Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Hidrobiológica
versión impresa ISSN 0188-8897
Hidrobiológica vol.22 no.3 Ciudad de México sep./dic. 2012
Artículos
Effects of land use on water quality and Ceriodaphnia dubia reproduction
Efectos del uso del suelo sobre la calidad del agua y la reproducción de Ceriodaphnia dubia
Patricia L. García-García,1 Fernando Martínez-Jerónimo,2 Gabriela Vázquez,1 Mario E. Favila3 and Rodolfo Novelo-Gutiérrez4
1 Red de Ecología Funcional, Instituto de Ecología, A. C. Carretera Antigua a Coatepec 351, Congregación El Haya, Xalapa, Veracruz. 91070. México
2 Laboratorio de Hidrobiología Experimental, Escuela Nacional de Ciencias Biológicas, IPN, Carpio esq. Plan de Ayala s/n, Col. Sto. Tomás, D. F. 11340. México
3 Red de Ecoetología, Instituto de Ecología, A. C. Carretera Antigua a Coatepec 351, Congregación El Haya, Xalapa, Veracruz. 91070. México
4 Red de Biodiversidad y Sistemática, Instituto de Ecología, A. C. Carretera Antigua a Coatepec 351, Congregación El Haya, Xalapa, Veracruz. 91070. México. E-mail: patricia.lucero.garcia2@gmail.com
Recibido: 7 de diciembre de 2011.
Aceptado: 27 de julio de 2012.
ABSTRACT
This study evaluated the effect of water quality of streams from micro-watersheds with different land use (cloud forest, coffee plantation, pasture and under urban influence) during the dry and rainy seasons, lying within the upper watershed of the La Antigua river in Veracruz, Mexico. Water characteristics were measured and laboratory subchronic toxicity tests were performed to evaluate average accumulated progeny, broods per female, and non-reproductive females of Ceriodaphnia dubia. The cloud forest contained chemically undisturbed streams, while the lowest levels of chemical alteration were detected in pasture streams: low fecundity of C. dubia was observed in both types of streams. The most disturbed streams were those associated with coffee plantations and under urban influence, which resulted in enhanced C. dubia fecundity; however, the highest chemical disturbance, found in a stream with urban influence, led to reproduction suppression in the dry season. The most favorable conditions for reproduction were provided by nutrient and probably organic enrichment in streams associated with urban environments and coffee plantations, while in cloud forest and pasture streams, the natural, and close to natural water chemistry caused a reduction in fecundity. Female fecundity was higher during the rainy season.
Key words: Fecundity, Mexico, rural and urban streams, toxicity test, water quality.
RESUMEN
Durante las temporadas de estiaje y de lluvias, se evaluó el efecto de la calidad del agua sobre la reproducción de Ceriodaphnia dubia, en arroyos de microcuencas en la cuenca alta del río La Antigua, Veracruz, México, con distintos usos de suelo (bosques, pastizales, cafetales y con influencia urbana). Se determinaron diferentes características de la calidad del agua y se efectuaron pruebas de toxicidad subcrónica en condiciones de laboratorio para evaluar los promedios de progenie total y de camadas por hembra, así como el número de hembras no reproductivas. Los arroyos de bosque no resultaron perturbados y los menos alterados fueron los arroyos de pastizales. En ambos tipos de arroyos se encontró una baja fecundidad de C. dubia. En los arroyos asociados a cafetales y con influencia urbana la calidad del agua fue menor, pero se registró un aumento de la fecundidad. Sin embargo, para el río con influencia urbana y la menor calidad del agua, la reproducción del cladócero se inhibió en la época de estiaje. El enriquecimiento de nutrientes y probablemente de materia orgánica en los arroyos de cafetales y con influencia urbana, favoreció la fecundidad del cladócero, mientras que la química natural de los arroyos de bosques y de pastizales explica la disminución de la reproducción de este organismo de prueba. La fecundidad de C. dubia fue mayor durante la época de lluvias.
Palabras clave: Arroyos urbanos y rurales, calidad del agua, fecundidad, México, pruebas de toxicidad.
INTRODUCTION
Land use changes can be the major factor in determining watershed water chemistry, affecting the habitat, water chemistry and aquatic biota (Allan & Castillo, 2007; Bücker et al., 2010). Land use effects depend on the hydrological regime of the watercourses, which can vary seasonally, especially during dry and rainy seasons.
During the rainy season, if the riparian vegetation has been reduced and simplified, it is more likely for storm runoff to carry waste, agrochemicals, animal feces, and other chemical pollutants into the water channel, along with the increased stream discharge (Chapman, 1992), whereas during dry season pollutants may concentrate in a smaller discharge, resulting in concentrations that may exceed levels that pose a toxicity risk to aquatic organisms (Duke et al., 1999).
The tropical montane cloud forest of Veracruz has been replaced, over recent decades, by coffee and sugar cane plantations, and cattle pasture (Muñoz-Villers & López-Blanco, 2007; Williams-Linera, 2007). Herbicides, fertilizers, and insecticides are widely used by coffee producers (Nestel, 1995; Guadarrama-Zugasti, 2000). When it rains, fertilizers leach or wash from crop soils into nearby waterways, releasing nutrients, while insecticides release toxic compounds.
The coffee plantation soils of the area are rich in phosphorus and potassium, as a consequence of fertilizer application and habitat transformation (Geissert & Ibañez, 2008). In the rainy season, soil can also be eroded leading to sedimentation of the watercourse, as reflected by the suspended solids in coffee plantation streams in the area (Martínez et al., 2009; Vázquez et al., 2011). Soil erosion causes leaching of suspended solids that contain organic matter (Bellanger et al., 2004).
In pastures dedicated to livestock grazing, nitrogen and phosphorus increase in streams where there is no control of the disposal of animal manure to the stream channel, as is the case with the pastures in this study.
Urbanization is also increasing in the region, although it occupies less than 7% of the La Antigua river upper watershed area (Muñoz-Villers & López-Blanco, 2007). The urban areas of this study are not influenced by industry, but also do not feature a complete municipal sewage collection network necessary to prevent untreated household wastewaters being discharged directly into the watercourses. This is the cause of increased loads of organic matter (wastes), nitrates (garden fertilizers), phosphates (fertilizers and detergents), suspended solids (wastes and runoff), chloride (bleach, urine, feces), ammonium and sulfate (urine and feces) (Tchobanoglous et al., 1991; Chapman, 1992; Burks & Minnis, 1994; Kumar, 2002; Jonsson et al., 2005).
Nutrient and organic compound enrichment caused by manure, fertilizers and sewage input into watercourses, can cause negative responses in crustacean cladoceran communities (in terms of species number, density, biomass, body length) (Yufeng et al., 1998) while sometimes enhancing their reproduction (Nanazato & Yasuno, 1985; Kuhl et al., 2010).
Laboratory toxicity tests under controlled conditions have been widely used to demonstrate the adverse effects of chemicals on aquatic biota (Adams, 2003). Certain taxa, known as surrogate organisms, have been selected for this purpose due to their particular sensitivity to environmental and chemical stressors (Niemi & McDonald, 2004). Ceriodaphnia dubia Richard 1894 (Crustacea: Branchiopoda: Cladocera) is widely used for toxicity tests in North America (Bazin et al., 2009). The advantages of using this cladoceran lie in its importance as a link in aquatic food chains, short life cycle, low breeding costs, high sensitivity to toxic substances, and the low test water volume required to run bioassays (Bazin et al., 2009). The species is considered representative of freshwater zooplankton; even though it is more common in lakes and ponds, it also inhabits the quiescent sections of streams and rivers (Kim & Joo, 2000), where the flowing water flushes out the zoo- and phytoplankton on which it feeds (Sa-ardrit & Beamish, 2005). As a filter-feeding species, it mainly feeds on phytoplankton, but can also ingest bacteria, protozoa, organic debris and other suspended particles (Monakov, 2003).
Large-scale ecotoxicological research on aquatic ecosystems has focused mainly on temperate countries (Lacher & Goldstein, 1997). In Mexico, although there is an increasing use of toxicity tests as a tool complementary to the commonly used physicochemical water quality assessments (Mendoza-Cantú et al., 2007; Ramírez-Romero et al., 2007), to our knowledge, these tests have not been used to determine the impact on aquatic life caused by land use activities in watercourses.
The aim of this study was to evaluate the effect of water quality of streams in micro-watersheds of various land uses on the fecundity of C. dubia, during the dry and the rainy seasons. We tested the hypothesis that water from streams located in urban areas should cause a strong decrease in C. dubia fecundity, followed by coffee plantation stream water, and finally pasture stream water. Fecundity should be optimum in undisturbed cloud forest stream water. Regarding seasonality, we assumed that rain runoff would increase the concentration of nutrients in the streams, but also the increased stream discharge caused by the rains should produce a dilution effect on physicochemical indicators, leading to higher C. dubia reproduction relative to the dry season.
MATERIALS AND METHODS
Study area. We selected two streams for each of the four land uses in the upper watershed of the La Antigua river, located in the state of Veracruz, Mexico: tropical montane cloud forest, named cloud forest streams (F1, F2), cattle pastures, named pasture streams (P1, P2), coffee plantation streams (C1, C2), and streams with mixed land uses but greater urban influence, named urban influenced streams (U1, U2) (Fig. 1). These streams descend the eastern slope of the Cofre de Perote and Pico de Orizaba volcanoes, and supply water to the surrounding urban, agriculture and pasture areas. The streams range from first to third order in morphological hierarchy (Table 1). Elevation ranges from 1000 to 1600 masl. All of the sites feature humic andosols and are located in micro-watersheds with at least 50% of the area covered by the corresponding assigned land use. Urban streams were surrounded by a considerable area of coffee and sugar cane plantations (Table 1), but the water samples were collected in the urban area of influence. The climate in the region is characterized by three main seasons: a humid-cold season (featuring northerly cold fronts "nortes", November-March), a dry season (April-June), and a rainy season (July-October) (Williams-Linera, 2007).
Water sampling. Water samples for bioassays and water quality assessment were collected in April (dry season) and October (rainy season) 2010. Sampling sites were selected where each land use was represented in the local riparian zone. For bioassays, 2 L of water were collected from the center of the stream channel and for water quality measurements, 4 L of water (2 from runs and 2 from pools in each stream) were collected for chemical assessment. Samples were taken from each stream in sterilized polypropylene flasks (except for those to determine phosphorus, which were taken in 250 ml glass bottles) and then transported to the laboratory in coolers with ice to prevent microbial degradation and chemical transformation. Samples for water quality assessment were kept refrigerated for a maximum of 24 h before analysis (APHA, 1998). Samples for bioassays were kept in a freezer at -20 ºC and defrosted one day prior to preparation of test solutions. As no volatile or semi-volatile toxic substances were expected, samples were frozen for better preservation of any chemical with potentially toxic effects.
Physical and chemical variables. Discharge (Q), depth and instantaneous velocity were measured through a transversal transect of each stream using a meter stick and a flow meter (Probe 101FP201). Discharge was calculated as Q = Av, where A is the transversal area and v is the flow (m3 s-1) (Hauer & Lamberti, 1996). Temperature (T, °C), dissolved oxygen (O2, mg L-1), and conductivity (Cond, µS cm-1) were determined with a combination probe (YSI, Model 85). A potenciometer was used to determine pH in situ (Oakton, pH 11 series). We used the APHA methodologies to determine physicochemical variables in each stream (American Public Health Association, 1998). Total suspended solids (TSS, gravimetric method), total hardness (Hard, HACH titration method using EDTA), total alkalinity (Alk, phenolphthalein method), ammonium (NH4+, Nessler method), nitrates and nitrites (NO3- + NO2-, colorimetric method), total phosphorus (TP, persulfate digestion and colorimetry with the ascorbic acid method), chlorides (Cl-, colorimetry), and sulfates (SO42-, ion chromatography) were determined in the laboratory.
Toxicity tests. The test method used in this study was a subchronic toxicity assessment based on the short-term method of the U.S. Environmental Protection Agency (US EPA, 2002). For each test solution, we used ten Ceriodaphnia dubia female neonates (age < 24 h) obtained from controlled cultures of adult parthenogenetic females; these neonates were individually distributed in translucent 30 ml polystyrene cups. The green microalga Pseudokirchneriella subcapitata (Korshikov) Hindák was supplied as food during the bioassays at cell density of 1 million cells mL-1, in accordance with the US EPA (2002). Cladocerans were kept in environmental chambers at 25 ºC and with a 16:8 h photoperiod.
Thirty two test solutions were prepared as follows: two streams for each land use (cloud forest, pastureland, coffee plantation, and urban influenced), and four water concentrations: 12.5, 25, 50 and 100%, to evaluate the effects on cladoceran reproduction. Organisms were examined every day for eight days and the neonates produced by each female were counted and then discarded.
Each solution and food was renewed every day. Toxicity assessments were conducted with water samples from the dry and rainy seasons.
Fecundity per female was measured considering the following dependent variables: average accumulated progeny released per female (named progeny), average number of broods (named broods), and number of non-reproductive females (measured as the proportion from the total females, and named non-reproductive females) during the entire test period (eight days).
The dilution water was reconstituted soft water (42 mg L-1 CaCO3), since hardness analyses revealed that all of the streams had soft water (from 9 to 45 mg L-1 CaCO3). Fifteen control females, raised on hard reconstituted water of 160-180 mg L1 CaCO3 as suggested by US EPA guidelines, accompanied every series of tests, but were only used to validate the sensitivity of the C. dubia strain in use as the test organism, as our goal was to compare the types of streams in relation to the pristine cloud forest associated streams. Low water hardness can be a factor that affects C. dubia reproduction (Cowgill & Milazzo, 1991b; Harmon et al., 2003; Lasier et al., 2006); therefore, to evaluate a possible water hardness effect of dilution water on C. dubia reproduction (previously reared on hard water), we compared the cladoceran fecundity in the rainy season with previous toxicity assessment results using water samples obtained during the preceding rainy season (2009) and the same water test concentrations, where hard water was used as dilution water (160-180 mg L-1 CaCO3) (unpublished data). General Linear Model (GLM) analyses, with Poisson's error, were conducted to compare fecundity of C. dubia females in hard and soft water at the different concentrations tested. Fecundity was the same regardless the hardness of dilution water (progeny: G2 = 2.8, p = 0.25; broods: G2 = 0.5, p = 0.78; G2 = likelihood ratio Chi-square). We can thus be assured that the water hardness of dilutions in our experiments did not affect the C. dubia reproduction.
Statistical analysis. In order to determine differences between streams and seasons based on the water quality variables, we used principal component analysis (PCA) applying the Multi-Variate Statistical Package program (MVSP 3.1; Kovach, 1999). Logarithms were calculated for each variable (except pH). GLM were used to analyze the effect of the following independent variables on each of the fecundity dependent variables: land use (cloud forest, pastureland, coffee plantation, and urban influenced), water concentration (12.5, 25, 50 and 100%) and seasons (dry and rainy). Water concentration was analyzed as a nested variable on each stream. We used a Poisson's distribution for the GLM analysis of progeny; the broods presented over-dispersion, so we used a quasi-Poisson distribution. For the "non-reproductive females" variable, we used an arcsine transformation and then conducted a nested ANOVA, as recommended by Crawley (2002) for certain types of proportions. Only the second-degree interactions of the independent variables were tested. Akaike Information Criteria (AIC) was used to obtain the optimal model. The best-supported model has the lowest AIC compared to other models (Crawley, 2002). However, analysis of deviance was used to obtain the optimal model for progeny. Statistical analyses were carried out using R 2.9.0 software (R development Core Team, 2006).
RESULTS
Water quality. Table 2 presents the average values of the physicochemical variables of water from each stream from April (dry season) and October (rainy season), separately. In this study, the cloud forest streams showed the coolest temperatures and highest oxygen values (Table 2). In addition, F1 had the lowest total alkalinity. P1 showed higher values of temperature, conductivity, total hardness, total alkalinity, and ammonium than P2, but not as high as those observed in the coffee plantation and urban influenced streams; only total alkalinity was very similar. P2 showed total phosphorus as high as U1, but presented the lowest conductivity, total hardness, and ammonium, together with the cloud forest streams. P2 was thus as undisturbed as the cloud forest streams, but P1 was moderately altered. Stream U1 presented among the highest values of total suspended solids, ammonium, and total phosphorus. Along with U2, the stream C1 had the highest values of conductivity and total hardness. Both coffee plantation streams had the highest discharge and the highest nitrite and nitrate concentrations. Coffee plantation and urban influenced streams contained at least twice the amount of total suspended solids of the other streams. The urban stream U2 had the highest average values of more numbers of variables; conductivity, total suspended solids, ammonium, total phosphorus, chloride, and sulfate, but presented the lowest oxygen concentration of all the streams.
Based on the physicochemical variables measured with separated seasonal values, the PCA (Fig. 2) shows a relationship between stream condition and season. Axis 1 explains 55.6% of the variance and suggests a disturbance gradient in the streams. Both cloud forest streams were associated with the highest concentrations of DO (negative scores in axis 1), while pasture streams were not associated with any particular variable. Meanwhile, coffee plantation streams were related to the highest values of conductivity and hardness, and U2 had the highest concentrations of chloride, sulfate and ammonium (positive scores). Axis 2 explained 23.1% of the variance and was associated with an eutrophication gradient. Coffee plantation streams showed high concentrations of NO3 -+ NO2 -, alkalinity, and pH (positive scores) and U2 showed the highest concentrations of total phosphorus (negative scores). The remaining parameters presented lower values. April scores were always located to the right of the PCA, and October ones to the left. Thus, half of the water quality parameters tended to be lower during the dry than during the rainy season, and this was positively related to the discharge found for each stream and season. However, suspended solids, alkalinity, nitrates and nitrites and pH all lacked this tendency. Dissolved oxygen and phosphorus were higher in the rainy season than in the dry season (Table 2).
Discharge tended to be greater during the rainy than during the dry season, except for urban influenced streams: U1 presented higher discharge during the dry season, and U2 presented the same discharge in both seasons. During the dry season, U1 had the highest discharge followed by C2 and forest streams. Pasture streams, C1, and U2 had the lowest discharges. In the rainy season, coffee plantation streams clearly showed the highest discharge, while U2 presented the lowest discharge of all the streams during this season (Fig. 3).
Toxicity tests. Test acceptability criteria were met, according to the US EPA guidelines (US EPA, 2002), since 93% of the control cladocerans survived (14 out of 15), and mean number of offspring was more than 15 neonates per female (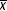 April = 16.9 ± 0.9;
April = 16.9 ± 0.9; 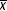 October = 22.3 ± 1.1). Routine parameters of test solutions were controlled, and fluctuated as follows: Oxygen (mg L-1): Average = 4.27, Min = 3.9, Max = 5.42; pH: Average = 8.01, Min = 6.07, Max = 8.75; Conductivity (µS cm-1): Average = 140.02, Min = 40.7, Max = 543; and Salinity (ups): Average = 0.09, Min = 0, Max = 0.3.
October = 22.3 ± 1.1). Routine parameters of test solutions were controlled, and fluctuated as follows: Oxygen (mg L-1): Average = 4.27, Min = 3.9, Max = 5.42; pH: Average = 8.01, Min = 6.07, Max = 8.75; Conductivity (µS cm-1): Average = 140.02, Min = 40.7, Max = 543; and Salinity (ups): Average = 0.09, Min = 0, Max = 0.3.
The minimal model for the reproduction variables (progeny and broods) showed that land use, season, the interaction between these two factors, and between land use and dilutions, significantly influenced fecundity (Table 3). There were more progeny for urban influenced streams, followed by coffee plantation streams than for pasture and cloud forest streams (Fig. 4a). There were significantly more progeny during the rainy season than during the dry season (Fig. 4b). The land use-season interaction showed that progeny increased with stream alteration gradient during the dry season (Fig. 4c). During the rainy season there was a similar pattern, but in coffee plantation streams progeny increased significantly, and was lowest in pasture streams. However, with natural water (100% concentration) of U2 in the dry season, C. dubia reproduction was inhibited in all the test females. In general, for all the water concentrations the pattern of progeny was similar to that showed in all land uses (Fig. 4d). Only in the cloud forest and pastureland streams at 100% concentration, there was significantly less progeny relative to the other concentrations (Fig. 4d).
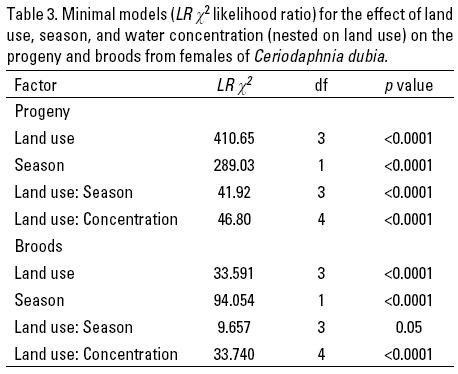
Regarding the "broods" variable, C. dubia had less broods in cloud forest and pasture streams than in coffee plantation and urban streams (Fig. 5a). Number of broods was significantly higher in the rainy than in the dry season (Fig. 5b). In a similar pattern to that observed in progeny, the number of broods from cloud forest streams increased significantly in the dry season, but in the rainy season broods increased significantly in cloud forest streams, and was lowest in pasture streams (Fig. 5c). Only in 100% of water concentration of the coffee plantation, pasture and urban influenced streams was the number of broods of C. dubia significantly lower relative to the other water concentrations (Fig. 5d).
Season had influence on the number of non-reproductive females (p = 0.01): there were more in the dry than in the rainy season (Dry = 15.9%, Rainy = 8.6% of the total number of females).
Moreover, in the rainy season, at least one female reproduced in each test solution.
DISCUSSION
In this study, cloud forest streams were the most oxygenated, and had the coolest temperatures and lowest values of physicochemical variables. This agrees with the results obtained by Martínez et al. (2009) and Vázquez et al. (2011), suggesting that the cloud forest streams studied herein are a good representation of an undisturbed ecosystem. Also, cloud forest streams had the lowest conductivity, hardness, and alkalinity concentrations, together with one of the pasture streams. Several studies at the La Antigua upper watershed have found that the streams and rivers of this region have similar chemical characteristics (Astudillo-Aldana, 2009; Cortés-Soto, 2010; Vázquez et al., 2011).
A 1 m wide vegetated buffer can significantly reduce concentrations of fecal coliform bacteria from cow manure in runoff (Sullivan et al., 2007). Local cattle producers leave some trees along the riverside (first author pers. obs.) and do not treat their pastures (they do not apply chemical fertilizers or manure for pasture fertilization, and avoid the burning treatment to enhance pasture growth: local cattle farmers, pers. com). This practice probably helps to maintain the chemistry of the streams closer to that of natural conditions. However, total phosphorus was high for one of the pasture streams compared to the cloud forest and coffee plantation streams. This could be due to the presence of cow manure near the stream channel (first author pers. obs.) as a result of the free access of livestock to the stream for drinking water (no other source of drinking water was present on the fields). On the other hand, phosphorus is released from cattle manure trough runoff mostly during the rainy season (Sharpley et al., 1998; Soupir et al., 2006). In fact, we found that for both pasture streams, TP was greater during the rainy season than during the dry season (P1April = 0.03, P1October = 0.2; P2April = 0.02, P2October = 0.8).
Coffee plantation streams showed the highest concentrations of nutrients, almost certainly a result of fertilizer use (Nestel, 1995; Guadarrama-Zugasti, 2000), and one of these streams also showed high values of conductivity, total suspended solids, total hardness and total alkalinity. At the La Antigua upper watershed, Martínez et al. (2009) and Vázquez et al. (2011) also found that suspended solids, conductivity and nutrients were higher in coffee plantation than in pasture streams, whereas cloud forest streams had the lowest values. This occurs because riparian forests and grasslands can delay or prevent nutrient transport from nearby areas to streams (Osborne & Kovacic, 1993), since the understory vegetation retains the soil runoff.
As we hypothesized, the urban influenced streams studied presented the highest values for several physicochemical variables, but U2 in particular was the most chemically altered stream, reaching the highest values of the water quality measurements such as total suspended solids, ammonium, chloride, sulfate, and the lowest oxygen concentration. These variables are indicators of chemical alterations caused by the input of domestic wastewaters (Tchobanoglous et al., 1991; Burks & Minnis, 1994; USGS, 1999; Kumar, 2002; Jonsson et al., 2005).
A significant proportion of land use at both mixed micro-watersheds (U1 and U2) is coffee and sugar cane plantations, where the use of pesticides is frequent (Nestel, 1995; Guadarrama-Zugasti, 2000); however, the sampling sites were also directly influenced by domestic wastewaters, since both streams cross an urban area of influence from 0.6 to 1 km upstream from the sampling site. In this area, sewage collection is either non-existent or inefficient at best (first author pers. obs.). In short, cloud forest streams represent the natural condition of the streams, the stream least altered from this condition was P2, and the most altered was U2. The rest were moderately disturbed.
In relation to water quality parameters and season, non-seasonal changes in levels of nitrates and nitrites could be a result of pesticide application by coffee producers (against the coffee berry borer) and fertilizers in the studied areas in early April and September 2010 (local coffee producers' pers. com.). Suspended solids would have had no apparent seasonal changes because sampling took place several days after the last rain, giving time for most of the sediment carried by runoff to precipitate. Nonetheless, total phosphorus also showed higher concentrations in the rainy season in coffee plantation streams. This could be the result of fertilizers entering the channel via soil runoff. Other authors also report an increase of nutrients in rivers during the rainy season (Wang et al., 2009).
Generally lower water quality parameters in the dry season could be positively related to the discharge levels found for each stream and season, which suggests dilution of contaminants takes place during the rainy season. However, the discharge was greater for U1 during the dry season, while for U2 discharge was the same in both seasons. This is evidence of a non-seasonal alteration in the urban influenced streams, as they depend on intermittent household discharges (Schiff & Tiefenthaler, 2003). Greater discharge took place in the coffee plantation streams, and is related to their third hierarchy order condition. However, even though these were the largest streams, their water quality was entirely related to the coffee plantations in the watersheds to which they belong. This water quality is characterized by higher concentrations of suspended solids and nitrates and nitrites (Vázquez et al., 2011). Therefore, no other land use effect had enough influence on the water quality of these bigger streams.
Regarding the toxicity tests, and contrary to the original hypothesis, the results revealed that fecundity (progeny and number of broods) among land uses was lower for cloud forest and pasture streams than for coffee plantation and urban influenced streams (except for U2 during the dry season, with 100% concentration). These results can be related to the higher values of conductivity, ammonium, suspended solids, and lower dissolved oxygen of the coffee plantation and urban influenced streams which, as indirect indicators of organic matter, could have had benefits for cladoceran fecundity through the additional supply of food. This species is not a selective filter feeder (Monakov, 2003) and, in response to the increase of potentially assimilable organic particles (food augmentation), females could grow and reproduce better (Rose et al., 2000).
C. dubia can also feed on bacteria (Anderson & Benke, 1994), other algae (Wylie & Currie, 1991), and particulate organic matter (Kirk, 1991); however, in addition to the supplied food (P. subcapitata microalgae), the cladocerans probably fed on other particles, such as organic matter present in suspended solids, as has been observed by other authors (Salonen & Hammar, 1986; Santos et al., 2006). The females thus enhanced their fecundity, particularly when grown in coffee plantation and urban stream samples, which had the highest values of suspended solids. In addition, coffee plantation streams showed the highest levels of nitrates and nitrites, while U2 had the highest levels of phosphorus; both N and P lead to a nutrient enriched medium that could also explain the cladoceran fecundity results. Kuhl et al. (2010) found that in some nutrient-enriched rural and urban Brazilian streams fecundity higher than that of the controls. In fact, Ceriodaphnia sp. has been found to be a useful bioindicator of watercourse eutrophication provoked by sewage discharges (Kumar, 2002).
In this study cloud forest and pasture streams, along with the lower levels of nutrients and possibly limited organic matter in comparison with coffee plantation and urban influenced streams, presented very low water hardness (from 8 to 22 mg L-1 CaCO3) and, since calcium ion is essential for the composition of the crustacean exoskeleton (Greenaway, 1985), calcium deficiency could have prevented exoskeleton formation during molting, and therefore, reproduction.
While the use of herbicides, fertilizers, and insecticides is widespread by coffee producers in the region (Nestel, 1995; Gua-darrama-Zugasti, 2000), the overall impact of fertilizers and pesticides applied to coffee crops did not lead to lower fecundity of C. dubia. Coffee producers used pesticides and fertilizers in the watersheds of the streams studied in early April and September 2010, during the rainy season but a couple of weeks before the water was sampled for toxicity tests. During rainy periods, relatively high concentrations of pesticides in the streams would be present only for a short period of time (first-flush), after which a rapid decrease in concentration takes place (Cooman et al., 2005). Hall (1993) compared C. dubia reproduction between first-flush periods and post first-flush periods during the rainy season, and found that reproduction decreased during the first-flush, but increased during the post-flush; he suspected that this was the effect of organic pesticides contained in the effluent from a wastewater treatment plant. Gersberg et al. (2004) found peak toxicity values within the first 1-2 h after initiation of rain events, and suggested that non-polar organic compounds could be responsible for such toxicity. Both previous studies suggest that it is possible that no toxicity was found for C. dubia in coffee plantation stream waters, even when pesticides were used before sampling. This is because the first flush events, containing the peak in pesticides caused by runoff, were missed when water was sampled.
Fecundity was significantly higher (more progeny, more broods, and more reproductive females) during the rainy than during the dry season, probably due to the generally higher water quality and discharge in the streams during the rainy season. In addition, when chemical alteration reached its highest levels (U2 100% concentration, in the dry season) reproduction was inhibited in C. dubia. Obtaining results similar to ours, Vitale (2007) documented greater toxicity for C. dubia during the dry than during the rainy season, but related it to greater hardness and total dissolved solids (1429 mg L-1) during the dry season.
Consistent with the Mexican guidelines (CE-CCA, 1989; CONAGUA, 2008), which shows the maximum levels of several water quality parameters for the protection of aquatic life in freshwater ecosystems; DO for U2 in the dry season was lower than the minimum level (5 mg L-1); TSS maximum level (20-30 mg L-1) was exceeded by the coffee plantation and urban influenced streams; NH4 (0.06 mg L-1) was exceeded by the F2, P1, coffee plantation and urban influenced streams; TP (0.05 mg L1) was surpassed by the C1, cloud forest, pasture and urban influenced streams; and sulfate was exceeded in all the streams (0.005 mg L-1). There are no Mexican criteria available for conductivity, hardness, alkalinity, nitrates and nitrites for aquatic life protection, and the other parameters were not exceeded in any stream. The maximum level of ammonia was surpassed to the greatest extent by U2 in the dry season, when no C. dubia reproduction was recorded. Nimmo et al. (1989) reported a 48-h LC50 value of > 1.43 mg L-1 un-ionized ammonia, while Cowgill & Milazzo (1991a) found a 48-h LC50 of 9 mg L-1 total ammonia and Andersen & Buckley (1998) reported a 48-h LC50 of 1.18 mg L-1 of un-ionized ammonia: these three studies used survival as the endpoint of C. dubia toxicity effects. Our data showed a maximum average concentration of 13.8 mg L-1 of ammonium (±0.3 s.e.) for U2, but once converted to the un-ionized form (0.36 mg-1) it is lower than the toxic concentration found in these surveys.
Toxic conductivity levels for freshwater fauna are considered above 500 µS cm-1 (Pond et al., 2008), but the highest average in U2 was 317.12 µS cm-1 (±0.89 s.e.), so it is possible that this variable did not affect reproduction of C. dubia. Conductivity greater than 1000 µS cm-1 is negatively related with C. dubia reproduction (Mitchelmore, 2010), and a significant decrease in the richness and abundance of riverine macroinvertebrate taxa has been found with conductivity above 500 µS cm-1 (Kefford et al., 2006; Pond et al., 2008; Pond, 2010). Furthermore, conductivity can be correlated with sulfate values (Bryant et al., 2002; WVDEP, 2008). Sulfate values higher than 100 mg L-1 are considered toxic to freshwater fauna in British Columbia (Singleton, 2000), but this parameter was recorded at 50.3 mg L-1 (± 2.89 s.e.) in U2, and was therefore unlikely to be toxic for the test organism we used.
In the present study, the most favorable conditions for C. dubia reproduction were provided by nutrient and probably organic enrichment through sewage and organic sediments in urban and coffee plantation associated streams, while in the tropical montane cloud forest and pasture streams, the unpolluted and close to unpolluted water chemistry, along with natural soft waters, caused a reduction in fecundity, probably through the reduced availability of food particles, and a deficiency in ions necessary for growth. Thus, by using a test involving not only physicochemical parameters but also living organisms, the results show that chemical alterations can be positive for some aquatic organisms. Subchronic toxicity tests performed yielded useful information about the changes in physicochemical water parameters due to anthropic activity and the positive impacts of these water quality alterations on the secondary productivity of stream ecosystems. These positive impacts have a limit, however, at which an excess of organic matter will reduce water quality and impair the natural aquatic biota.
ACKNOWLEDGMENTS
Supported by Consejo Nacional de Ciencia y Tecnología (CONA-CyT), through a doctoral grant No. 168443 to the first author and project 101542. We thank Laura Martínez-Jerónimo for laboratory bioassays, Ariadna Martínez Virués and Daniella Cela Cadena for their support in water analysis and José Antonio Gómez Anaya and Javier Tolome Romero for support in field sampling. Rosario Landgrave helped with GIS analysis. The comments by two anonymous reviewers greatly improved the manuscript. Xochitl Ponce Wainer and Keith MacMillan revised the the English text.
REFERENCES
Adams, S. M. 2003. Establishing causality between environmental stressors and effects on aquatic ecosystems. Human Ecology Risk Assessment 19: 17–35. [ Links ]
Allan, J.D. & M. M. Castillo. 2007. Human impacts. In: Allan, J. D. & M. M. Castillo (Eds.). Stream ecology, structure and function of running waters. 2nd ed. Springer, Dordecht, pp. 317-357. [ Links ]
APHA (American Public Health Association). 1998. Standard methods for the examination of water and wastewater. 20th ed. APHA-AWWA WEF. Washington D. C. 1220 p. [ Links ]
Andersen, H. B. & J. A. Buckley. 1998. Acute toxicity of ammonia to Cerio daphnia dubia and a procedure to improve control survival. Bulletin of Environmental Contamination and Toxicology 61: 116-122. [ Links ]
Anderson, D. H. & A. C. Benke. 1994. Growth and reproduction of the cladoceran Ceriodaphnia dubia from a forested floodplain swamp. Limnology and Oceanography 39: 1517-1527. [ Links ]
Astudillo-Aldana, M. R. 2009. Diversidad filogenética de Odonata (Insecta) en el río Huehueyapan en Coatepec, Veracruz, México y su relación con factores físico-químicos. Master in Science thesis, Instituto de Ecología, A.C., Xalapa, Veracruz. 51 p. [ Links ]
Bazin, C., P. Pandard, A. M. Charissou & Y. Barthel. 2009. Ceriodaphnia dubia chronic toxicity tests. In: Moser H. & J. Römbke (Eds.). Ecotoxicological characterization of waste. Part 2, Springer, New York, pp. 161–164. [ Links ]
Bellanger, B., S. Huon, F. Velasquez, V. Vallès, C. Girardin & A. Mariotti. 2004. Monitoring soil organic carbon erosion with d13C and d15N on experimental field plots in the Venezuelan Andes. CATENA 58: 125-150. [ Links ]
Bryant, G., S. Mcphilliamy & H. Childers. 2002. A survey of the water quality of streams in the primary region of mountaintop / valley fill coal mining. Mountaintop mining / valley fill programmatic environmental impact statement. Region 3, US Environmental Protection Agency (USEPA), Philadelphia, Pennsylvania. 65 p. [ Links ]
Bücker, A., P. Crespo, H. G. Frede, K. Vaché, F. Cisneros & L. Breuer. 2010. Identifying controls on water chemistry of tropical cloud forest catchments: combining descriptive approaches and multivariate analysis. Aquatic Geochemistry 16: 127-149. [ Links ]
Burks, B. D. & M. M. Minnis. 1994. On site wastewater treatment systems. Hogarth House, Madison, Wisconsin, 248 p. [ Links ]
Chapman, D. 1992. Water quality assessments. A guide to the use of biota, sediments and water in environmental monitoring. UNESCO, WHO and UNEP. Chapman & Hall, London. 585 p. [ Links ]
Cooman, K., P. Debels, M. Gajardo, R. Urrutia & R. Barra. 2005. Use of Daphnia spp. for the ecotoxicological assessment of water quality in an agricultural watershed in South-Central Chile. Archives of Environmental Contamination and Toxicology 48: 191-200. [ Links ]
Cortés-Soto, N. G. 2010. Diagnóstico de la calidad del agua del río Pixquiac en la congregación Zoncuantla, Municipio de Coatepec, Veracruz. Especialidad en diagnóstico y gestión ambiental. Facultad de Ingeniería Química. Universidad Veracruzana, Xalapa. 31 p. [ Links ]
Cowgill, U. M. & D. P. Milazzo. 1991a. The response of the three brood Ceriodaphnia test to fifteen formulations and pure compounds in common use. Archives of Environmental Contamination and Toxicology 21: 35-40. [ Links ]
Cowgill, U. M. & D. P. Milazzo. 1991b. The sensitivity of two cladocerans to water quality variables: salinity <467 mg NaCl/L and hardness <200 mg CaCO3/L. Archives of Environmental Contamination and Toxicology 21: 218-223. [ Links ]
Crawley, M. J. 2002. Statistical computing: An introduction to data analysis using S-PLUS. John Wiley & Sons, New York. 761 p. [ Links ]
CONAGUA (Comisión Nacional del Agua). 2008. Ley federal de derechos. Disposiciones aplicables en materia de aguas. Diario Oficial de la Nación. 13 de Noviembre 2008. México, D.F. 97 p. [ Links ]
Criterios de Calidad del Agua de la Comisión Nacional del Agua. CE-CCA001/89 Diario Oficial de la Nación 2 de diciembre de 1989. México, D.F. [ Links ]
Duke, L. D., T. S. Lo & M. W. Turner. 1999. Chemical constituents in storm flow vs. dry weather discharges in California storm water conveyances. Journal of the American Water Resources Association 35: 821-836. [ Links ]
Geissert, D. & A. Ibáñez. 2008. Calidad y ambiente físico-químico de los suelos, Chapter 15. In: Manson R. H., V. Hernández-Ortiz, S. Gallina, & K. Mehltreter (Eds.). Agroecosistemas cafetaleros en Veracruz, biodiversidad, manejo y conservación. Instituto de Ecología A.C. (INECOL) & Instituto Nacional de Ecología (INE-SEMARNAT), México, pp. 213-221. [ Links ]
Gersberg, R. M., D. Daft & D. Yorkey. 2004. Temporal pattern of toxicity in runoff from the Tijuana River Watershed. Water Resources 38: 559-568. [ Links ]
Greenaway, P. 1985. Calcium balance and moulting in the crustacean. Biological Reviews 60: 425-454. [ Links ]
Guadarrama-Zugasti, C. 2000. The transformation of coffee farming in Central Veracruz, México: sustainable strategies? PhD Thesis, University of California, Santa Cruz. 187 p. [ Links ]
Hall, D. B. 1993. Temporal and spatial comparisons of ambient toxicity of the Trinity River in relationship to an effluent. PhD Thesis.University of North Texas. University Microfilms International, Ann Arbor, Michigan, 326 p. [ Links ]
Harmon, S. M., W. L. Specht & G. T. Chandler. 2003. A comparison of the daphnids Ceriodaphnia dubia and Daphnia ambigua for their utilization in routine toxicity testing in the southeastern United States. Archives of Environmental Contamination and Toxicology 45: 79-85. [ Links ]
Hauer, R. M. & G. L. Lamberti. 1996. Methods in stream ecology. Academic Press, Inc., San Diego, C.A. 739 p. [ Links ]
Jonsson, H., A. Baky, U. Jeppsoon, D. Hellström, & E. Karrman. 2005. Composition of urine, faeces, greywater and biowaste for utilization in the URWARE model. Urban water report of the MISTRA Programme, Chalmers University of Technology, Gothenburg, Sweden. 45 p. [ Links ]
Kefford, B. J., D. Nugegoda, L. Metzeling & E. J. Fields. 2006. Validating species sensitivity distributions using salinity tolerance of riverine macroinvertebrates in the southern Murray-Darling Basin (Victoria, Australia). Canadian Journal of Fisheries and Aquatic Sciences 63: 1865-1877. [ Links ]
Kim, H. & G. Joo. 2000. The longitudinal distribution and community dynamics of zooplankton in a regulated large river: a case study of the Nakdong river (Korea). Hydrobiologia 438: 171-184. [ Links ]
Kirk, K. L. 1991. Inorganic particles alter competition in grazing plankton: The role of selective feeding. Ecology 72: 915-923. [ Links ]
Kovach, W. L. 1999. MVSP - A Multivariate Statistical Package for Windows, Version 3.1. Kovach Computing Services, Pentraeth, Wales. 133 p. [ Links ]
Kuhl, A. M., M. S. C. da Rocha, C. L. Gaeta-Espindola & F. A. Lansac-Toha. 2010. Rural and urban streams: anthropogenic influences and impacts on water and sediment quality. International Review of Hydrobiology 95: 260-272. [ Links ]
Kumar, A. 2002. Biomonitoring of sewage pollution. A P H publishing Corporation. Inc. New. Delhi, pp. 39-103. [ Links ]
Lacher, T. E. Jr. & M. I. Goldstein. 1997. Tropical ecotoxicology: needs. Environmental Toxicology and Chemistry 16: 100-111. [ Links ]
Lasier, P. J., P. V. Winger & I. R. Hardin. 2006. Effects of hardness and alkalinity in culture and test waters on reproduction of Ceriodaphnia dubia. Environmental Toxicology and Chemistry 25: 2781-2786. [ Links ]
Martínez, M. L., O. Pérez-Maqueo, G. Vázquez, G. Castillo-Campos, J. García-Franco, K. Mehltreter, M. Equihua & R. Landgrave. 2009. Effects of land use change on biodiversity and ecosystem services in tropical montane cloud forests of Mexico. Forest Ecology and Management 258: 1856-1863. [ Links ]
Mendoza-Cantú, A., P. Ramírez-Romero & Y. Pica-Granados. 2007. Environmental legislation and aquatic ecotoxicology in Mexico: Past, present and future scenarios. Journal of Environmental Science and Health Part A 42: 1343-1348. [ Links ]
Mitchelmore, C. L. 2010. Evaluation of the chronic toxicity tests carried out by the US Environmental protection Agency at various sites in the coalfields of Kentucky and West Virginia, USA. Report on the U.S. Environmental Protection Agency's chronic whole effluent toxicity testing at selected sites in the coalfields of Kentucky and West Virginia. University of Maryland Center for Environmental Science. 5 p. [ Links ]
Monakov, A. B. 2003. Water fleas-Cladocera. In: Monakov A. B. (Ed.). Feeding of freshwater invertebrates. Kenobi Productions, Ghent, Belgium, pp. 133-172. [ Links ]
Muñoz-Villers, L. E. & J. López-Blanco. 2007. Land use/cover changes using Landsat TM/ETM images in a tropical and biodiverse mountainous area of central-eastern Mexico. International Journal of Remote Sensing 29: 71-93. [ Links ]
Nanazato, T. & M. Yasuno. 1985. Population dynamics and production of cladoceran zooplankton in the highly eutrophic Lake Kasumigaura. Hydrobiologia 124 (1): 13-22. [ Links ]
Nestel, D. 1995. Coffee and Mexico: International market, agricultural landscape, and ecology. Ecological Economics 15: 165-179. [ Links ]
Niemi, G. J. & M. E. McDonald. 2004. Application of ecological indicators. Annual Review of Ecology, Evolution and Systematics 35: 89-111. [ Links ]
Nimmo, W. R. De, D. Link, L. P. Parrish, G. J. Rodriguez, W. Wuerthele & P. H. Davies. 1989. Comparison of on-site and laboratory toxicity tests: derivation of site-specific criteria for un-ionized ammonia in a Colorado transitional stream. Environmental Toxicology and Chemistry 8: 1177-1189. [ Links ]
Osborne, L. L. & D. A. Kovacic. 1993. Riparian vegetated buffer strips in water quality restoration and stream management. Freshwater Biology 29: 243-258. [ Links ]
Pond, G. J. 2010. Patterns of Ephemeroptera taxa loss in Appalachian headwater streams (Kentucky, USA). Hydrobiologia 641: 185-201. [ Links ]
Pond, G. J., M. E. Passmore, F. A. Borsuk, L. Reynolds & C. J. Rose. 2008. Downstream effects of mountain top coal mining: Comparing biological conditions using family- and genus-level macroinvertebrate bioassessment tools. Journal of North American Benthological Society 27: 717-737. [ Links ]
R Development Core Team. 2006. R: A language and environment for statistical computing. R foundation for statistical computing, Vienna, Austria. ISBN 3-900051-07-0, URL Available online at: http://www.R-project.org/ (downloaded January 12, 2011). [ Links ]
Ramírez-Romero, P., Y. Pica-Granados & F. Martínez-Jerónimo. 2007. In-forme final: Pruebas biológicas para la evaluación ecotoxicológica de las sustancias químicas, tercera etapa: Desarrollo de pruebas biológicas con especies nativas y ejercicio de intercalibración. SEMARNAT, INE, México, D.F. 342 p. [ Links ]
Rose, R. M., M. St. Warne & R. P. Lim. 2000. Life history of the cladoceran Ceriodaphnia cf. dubia to variation in food concentration. Hydrobiologia 427: 59-64. [ Links ]
Sa-ardrit, P. & F. W. H. Beamish. 2005. Cladocera diversity, abundance and habitat in a western Thailand stream. Aquatic Ecology 39: 353-365. [ Links ]
Salonen, K. & T. Hammar. 1986. On the importance of dissolved organic matter in the nutrition of zooplankton in some lake waters. Oecologia 68: 246-253. [ Links ]
Santos, M. A. P. F, M. G. G. Melão & A. T. Lombardi. 2006. Life history characteristics and production of Ceriodaphnia silvestrii Daday (Crustacea, Cladocera) under different experimental conditions. Acta Limnologica Brasiliensia 18: 199-212. [ Links ]
Schiff, K. & L. Tiefenthaler. 2003. Contributions of organophosphorus pesticides from residential land uses during dry and wet weather. Technical Report 406. Southern California Coastal Water Research Project. Westminster, C.A. 91 p. [ Links ]
Sharpley, A. N., J. J. Meisinger, A. Breeuwsma, J. T. Sims, T. C. Daniel & J. S. Schepers. 1998. Impacts of animal manure management on ground and surface water quality. In: Hatfield J. (Ed.). Effective management of animal waste as a soil resource. Ann Arbor Press, Michigan, pp. 173-242. [ Links ]
Singleton, H. 2000. Ambient water quality guidelines for sulphate: technical appendix. Ministry of Environment, Lands and Parks, Province of British Columbia, Canada. Available online at: http://www.env.gov.bc.ca/wat/wq/BCguidelines/sulphate/ (downloaded January 12, 2011). [ Links ]
Soupir, M. L., S. Mostaghimi, E. R. Yagow, C. Hagedorn & D. H. Vaughan. 2006. Transport of fecal bacteria from poultry litter and cattle manures applied to pastureland. Water, Air and Soil Pollution 69: 125-136. [ Links ]
Strahler, A. N. 1957. Quantitative analysis of watershed geomorphology. Transactions of the American Geophysical Union 38: 913-920. [ Links ]
Sullivan, T. J., J. A. Moore, D. R. Thomas, E. Mallery, K. U. Snyder, M. Wustenberg, J. Wustenberg, S. D. Mackey & D. L. Moore. 2007. Efficacy of vegetated buffers in preventing transport of fecal coliform bacteria from pasturelands. Environmental Management 40: 958-965. [ Links ]
Tchobanoglous, G., L. B. Franklin, S. H. David (eds.). 1991. Wastewater Engineering. Treatment Disposal Reuse. 3rd ed. Metcalf & Eddy, Inc. McGraw-Hill, New York. 1820 p. [ Links ]
US EPA (United State Environmental Protection Agency). 2002. Daphnid, Ceriodaphnia dubia, survival and reproduction test. Method 1002.0. Short-term methods for estimating the chronic toxicity of effluents and receiving waters to freshwater organisms. Fourth Edition. EPA821-R-02-013. [ Links ]
USGS (United State Geological Survey). 1999. The quality of our nation's waters, nutrients and pesticides. US Geological Survey Circular 1225. Available online at: http://pubs.usgs.gov/circ/circ1225/ (downloaded January 11, 2011). [ Links ]
Vázquez, G., J. A. Aké-Castillo & M. E. Favila. 2011. Algal assemblages and their relationship with water quality in tropical Mexican streams with different land uses. Hydrobiologia 667: 173-189. [ Links ]
Vitale, P. 2007. Lake Elsinore sediment and water column toxicity study. Technical report. Surface Water Ambient Monitoring Program, Santa Ana Regional Water Quality Control Board, California. 12 p. [ Links ]
Wang, X., Y. Ou, P. Dou, & X. Fang. 2009. Relationship between the variation of water quality in rivers and the characteristics of watershed at Miyun, Beijing, China. Chinese Journal of Geochemistry 28 (1): 112-118. [ Links ]
WVDEP (West Virginia Department of Environmental Protection). 2008. Integrated water quality monitoring and assessment report. Division of Water and Waste Management, Virginia. 52 p. [ Links ]
Williams-Linera, G. 2007. El bosque de niebla del centro de Veracruz: ecología, historia y destino en tiempos de fragmentación y cambio climático. INECOL-CONABIO, México. 208 p. [ Links ]
Wylie, J. L. & D. J. Currie. 1991. The relative importance of bacteria and algae as food sources for crustacean zooplankton. Limnology and Oceanography 36: 708-728. [ Links ]
Yufeng, Y., Xiangfei, H. & L. Juankang. 1998. Long-term changes in crustacean zooplankton and water quality in a shallow, eutrophic Chinese lake densely stocked with fish. Hydrobiologia 391: 193-201. [ Links ]














