season when sampling took place soon after heavy rains. Average annual supersaturation index values of calcite were between 0.25 and 0.85.
Calcification. All studied populations were characterized by calcium carbonate precipitation around the sheaths and were found in shallow water conditions, even when substantial parts of the mat were outside the water column, facilitating evaporation processes and cementation of minerals. Variability in precipitation was found between populations at different sites. Samples from sites 1 and 4 had the highest mean carbonate precipitation regardless of sampling season (31 and 36%, respectively), whereas populations from sites 2 and 3 showed the lowest precipitation (13 and 16%, respectively). The eigenvalues of the first two RDA axes were high (RDA1, 0.66 and RDA2, 0.33) when compared with subsequent axes, and they accounted for 99% of the variance in carbonate precipitation (Fig. 3). Both axes accounted for 99% of the variance in the relationship between carbonate precipitation at the four sites and environmental variables. The results of the permutation tests revealed the statistical significance (p ≤ 0.05) of the effects of current velocity and N-NO2 - at sites 2 and 3; total alkalinity, K25, SO42-, Ca2+, Mg2+, temperature, P-PO43- and SI at site 1, and pH and SI at site 4. No differences in total alkalinity among the seasons at site 1 were associated with the direction of the distribution pattern of carbonate precipitation.
Calcification occurred as dense, thick encrustation around the sheaths, creating micritic (metabolically induced, but environmentally dependent) tubes typically 10-25 µm thick enclosing B. ganeshii filaments (Figs. 4A, C). The calcareous tubes around the filaments were separate from each other. Distal ends of the filaments remained uncalcified, and allowing trichomes to be visible (Fig. 4E). Calcite crystals displayed a rhombohedral form with triangular surfaces and irregular disposition (Figs. 4C-D), forming the "gothic arch crystals" described by Rainey and Jones (2009). Scanning-electron-microscopic examination of the initial precipitate nucleation indicates the presence of calcium carbonate particles with diameters that range from several tenths of a micrometer to as much as 1 micrometer on the polysaccharide sheath (Fig. 4B). EDXS of these precipitates showed they contained a lower Ca2+ percentage than the fully formed calcite crystals (Fig. 5A-B). Inclusions contained Ca2+ as a major element. Around the sheaths, the calcite coating was enlarged (up to five times the diameter of the trichome) mainly by the addition of calcite crystals, which grouped themselves in cylindrical tubes (Fig. 4F).
X-ray diffraction showed a high number of minerals (carbonates, silicates and oxides) deposited on B. ganeshii sheaths (Table 3). XRD analyses indicated that calcite was the only phase of CaCO3 present in readily detectable amounts at all sites. Sites 1, 3 and 4 had the highest proportion of calcite (average = 95, 81 and 88%, respectively). A temporal variation in precipitate minerals around the sheaths was recorded in all sampling sites. A comparison among seasons showed that major calcification occurred in the dry season, when higher SI values were found, except in site 3, which had a negative SI value.
DISCUSSION
The formation of travertine in tropical streams of the central region of Mexico evidently is stimulated by calcite supersaturation in the water. In particular, B. ganeshii populations occurred under conditions of alkaline water and the presence of abundant EPS and ephyphitic species that promote the absorption of ions and mineral nucleation.
High SO42- concentrations occurred in the water at all sampling sites, resulting from solution of underlying gypsum deposits (Consejo de Recursos Minerales 1992, 1993, 2000). According to Carmona et al. (2005) sulfates are an essential component of B. ganeshii sheaths as well as of several other cyanobacteria. Our analysis shows that Ca2+, HCO3 - and Mg2+ are the prevailing ions in the studied streams, as would be expected for water in contact with limestone and dolomite (Consejo de Recursos Minerales 1992, 1993, 2000; Ferrusquía-Villafranca, 1998). Na+, K+ and Cl- are present in low concentrations at the studied localities. Similar water composition has been described in thermal carbonate spring deposits in Canada and Mexican maar-crater lakes (Vilaclara et al., 1993; Armienta et al., 2008; Rainey & Jones, 2009). The most abundant nutrient in water from all sampling sites is nitrogen as nitrate. Phosphate concentrations at all sites are low due to the presence of high concentrations of Ca2+. This condition has been observed in similar karstic systems elsewhere (Reddy, 1988) due to the co-precipitation of phosphates with calcium carbonate (Kleiner, 1990).
Mineral formation in B. ganeshii could be explained by the reduction of CO2 in the water due to photosynthetic activity. As the water in which these cyanobacteria live is supersaturated with respect to calcium carbonate, CO2 reduction induces mineralization and increases in pH values, alkalinity and CO32- ion concentration, thus principally promoting calcite precipitation. EPS excreted by cyanobacterial cells favor calcium carbonate encrustation by providing an ideal surface for the adsorption of ions and mineral nucleation (Emeis et al., 1987; Braissant et al., 2003; Dittrich & Sibler, 2010). EPS possess the ability to concentrate Ca2+ cations from solution due to the net-negative surface charge that occurs on several cyanobacteria, including Pleurocapsa sp., Plectonema sp. and Scytonema sp. (Golubić, 1973; Merz-Preiß & Riding, 1999; Riding, 2000; Frankel & Bazylinski, 2003; Pentecost, 2005); possibly the same phenomenon occurs in B. ganeshii. Pentecost (1978) and Konhauser (2007) proposed that cyanobacterial species that produce sheaths or EPS generally precipitate more calcium carbonate than those species without such structures.
Merz-Preiß and Riding (1999) report that calcium carbonate precipitation in freshwater streams becomes conspicuous where average annual supersaturation index values exceed 0.75. However, all B. ganeshii populations showed extracellular mineral formation, although relatively lower values were measured in several seasons, particularly in the warm rainy season. Nucleation can be disadvantaged by the mechanical removal of minerals related to high flow velocity.
The evaporation processes occurring on exposed surfaces of benthic mats could explain the plentiful calcification. According to Schneider and Le Campion-Alsumard (1999), boundary layers are the most important environments for cyanobacterial activity during the formation of carbonates.
The Blennothrix ganeshii calcification process involves two main phases: first, the formation of a solid nucleus from dissolved ions and second, the addition of ions to the nucleus to form crystals of calcium carbonate. During the first phase, ions are positioned over the sheath or epiphytic species and resist rapid dissolution with a posterior crystalline phase. According to Merz-Preiß and Riding (1999), Schneider and Le Campion-Alsumard (1999), Pedley (2009), Pedley et al. (2009) and Rainey and Jones (2009) microbes do not contribute to the establishment of elevated supersaturation states but passively serve as substrates upon which calcite precipitates. Filaments of B. ganeshii were used as substrate by several cyanobacteria, diatoms and epiphytic species of red algae present throughout the entire study, including Chamaesiphon confervicola A. Braun, Chamaecalyx swirenkoi (Sirsov) Komárek et Anagnostidis, Stichosiphon sansibaricus (Hieronymus) Drouet et Daily, Xenococcus bicudoi Montejano, Gold et Komárek, X. willei Gardner, Cocconeis placentula Ehrenberg var. placentula, Gomphonema gracile Ehrenberg, Surirella linearis W. Smith, Synedra ulna (Nitzsch) Ehrenberg var. ulna, Terpsinoë musica Ehrenberg, Audouinella meiospora (Skuja) Garbary and Compsopogon coeruleus (C. Agardh) Montagne. Several of them have been reported in similar tropical and alkaline environments (Golubic, 1973; Prins & Elzenga, 1989; Freytet & Verrecchia, 1998; Merz-Preiß & Riding, 1999; Beltrán-Magos et al., 2005). According to Emeis et al. (1987), epiphytic diatoms excrete mucilage enriched in aspartic acid as a response to high Ca2+ concentrations. The mucilage traps micrite particles that are suspended in the water, which then act as crystal seeds for inorganic calcite precipitation. The second phase includes the precipitation mainly of calcium carbonate ions and the formation of micritic tubes of calcite minerals that enclose sheaths. Pentecost (1978), Tavera and Komárek (1996), Freytet and Verrecchia (1998), Schneider and Le Campion-Alsumard (1999) and Pentecost (2005) reported this type of calcification is the most common on travertine surfaces, and similar trends have been observed in several filamentous cyanobacteria: Tapinothrix janthina (Bornet et Flahault) Bohunická et Johansen (H. janthina Bornet et Flahault), Lyngbya aerugineo-caerulea Gomont, Microcoleous vaginatus Gomont ex Gomont, Phormidium incrustatum (Nägeli) Gomont ex Gomont, Plectonema gloeophilus Borzi, P. gracillimum Zopf ex Hansgirg, P. phormidioides Hansgirg ex Forti, Rivularia haematites (De Candolle) Agardh ex Bornet et Flahault, R. varians Obenlüneschloss, Schizothrix calcicola Gomont and Scytonema myochrous (Dillwyn) C.A. Agardh ex Bornet et Flahault. The extracellular biomineralization reported in these species and the intracellular calcification in a cyanobacterium belonging to Gloeobacterales confirm the importance of cellular control and physicochemical parameters on the formation and morphology of different types of travertine (Tavera & Komárek, 1996; Couradeau et al., 2012).
Cyanobacterial mats of B. ganeshii in the central region of Mexico create ideal conditions for biologically induced mineralization of calcite and have presumably played a significant role in the development of these natural calcium carbonate environments. This facultative but environmentally dependent calcification reflects the saturation state of the water, as well as the oxic sediment role of B. ganeshii sheaths. The present investigation also highlights the significance of the study of physical and chemical seasonal variations in freshwater alkaline streams and the calcification processes of cyanobacteria so as to understand their functioning and prevalence in tropical streams.
ACKNOWLEDGEMENTS
The authors are indebted to H. Sergio Castillo Sandoval (ICMyL-UNAM) for nutrient analysis; to Q. F. B. Nora Elia Ceniceros B., I. Q. Alejandra Aguayo Ríos and Q. F. B. Olivia Cruz R. (IGeof-UNAM) for carrying out mayor ion analysis; to M. C. Leticia Baños López (IIMUNAM) for X-ray diffraction analysis and to Dr. Rafael Quintanar (FES Iztacala-UNAM) for SEM and EDXS. Special thanks are given to Posgrado en Ciencias Biológicas, Universidad Nacional Autónoma de México. This paper constitutes a partial fulfillment of the Graduate Program in Biological Sciences of the National Autonomous University of México (UNAM). Y. P. Beltrán-Magos acknowledges the scholarship and financial support provided by the National Council of Science and Technology (CONACyT) (175787), and UNAM.
J. Carmona received financial support from research grant AECI (A/010529/07 and A/016417/08).
REFERENCES
Anagnostidis, K. & J. Komárek. 1988. Modern approach to the classification system of cyanophytes. 1. Introduction. Archiv für Hydrobiologie Supplement 71 (Algological Studies 38-39): 291-302. [ Links ]
APHA (American Public Health Association). 1995. Standard Methods for the Examination of Water and Wastewater. 19th ed., USA, New York. 1100 p. [ Links ]
Armienta, M. A., G. Vilaclara, S. De la Cruz-Reina, S. Ramos, N. Ceniceros, O. Cruz, A. Aguayo & F. Arcega-Cabrera. 2008. Water chemistry of lakes related to active and inactive Mexican volcanoes. Journal of Volcanology and Geothermal Research 178: 249-258. [ Links ]
ASTM (American Society for Testing and Materials). 1989. Annual book at ASTM standards. U.S.A. Philadelphia. 4100 p. [ Links ]
Beltrán-Magos, Y., J. Carmona & G. Vilaclara. 2005. Microhabitat and morphological variation in freshwater Blennothrix ganeshii (Oscillatoriaceae, Cyanophyceae) populations in streams of central Mexico. Archiv für Hydrobiologie Supplement 159 (Algological Studies 117): 133-146. [ Links ]
Boyle, J. 2004. A comparison of two methods for estimating the organic matter content of sediments. Journal of Paleolimnology 31: 125-127. [ Links ]
Braissant, O., G. Cailleau, C. Dupraz & E. P. Verrecchia. 2003. Bacterially Induced Mineralization of Calcium Carbonate in Terrestrial Environments: The Role of Exopolysaccharides and Amino Acids. Journal of Sedimentary Research 73: 485-490. [ Links ]
Cantoral, U. E. & S. M. Aboal. 2001. El marjal Pego-Oliva: Evolución temporal de la flora de microalgas. Limnetica 20 (1): 159-171. [ Links ]
Carmona, J., Y. Beltrán-Magos & L. Collado-Vides. 2005. Taxonomy and distribution of freshwater Blennothrix ganeshii Watanabe & Komárek (Oscillatoriaceae, Cyanophyceae) from central Mexico. Nova Hedwigia 80: 323-333. [ Links ]
Consejo de Recursos Minerales. 1992. Monografía geológico-mineras del estado de Hidalgo. Secretaría de energía, minas e industria paraestatal, Subsecretaría de minas e industria básica, México, D.F. 95 p. [ Links ]
Consejo de Recursos Minerales. 1993. Monografía geológico-mineras del estado de San Luis Potosí. Secretaría de energía, minas e industria paraestatal, Subsecretaría de minas e industria básica, México, D.F. 217 p. [ Links ]
Consejo de Recursos Minerales. 2000. Monografía geológico-mineras del estado de Morelos. Secretaría de Comercio y Fomento Industrial. Coordinación General de Minería, México, D.F. 209 p. [ Links ]
Couradeau, E., K. Benzerara, E. Gérard, D. Moreira, S. Bernard, G. E. Brown Jr. & P. López-García. 2012. An early-branching microbialite cyanobacterium forms intracelular carbonates. Science 336: 459-462. [ Links ]
Decho, A. W. 1990. Microbial exopolymer secretions in ocean environments: their role(s) in food webs and marine processes. Oceanography and Marine Biology, Annual Review 29: 73-153. [ Links ]
Dittrich, M. & S. Sibler. 2010. Calcium carbonate precipitation by cyanobacterial polysaccharides. Geological Society 336: 51-63. [ Links ]
Emeis, K.C., H. H. Richnow & S. Kempe. 1987. Travertine formation of Plitvice National Park, Yugoslavia: chemical versus biological control. Sedimentology 34: 595-609. [ Links ]
Ferrusquía-Villafranca, I. 1998. Geología de México: Una sinopsis. In: Ramamoorthy, T. P., R. Bye, A. Lot & J. Fa (Eds.). Diversidad biológica de México: orígenes y distribución. Instituto de Biología, Universidad Nacional Autónoma de México, México, D.F., pp. 3-108. [ Links ]
Frankel, R. B. & D. A. Bazylinski. 2003. Biologically Induced Mineralization by Bacteria. In: Dove, P. M., S. Weiner & J. J. De Yoreo (Eds.). Biomineralization. Mineralogical Society of America, Washington, D.C., pp. 95-114. [ Links ]
Freytet, P. & E. P. Verrecchia. 1998. Freshwater organisms that build stromatolites: a synopsis of biocrystallization by prokaryotic and eukaryotic algae. Sedimentology 45: 535-563. [ Links ]
García, E. 2004. Modificaciones al sistema de clasificación climática de Köppen. Instituto de Geografía, Universidad Nacional Autónoma de México, México, D.F. 90 p. [ Links ]
Golubic, S. 1973. The relationship between blue-green algae and carbonate deposits. In: Carr, N. G. & B. A. Whitton (Eds.). The Biology of Blue-Green Algae Botanical Monographs, Vol. 9. University of California Press, Great Britain, pp. 434-472. [ Links ]
Heiri, O., A. F. Lotter & G. Lemcke. 2001. Loss on ignition as a method for estimating organic and carbonate content in sediments: reproductibility and comparability of results. Journal of Paleolimnology 25: 101-110. [ Links ]
INEGI (Instituto Nacional de Estadística, Geografía e Informática). 1985. Síntesis Geográfica del estado de San Luis Potosí. Instituto Nacional de Estadística, Geografía e Informática, México. D.F. 186 p. [ Links ]
INEGI (Instituto Nacional de Estadística, Geografía e Informática). 1992. Síntesis Geográfica del estado de Hidalgo. Instituto Nacional de Estadística, Geografía e Informática, México. D.F. 134 p. [ Links ]
Kleiner, J. 1990. Calcite precipitation-regulating mechanisms in hardwaters lakes. Verhandlungen Internationale Vereinigung für Theoretische und Angewandte Limnologie 24: 136-139. [ Links ]
Komárek, J. 1998. Validity of the genus Blennothrix Kütz. 1843, and its position in the oscillatoriacean cyanoprokaryotes. Anais IV Congreso Latino-Americano, II Reunião Ibero-Americana y VII Reunião Brasileira. Conservaçao da biodiversidade e Novas Tecnologías. Promesas e Perigos 1: 341-352. [ Links ]
Konhauser, K. 2007. Introduction to Geo Microbiology. Willey-Blackwell Publishing. U.S.A., New Jersey. 425 p. [ Links ]
Levesque, R. 2006. SPSS Programming and Data Management, 3rd Edition. Chicago, IL. 388 p. [ Links ]
Merz-Preiß, M. & R. Riding. 1999. Cyanobacteria tufa calcification in two freshwater streams: ambient environment, chemical thresholds and biological processes. Sedimentary Geology 126: 103-124. [ Links ]
Montejano, G., J. Carmona & E. Cantoral. 2000. Algal communities from calcareous springs and streams in La Huasteca, central Mexico: A synthesis. In: Munawar, M., S. G. Lawrence, I. F. Munawar & D. F.Malley (Eds.). Aquatic Ecosystems of Mexico: Status and Scope. Ecovision World Monograph Series. Backhuys Publishers, The Netherlands, Leiden, pp. 135-149. [ Links ]
Pedley, M. 2009. Tufas and travertines of the Mediterranean region: a testing ground for freshwater carbonate concepts and developments. Sedimentology 56: 221-246. [ Links ]
Pedley, M., M. Rogerson & R. Middleton. 2009. Freshwater calcite precipitates from in vitro mesocosm flume experiments: a case for bioremediation of tufas. Sedimentology 56: 511-527. [ Links ]
Pentecost, A. 1978. Blue-green algae and freshwater carbonate deposits. Proceedings of the Royal Society of London 200: 43-61. [ Links ]
Pentecost, A. 2005. Travertine. Springer. U.S.A. New York. 445 p. [ Links ]
Prins, H. B. A. & J. T. M. Elzenga. 1989. Bicarbonate utilization: Function and mechanism. Aquatic Botany 34: 59-83. [ Links ]
Rainey, D. K. & B. Jones. 2009. Abiotic versus biotic controls on the development of the Fairmont Hot Springs carbonate deposit, British Columbia, Canada. Sedimentology 56: 1832-1857. [ Links ]
Reddy, M. M. 1988. Physical-chemical mechanisms that affect regulation of crystallization. In: Sikes, C. S. & A. P. Wheeler (Eds.). Chemical aspects of regulation of mineralization. University of South Alabama Publication Services, Alabama, pp. 3-8. [ Links ]
Riding, R. 2000. Microbial carbonates: the geological record of calcified bacterial-algal mats and biofilms. Sedimentology 47: 179-214. [ Links ]
Schneider, J. & T. Le Campion-Alsumard. 1999. Construction and destruction of carbonates by marine and freshwater cyanobacteria. European Journal of Phycology 34: 417-426. [ Links ]
SPP (Secretaría de Programación y Presupuesto). 1981. Síntesis Geográfica del Estado de Morelos. Secretaría de Programación y Presupuesto. México. D. F. 110 p. [ Links ]
Tavera, R. & J. Komárek. 1996. Cyanoprokaryotes in the volcanic lake of Alchichica, Puebla State, Mexico. Archiv für Hydrobiologie Supplement 117 (Algological Studies 83): 511-538. [ Links ]
Turner, E. C. & B. Jones. 2005. Microscopic calcite dendrites in cold-water tufa: implications for nucleation of micrite and cement. Sedimentology 52: 1043-1066. [ Links ]
Valadez-Cruz, F., J. Carmona & E. Cantoral-Uriza. 1996. Algas de ambientes lóticos en el estado de Morelos, México. Anales del Instituto de Biología, Universidad Nacional Autónoma de México, Serie Botánica 67 (2): 227-282. [ Links ]
Vilaclara, G., M. Chávez, A. Lugo, H. González & M. Gaytan. 1993. Comparative description of crater-lakes. Basic Chemistry in Puebla State, Mexico. Verhandlungen Internationale Vereinigung für Theoretische und Angewandte Limnologie 25: 435-440. [ Links ]
Watanabe, M. & J. Komárek. 1989. New Blennothrix-species (Cyanophyceae/ Cyanobacteria) from Nepal. Bulletin of the National Science Museum 15 (3): 67-79. [ Links ]
Wetzel, R. G. & G. E. Likens. 1991. Limnological Analyses. Springer-Verlag, U.S.A., New York. 391 p. [ Links ]
XLSTAT. 2004. XLSTAT-Pro. User's Manual. Addinsoft, Paris, France. 230 p. [ Links ]














