Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Hidrobiológica
versión impresa ISSN 0188-8897
Hidrobiológica vol.22 no.3 Ciudad de México sep./dic. 2012
Artículos
Protein requirement in masculinized and non-masculinized juveniles of Bay Snook Petenia splendida
Requerimiento de proteína en juveniles masculinizados y no masculinizados de la mojarra tenguayaca Petenia splendida
Arkady Uscanga-Martínez,1 Carlos Alfonso Álvarez-González,2 Wilfrido Miguel Contreras-Sánchez,2 Gabriel Márquez-Couturier,2 Roberto Civera-Cerecedo,3 Héctor Nolasco-Soria,3 Alfredo Hernández-Llamas,3 Ernesto Goytortúa-Bores3 and Francisco Javier Moyano4
1 Laboratorio de Acuicultura, Campus del Mar, Universidad de Ciencias y Artes de Chiapas. Carretera Tonalá-Paredón km 0.5., Tonalá, Chiapas. 86039. México
2 Laboratorio de Acuicultura Tropical, División Académica de Ciencias Biológicas, Universidad Juárez Autónoma de Tabasco. Carretera Villahermosa-Cárdenas km 0.5., Villahermosa, Tabasco. 86039. México
3 Centro de Investigaciones Biológicas del Noroeste (CIBNOR). Mar Bermejo 195, Col. Playa Palo de Santa Rita, La Paz, B.C.S. 23090. México
4 Departamento de Biología Aplicada, Escuela Politécnica Superior. La Cañada de San Urbano, Universidad de Almería, Almería. 04120. Spain. E-mail: alvarez_alfonso@hotmail.com
Recibido: 16 de mayo de 2011.
Aceptado: 11 de junio de 2012.
ABSTRACT
The effect of the dietary protein level on growth and total body chemical composition of the native cichlid Bay snook (Petenia splendida), masculinized and non-masculinized, was studied. Five semi-purified diets with protein levels 35, 40, 45, 50 and 55% crude protein (CP) were formulated and evaluated by triplicate. Groups of 50 juveniles were each stocked in 70 L tanks and fed to apparent satiation for 42 days trial. At the end, weight gain (WG) (403.41%), body length (BL) (6.58 ± 0.10 cm) and specific growth rate (SRG) (1.67%/day) of the masculinized fish were obtained with the 45% CP diet, and they were significantly different (p = 0.002) from the other treatments. In the case of non-masculinized fish, the 45 and 55% CP treatments showed significant differences (p = 0.00001), with respect to other treatments, with a WG of 398 and 394%, SGR of 1.66 and 1.63%/day, protein productive value (PPV) of 28.91 and 29.21%, and feed conversion rate (FCR) of 1.23 and 1.08 respectively. Protein body composition for masculinized fish was different (p = 0.0001) only for fish fed 35% CP compared with fish at the beginning of the experiment. We conclude that the optimum protein requirement, estimated by the broken-line model for masculinized and non-masculinized P. splendida was 45 and 44.8% PC respectively.
Key words: Cichlid, larviculture, nutrition, protein, Tenguayaca.
RESUMEN
Se evaluó el efecto del nivel de proteína sobre el crecimiento y composición química proximal del cíclido nativo tenguayaca (Petenia splendida) masculinizados y no masculinizados, para lo que se formularon cinco dietas semipurificadas con diferentes niveles de proteína cruda: 35, 40, 45, 50 y 55% PC, colocando 50 peces por tina (70 L) los cuales fueron alimentados a saciedad aparente por 42 días. Al final del periodo experimental, el mayor (p = 0.002) peso promedio (AW, 3.31 ± 0.46 g), longitud (BL, 6.58 ± 0.10 cm), ganancia en peso (WG, 403.41%) y tasa específica de crecimiento (SGR, 1.67%/día) en peces masculinizados se obtuvo para los alimentados con 45% CP. Los peces no masculinizados alimentados con las dietas de 45 y 55% CP fueron estadísticamente diferentes (p = 0.00001) en relación al WG (398 y 394%), SGR (1.66 y 1.63%/día), valor productivo de la proteína (PPV, 28.91 y 29.21%) y factor de conversión alimenticio (FCR, 1.23 y 1.08). La proteína corporal de los peces masculinizados fue estadísticamente diferente (p = 0.0001) para los peces alimentados con 35% CP comparados con los peces al inicio del experimento. Concluimos que el nivel óptimo de proteína estimado con el modelo de línea quebrada para los juveniles masculinizados y no masculinizados de P. splendida fue de 45 y 44.8% CP respectivamente.
Palabras clave: Cíclidos, larvicultura, nutrición, proteína, Tenguayaca.
INTRODUCTION
The success of any aquacultural enterprise is mainly based on the selection of a species suitable for rearing and commercialization. Some introduced species such as carp (Ctenopharyngodon idella Valenciennes, 1844), tilapia (Oreochromis niloticus Linnaeus, 1758), and rainbow trout (Oncorhyncus mykiss Walbaum, 1792) are the most common cultured fish in Mexico. However, recent studies on native species such as the bay snook (Petenia splendida (Günther, 1862)) have generated a strong interest in aquaculturists due to its high price in the regional market 7 US$/kg. In addition, several studies have reported a decrease in wild populations of this specie due to its over-exploitation (Reséndez & Salvadores, 1983; Chávez et al., 1989; Caro et al., 1994; Ferreira & Gómez, 1994; Gamboa & Schmitter, 1997; Domínguez & Rodiles, 1998).
For these reasons, the rearing of this species has become a major issue within freshwater aquaculture research in Mexico and a number of recent studies have provided knowledge about different basic and applied aspects of its life cycle, controlled reproduction, larval culture, nutritional requirements, masculinization and adaptation to artificial feeds among others (Garcia, 2003; Real, 2003; Martínez, 2004; Chan, 2004; Vidal et al., 2009; Jiménez-Martínez et al., 2009).
P. splendida reaches commercial sizes of 250-300 g in approximately one and a half year when fed commercial trout foods. In order to improve the culture of this species, suitable balanced feed need to be developed to adequately cover its nutritional requirements. Protein is obviously the main nutrient for muscle growth (De la Higuera, 1987; Soler et al., 2000; Catacutan et al., 2001; Moon et al., 2001) as well as for other important biological functions such as structural, contractile catalytic, transport and regulation functions (Tacon, 1987; Barroso et al., 1994; Lehninger, 1995; Kang et al., 2005: Ali & Jauncey, 2005). In addition, providing adequate concentrations of nutrients and also digestibility aspects contributes in a reduction of production costs (NRC 1993; Lee et al., 2000; Kim et al., 2001; Ng et al., 2003; Cho et al., 2005). Several studies have been recently conducted to determine the protein requirement of species of carnivorous fishes including Japanese croaker (Nibea japonica, Temminck & Schlegel 1843) (Moon et al., 2001), Korean bullhead (Pseudobagrus fulvidraco, Richardson 1846) (Kim et al. 2005), turbot (Scophthalmus maximus, Linnaeus 1758) (Peresa & Oliva-Teles 2005), Amoy croaker (Nibea miichthioides, Bleeker 1863) (Wang et al., 2006) and Pike-perch (Sander lucioperca, Linnaeus 1758) (Bódis et al., 2007). In these cases, protein requirement varied from 37 to 50% of the diet depending on the species, type of rearing system, age and type of ingredients used in feed preparation (Akiyama, 1995; Guzmán, 2003).
The purpose of this study was to determine the protein level requirement using different biological indexes as weight gain, survival and total body chemical composition of masculinized and non-masculinized P. splendida juveniles using semi-purified diets.
MATERIALS AND METHODS
Experimental Organisms. Two parallel trials were conducted using 15,000 P. splendida embryos obtained from the native cichlid reproduction facility at the Laboratorio de Acuicultura Tropical, División Académica de Ciencias Biológicas, Universidad Juárez Autónoma de Tabasco, Mexico (DACBIOL-UJAT). Once yolk reserves were absorbed (day 3 post fertilization), 7500 larvae were maintained in two 2500 L cylindrical tanks provided with a recirculating water system. The masculinization of larvae was determined using the methodology described by Vidal et al. (2009), where 2 g of Artemia cyst where hydrated for one hour, and washed with chlorine solution (4.5%) to eliminate the corion, finally decapsule cyst where placed in a 5 L flask with marine water (28 ups) for 24 h until hatch. After hatching, nauplii were collected with 200 µm mesh. Previously, a stocking solution (6.67 mg mL-1) of 17 α-methyltestosterone (MT) was prepared by diluting 500 mg of MT in 75 mL of 70% ethylic alcohol. Artemia nauplii (450 nauplii mL-1 of marine water) where washed with the MT solution (20 mg of MT L-1) in artificial marine water (28 ups) for two hours for 15 days, followed by a commercial trout feed (Argent Laboratories) added with the same steroid (20 mg of MT Kg-1 and 52 g kg-1 crude protein) for 30 additional days.
The other group of 7500 larvae was fed following the same schedule, but without esteriod inclusion, either in Artemia nauplii or trout feed. The number of transformed males obtained in each treatment was determined following the Squash method (Contreras, 2001). A 99% of males were recorded for the group fed MT, while 56% males were obtained in the group fed without MT.
Experimental design and conditions. Masculinized fish (initial body weight of 0.65 ± 0.1 g) and non-masculinized fish (initial body weight of 0.50 ± 0.1 g) were stocked at 50 fish per tank. The fish were randomly placed in a recirculating system with 30 tanks (70 L) connected to a 2500 L reservoir with compartments for organic matter sedimentation, an air-lift section, a biological filter, two titanium heathers PSA brand, model R9CE37I, a filtering system model JWPA5D-230A Star-rite brand and a UV light lamp Emperor Aquatics model 02025 of 25 Watts (Jiménez-Martínez et al., 2009).
Temperature and dissolved oxygen, were daily measured using a YSI model 55 oxymeter, Yellow Springs, Ohio, USA) and maintained at 32 ± 1 ºC and 6.40 ± 0.28 g L-1, respectively. Ammonia (0.41 ± 0.16 g L-1), nitrate (0.28 ± 0.15 g L-1) and nitrite (0.04 ± 0.03 g/L) concentrations (Strickland & Parsons, 1972) were weekly measured. Feed was provided manually four times per day (8:00, 11:00, 13:00 and 17:00 h) to apparent satiation.
Diet preparation. Five levels of dietary protein (35, 40, 45, 50 and 55% CP) were tested in each experiment. The semi-purified diets were formulated in the laboratory of aquaculture nutrition, CIBNOR, according to the MIXIT-WIN V.5 (Agricultural Software Consultants Inc., San Diego, CA, USA), and prepared in the Laboratorio de Acuicultura Tropical - UJAT-DACBIOL, Tabasco, Mexico according to Álvarez-González et al. (2001). Diets were prepared using casein (NZMP, New Zealand) as main source of protein. The nutrimental composition of diets for both trial were obtained from the ingredients and proximate chemical composition, which was conducted at the Laboratory of Bromatology, CIBNOR (Table 1) according to AOAC techniques (1995). CP was determined using the micro-Kjeldahl method (APHA, 1989) and crude lipids were extracted with anhydrous ether in a soxhlet extraction system (Soxtec Avanti 2050, Foss Tecator®, Copenhague, Denmark) following Bligh and Dyer (1959). Ash was determined with a muffled furnace (AOAC 1995), and crude fiber by the phenol-sulfuric acid method (Myklestad & Haug 1972). Gross energy of the diets was determined using an adiabatic pump calorimeter (Model 1 261, Parr, Moline, IL, USA).
Sampling and growth and food quality indexes. The experimental trials lasted 42 days and growth was evaluated measuring the individual wet weight and total length of the total population every two weeks. At the end of the experiment, survival rate was calculated counting the total fish per tank. The following growth and food quality index were evaluated using the following formulae:
Specific growth rate (SGR): [(ln final weight − ln initial body weight) / days] x 100.
Protein efficiency ratio (PER): (fish wet weight gain, (g)) / (protein intake, (g)).
Protein productive value (PPV): (final% body protein x final body weight) – (initial% body protein x initial body weight) / (total protein intake (g) x weight gain (g) x 100).
Weight gain (WG): [(final mean weight – initial mean body weight) / (final mean weight)] x 100.
Condition factor (CF): (final mean body weight / final mean body length3) x 100.
Feed conversion ratio (FCR): (feed intake, g dry matter) / (fish weight gain, (g)).
Survival (%): (final fish number / initial fish number) x 100.
Each index was calculated using the data on food consumption per tanks, which were estimated as the difference between provided feed and left-overs. Siphoning was used to remove and estimate the unconsumed feed two hours after feeding. At the beginning and at the end of the experimental period, 10 fish per tank were randomly selected and sacrificed with an overdose of the anesthetic un-buffered tricaine methansulfonate (MS-222). Then fish were washed with distilled water, frozen at -20 ºC and freeze-dried, to determine proximate composition of whole fish at the Laboratory of Bromatology at CIBNOR (La Paz, B.C.S., Mexico).
Statistical analysis. Biometrical data were previously verified for normality (Kolmogorov-Smirnov test) and variance homogeneity (Levine test) and then analyzed by a one-way ANOVA, followed by a Duncan test to determine statistical differences among protein values. Survival of fish was analyzed expressed as final percentage of surviving fish, and compared using the hypothesis test between the proportions of the two populations previously transformed arcsine for all combinations. The index for growth, food quality and chemical composition of whole fish were compared using a Kruskal-Wallis test; in cases of significant differences, the Nemenyi multiple comparison tests was used (Zar, 1996). The broken-line model was applied to determine the optimum protein requirement for bout trials. Mean wet weight (g) and protein requirement followed a lineal model Y = a + bX, using the mean weight as the response variable (Y), "a" is the initial weight; "b" is the growth rate and the protein level as the independent variable (X) (Robbins et al., 1979). All statistical analyses were conducted with STATISTICA v.8 (StatSoft, Tulsa, OK, USA) using a significance value of 0.05.
RESULTS
The highest growth response of masculinized fish, expressed in terms of AW and BL was obtained when feed included 45% CP (weight 3.31 ± 0.46 g, length 6.58 ± 0.10 cm) (Table 2) (Duncan, p = 0.0001), These fish also presented significant differences WG (403.41%) (Nemenyi, p = 0.002). No significant differences were detected for FCR, SGR and final survival (Table 3) (Nemenyi and Duncan, p = 0.12 and p = 0.07).
In the case of the non-masculinized fish, significantly greater growth was obtained when fed on the diet including 40% CP (2.60 ± 0.46 g, length 6.02 ± 0.13 cm) (Table 2) (Duncan, p = 0.0003). There were significant differences WG (398 ± 34.8%) (Nemenyi, p < 0.00001), where the maximum response was measured in fish fed with 40% CP dietary (Table 3). On the other hand, fish fed with the 45 and 55% CP diets presented the greatest PPV (28.91 and 29.21%, respectively) and the lowest FCR (1.23 and 1.08, respectively) (Nemenyi, p = 0.00001). No significant differences were recorded for survival among treatments (Table 3) (Duncan, p = 0.11).
Significant differences (Nemenyi, p = 0.0001) in CP content for masculinized fish were detected between initial fish (55.40%) and fish feed 35% CP diet (51.74%). For ether extract and ash body content significant differences were not detected (Nemenyi, p = 0.08). In non-masculinized fish neither significant difference were detected for any nutrient content in body composition (Table 4) (Nemenyi, p = 0.12).
Fitting the broken-line model to the mean wet weight data yielded significant results, and the optimum level of protein in the diet for masculinized juveniles was estimated at 45% (R2 = 0.93), while 44.8% (R2 = 0.75) was the optimum for non-masculinized fish (Fig. 1a and b).
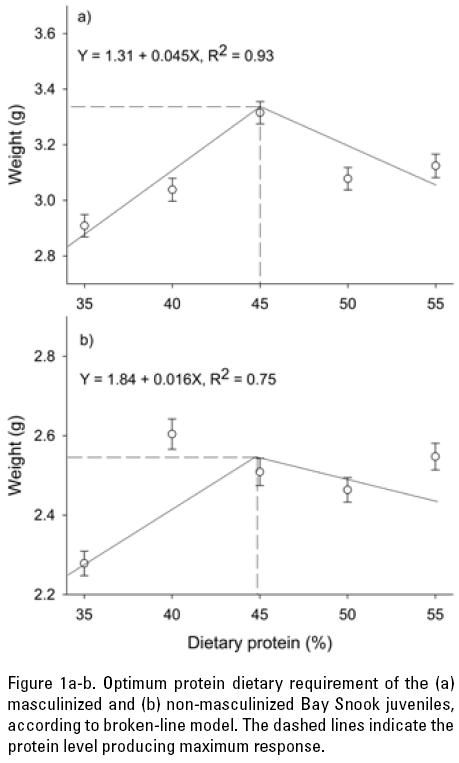
DISCUSSION
The highest growth recorded in this study for masculinized fish was obtained when P. splendida was fed with 45% CP, while in non-masculinized fish this was recorded when fed a diet between 40 to 55% CP. This high requirement of protein in P. splendida seems to be related to its carnivorous feeding habits (Resendez & Salvadores, 1983; Caro et al., 1994; Santiago et al., 1997; Valtierra & Schmitter, 2000).
The values of required protein obtained in the present experiments are similar to those reported for other species of similar feeding habits such as Japanese croaker N. japonica (45%; Moon et al., 2001), orange-spotted grouper (Epinephelus coioides Hamilton, 1822) (48%; Luo et al., 2004), bastard halibut (Paralichthys olivaceus Temminck & Schlegel, 1846) (46.4 to 51.2%; Lee et al., 2000; Kim et al., 2002, 2005), P. fulvidraco (42%; Kim & Lee, 2005), and mangrove red snapper (Lutjanus argentimaculatus Forsskål, 1775) (44%; Catacutan et al., 2001). This is in agreement with the reports of Koppe and Roem (1998), which indicate that carnivorous fish have a relatively high requirement of amino acids and require protein as a source of energy. Navas (1997) and Guzmán (2003) indicated that most carnivorous fish depend on a high content of dietary protein, since they have a low ability to digest and metabolize carbohydrates as energy source.
According to the results from the broken-line model, the dietary protein level supporting optimal growth of masculinized juveniles was 45% (44.8%, in the case of non-masculinized fish), which is slightly greater than the redhead cichlid (Vieja synspila Hubbs, 1935) (40.8%; Olvera-Novoa & Gasca-Leyva, 1996) and similar to the Mayan cichlid (Cichlasoma urophthalmus Günther, 1862) (45.3%; Martínez-Palacios et al., 1996). Fish fed on diets with CP content lower than the one producing maximum growth may be limiting due to a lower availability of some amino acids (Schuchardt et al., 2008); or in case of a weight loss due to the use of protein for maintaining the functions of vital tissues (Wilson, 2002). The optimal dietary protein level in fish diets is influenced by several factors: the optimal protein/energy ratio, the essential amino acid composition and digestibility of the test protein, as well as by the amount of non-protein energy sources in the diet (Meyer & Machado, 2004). In fact, although some nutritional studies tend to overestimate protein requirements as a result of an imbalanced amino acid composition or a low digestibility of the proteins used in the test diets (Ng et al., 2001). In other words, fish submitted to a lower energy content diet could be utilizing part of dietary protein as energy source. However, other studies have shown that if an excess of protein is supplied in the diet, only part of it is used to make body tissue protein, and the remainder will be metabolized as energy, without being effectively utilized for growth. This, in turn, results in increased ammonia and nitrogen excretion, which can deteriorate pond water quality and increase the cost of the of feeding (Vergara et al., 1996; Lovell, 1998; Kim et al., 2002; Zhou et al., 2007), however our water quality was not affected because we use a recirculating system for bout trials. This problem was overcome in the present study by using high-quality purified ingredients such as dextrin, gelatin and casein, which are widely use to determine protein requirements in aquatic organism because they are highly digestible and allow to get the maximum control of protein source (Lovell, 1998; Siccardi III et al., 2006) and supported for the low ammonia, nitrate and nitrite concentrations during the experiment (0.41 ± 0.16 g L-1, 0.28 ± 0.15 g L-1, and 0.04 ± 0.03 g L-1 respectively).
The maximum SGR was 1.67%/day for masculinized fish and at 1.69%/day for non-masculinized fish. These values are lower than those recorded for other species such as catfish (Clarias gariepinus Burchell, 1822) of 3.24%/day (Ali & Jauncey, 2005) and blue discus (Symphysodon aequifasciatus Pellegrin, 1904) of 1.84%/day (Chong et al., 2000). The PER values (masculinized: 2.46 and non-masculinized: 1.80) are greater here than those reported by Luo et al. (2004) for E. coioides juveniles (1.08), Lee et al. (2003) for Scophthalmus maximus juveniles (2.18) and Ng et al. (2001) for Asian redtail catfish (Mytus nemurus Valenciennes, 1840) juveniles (1.17). These differences may be explained by a number of factors: species, genetic selection, size, sex, age, nutritional requirement, initial fish weight, and handling during rearing (Jussila, 1997; Cruz-Suárez et al., 2002).
The FCR in the masculinized (1.10) and non-masculinized (1.08) P. splendida juveniles presented the typical inverse relationship between protein level and food consumption, where the most adequate diet for growth requires a smaller amount of feed to produce a unit of WG (Mohanty & Samantaray, 1996; Koppe & Roem, 1998). Increases in dietary protein levels increase fish growth, however, if the optimum level of protein is exceeded, the growth rate will remain constant or will decrease, since part of the dietary energy will be used to excrete the excess amino acids or the amino groups, and part will be used in lipid anabolism resulting in an accumulation of lipids in muscle and deformation in mesenteric tissue (Swann et al., 1994; Civera et al., 2002).
Protein level in the monosex group of P. splendida juveniles had a significant effect on development, with a greater WG of the males (Table 3) in comparison with mixed populations (males and females), which indicate that the response in WG to increasing dietary protein levels differed significantly between masculinized and non-masculinized fish. Particularly, the maximum growth (broken-line model) indicated that growth response in masculinized fish was greater with a lower protein requirement (45%) compared with the non-masculinized fish (44.8%) This is in agreement with Pandian and Sheela (1995) who found that adding steroids to feed, especially androgens, along with inducing sexual inversion, stimulate an accelerated growth as a result of their anabolic characteristics, which has been reported for tilapia (Blázquez et al., 1995; Fitzsimmons, 1997; Gale et al.,1999). Also it avoids non-desired reproductive effort that might cause a potentially negative impact in aquacultural system. Thus, feed components and energy are focused to body increasing in muscle mass and size, in order to avoid longer rearing times, over-population in ponds, and lower production (Green et al., 1997; Salgado et al., 1997). Sheehan et al. (1999) and Bastardo et al. (2003) found that mono-sex (females) populations of Oncorhyncus mykiss presented a greater yield in weight compared to mixed-sex populations.
On the other hand, a general trend to increase lipids and reduce protein in body composition was related to the increase in dietary protein in P. splendida, which has been associated to the quality of the diet and their ingredients. In this sense, when the protein is reduced a greater amount of food is required to satisfy the lack of that element. An increment in fat content is observed when the protein is exceeded, leading to low protein efficiency (Hepher, 1993). Lee et al. (2000) and Kim et al. (2002) recorded with Paralichthys olivaceus a lower percentage of protein in the fish body when fish were fed with a dietary with low level of protein. Serrano (2004) reported the accumulation of lipids in O. mykiss when the protein was in excess. In this sense, several factors may be explained when protein requirement is excessed, for example, when food is provided to apparent satiation with a higher protein content, the body fat increases and a low growth could be obtain (Abdelghany, 2000; Menghe et al., 2003). Additionally, there is a direct relationship between the protein and energy (protein sparing) when other sources of energy are use such as lipids. This balance should be carefully determined to avoid an excess of lipid deposited in tissues (Hepher et al., 1971; Hepher, 1993; Gómez et al., 1993; Shearer, 1994; Burel et al., 1998; Glencross et al., 2002; Serrano, 2004).
In conclusion, this study indicates that optimum protein level for masculinized and non-masculinized P. splendida juveniles is the same (45% CP). In this sense, mono-sex treatment in P. splendida showed an effect on weight gain compared with mix population.
ACKNOWLEDGEMENTS
The authors thank CONACyT for support through the grant program registered under No. 177248, Funding for this research was provided by the Aquaculture Collaborative Research Support. Program accession number # 1372. The Aquaculture CRSP is funded in part by United States Agency for International Development (USAID) Grant No. LAG-G-00-96-90015-00 and by participating institutions. The opinions expressed herein are those of the author(s) and do not necessarily reflect the views of the US Agency of International Development.
REFERENCES
Abdelghany, A. E. 2000. Replacement value of cystine for methionine in semi purified diets supplemented with free amino acids for the Nile tilapia, Oreochromis niloticus. L. In: K., Fitzsimmons (Eds.). Proceedings from the Fifth International Symposium on Tilapia Aquaculture, Rio de Janeiro, Brazil. pp. 109-119. [ Links ]
Akiyama, D. 1995. Nutrición, alimentos y alimentación de los peces. Soyanoticias 2: 22-25. [ Links ]
Ali, M. Z. & K. Jauncey. 2005. Approaches to optimizing dietary protein to energy ratio for African catfish Clarias gariepinus (Burchell, 1822). Aquaculture Nutrition 11: 95-101. [ Links ]
Alvarez-González, C.A., R. Civera-Cerecedo, J.L. Ortiz-Galindo, S. Dumas, M. Moreno-Legorreta & T. Grayeb-Del Alamo. 2001. Effect of dietary protein level on growth and body composition of juvenile spotted sand bass, Paralabrax maculatofasciatus, fed practical diets. Aquaculture 194: 151-159. [ Links ]
AOAC (Association of Official Analytical Chemists). 1995. Official methods of analysis of the association of official analytical chemists. Vol. I. 16th ed. Washington, DC, USA. 1244 p. [ Links ]
APHA. 1989. Standard Methods for the Examination of Water and Wastewater, 14th ed. American Public Health Association, Washington, DC, USA. 1325 p. [ Links ]
Barroso, B. J., S. L. García, J. Peregon, M. de La Higuera & A. J. Lupiañez. 1994. The influence of dietary protein on the kinetics of NADPH production systems in various tissues of rainbow trout Oncorhynchus mykiss. Aquaculture 124: 47-59. [ Links ]
Bastardo, H., R. B. Sara & B. Sofía. 2003. Growth of all-female and mixed sex trout in a Venezuelan fish hatchery. Zootecnia Tropical 21: 17-26. [ Links ]
Blazquez, M., F. Piferrer, S. Zanuy, M. Carrillo & E. M. Donaldson. 1995. Development of sex control techniques for European sea bass (Dicentrarchus labrax L.) aquaculture: effects of dietary 17-α methyltestosterone prior to sex differentiation. Aquaculture 135: 329-342. [ Links ]
Bódis, M., B. Kucska & M. Bercsényi. 2007. The effect of different diets on the growth and mortality of juvenile pikeperch Sander lucioperca in the transition from live food to formulated feed. Aquaculture International 15: 83-90. [ Links ]
Burel, C., T. Boujard, G. Corraze, S. Kaunshik, G. Boeuf, K. Mol, S. Van Der Geyte & E. Kühn. 1998. Incorporation of high levels of extruded lupin in diets for rainbow trout Oncorhyncus mykiss: nutritional value and effect on thyroid status. Aquaculture 163: 323-343. [ Links ]
Caro, C. C., A. A. Mendoza & C. M. Sánchez. 1994. Caracterización del ambiente de la Petenia splendida en las lagunas del sur de Quintana Roo. In: E. Mendoza, T. A. Galmiche & E. R. Meseguer (Eds.). Memoria de II Seminario sobre Peces Nativos con Uso Potencial en Acuacultura. H. Cárdenas, Tabasco, México. pp 1-12. [ Links ]
Catacutan, M. R., G. E. Pagador & S. Teshima. 2001. Effect of dietary protein and lipid levels and protein to energy ratios on growth, survival and body composition of the mangrove red snapper, Lutjanus argentimaculatus (Forsskal, 1775). Aquaculture Research 32: 811-818. [ Links ]
Chan R. 2004. Efecto de la temperatura sobre el consumo de oxígeno en tenguayaca (Petenia splendida Günther 1862). Tesis de Licenciatura. División Académica de Ciencias Biológicas, Universidad Juárez Autónoma de Tabasco, Mexico. 72 p. [ Links ]
Chávez, L. M. O., A. E. Mattheeuws & V. H. Pérez. 1989. Biología de peces del río San Pedro en vista de determinar su potencial para la piscicultura. Instituto Nacional de Investigación. Recursos Bióticos. Xalapa Veracruz, México. 222 p. [ Links ]
Cho, S. H., S. M. Lee & J. H. Lee. 2005. Effect of dietary and lipid levels on growth and body composition of juvenile turbot Scophthalmus maximus L reared under optimum salinity and temperature conditions. Aquaculture Nutrition 11: 235-240. [ Links ]
Chong, S. C. A., R. Hashim & A. B. Ali. 2000. Dietary protein requirements for discus Symphysodon spp. Aquaculture Nutrition 6: 275-278. [ Links ]
Civera-Cerecedo, R., J. L. Ortiz-Galindo, S. Dumas, H. Nolasco-Soria, C. A. Alvarez-González, B. Anguas, R. Peña, M. Rosales, V. Carrasco, R. García & E. Goytortúa. 2002. Avances en la nutrición de la cabrilla arenera Paralabrax maculatofasciatus. In: L. E. Cruz-Suárez, D. Ricque-Marie, M. Tapia-Salazar, M. G. Gaxiola-Cortés & N. Simoes (Eds.). Avances en Nutrición Acuícola VI. Memorias del VI Simposium Internacional de Nutrición Acuícola. Cancún, Quintana Roo, México. pp. 352-406. [ Links ]
Contreras, W. M. 2001. Sex determination in Nile tilapia, Oreochromis niloticus: gene expression, masculinization methods, and environmental effects. Tesis de Doctorado. Oregon State University. USA. 193 p. [ Links ]
Cruz-Suárez, E., M. D. Ricque, M. Tapia-Salazar, L. F. Martín-Saldivar, B. C. Guajardo, M. Nieto-López & A. Salinas-Miller. 2002. Historia y estatus actual de la digestibilidad y algunas características fisicoquímicas de los alimentos comerciales para camarón usados en México. In: L. E. Cruz Suárez, D. Ricque Marie, M. Tapia-Salazar, M. G. Gaxiola-Cortes & N. Simoes (Eds.). Avances en Nutrición Acuícola VI. Memorias del VI Simposium Internacional de Nutrición Acuícola. Cancún, Q. R. México. pp. 1-22. [ Links ]
De La Higuera, M. 1987. Requerimientos de proteínas y aminoácidos en peces: Nutrición en Acuacultura II. In: A. Espinosa & A. Labarta (Eds.). CAICYT. Madrid. España. pp. 53-98. [ Links ]
Domínguez-Cisneros, S. & R. Rodiles-Hernández. 1998. Guía de Peces del Río Lacanja, Selva Lacandona, Chiapas, México. ECOSUR, México. 69 p. [ Links ]
Ferreira, N.A. & N.G. Gómez. 1994. Estado actual del conocimiento sobre los cíclidos nativos de los cuerpos de agua epicontinentales del estado de Quintana Roo. In: Memoria IV Congreso Nacional de Ictiología, Morelia, Michoacán, México. 21 p. [ Links ]
Fitzsimmons, K. 1997. Introduction to tilapia sex-determination and sex reversal. In: K. Fitzsimmons (Ed.). Proceedings from the Fourth International Symposium on Tilapia in Aquaculture, Vol. 2. Orlando, Florida, USA. 37 p. [ Links ]
Gale, L.W., M.S. Fitzpatrick, M. Lucero, W.M. Contreras-Sánchez & C.B. Schreck. 1999. Masculinization of Nile tilapia (Oreochromis niloticus) by immersion in androgens. Aquaculture 178: 349.357. [ Links ]
Gamboa, P. H. C. & S. J. J. Schmitter. 1997. Distribución de las mojarras (Perciformes: Cichlidae) de la Laguna Bacalar, Quintana Roo. In: R.G. Ramírez (Ed.). Memoria V Congreso Nacional de Ictiología. Mazatlán, Sinaloa, México. 21 p. [ Links ]
García, M.A. 2003. Determinación de la temperatura preferencial y metabolismo de la rutina de la tenguayaca (Petenia splendida Günther, 1862). Tesis de Licenciatura, División Académica de Ciencias Biológicas, Universidad Juárez Autónoma de Tabasco, México. 42 p. [ Links ]
Glengross, B., J. Curnow, W. Hawkins & M. Felsing. 2002. Evaluation of yellow lupin Lupinus lateus meal as an alternative protein resource in diets for sea cage reared rainbow trout Oncorhynchus mykiss. Journal of the World Aquaculture Society 33: 287-296. [ Links ]
Gomez, E., G. Corraze & S. Kaushik. 1993. Effect of dietary incorporation of a co-extruded plant protein (rapeseed and peas) on growth, nutrient utilization and muscle fatty acid composition of rainbow trout (Oncorhyncus mykiss). Aquaculture 113: 339-353. [ Links ]
Green, B. W., K. L. Veverica & M. S. Fitzpatrick. 1997. Fry and Fingerling Production. In: H. Egna & C. Boyd. (Eds.). Dynamics of Pond Aquaculture. CRC Press, Boca Raton, Florida, USA. 39 p. [ Links ]
Guzmán, C. V. 2003. Efecto de diferentes niveles de proteínas y lípidos de las dietas en el crecimiento de adultos de Galaxias maculatus (Jenyns, 1842). Tesis de Licenciatura, Ciencias de la Acuacultura. Universidad Católica de Temuco, Chile. 84 p. [ Links ]
Hepher, B. 1993. Nutrición de peces comerciales en estanques. Editorial LIMUSA, México. 406 p. [ Links ]
Hepher, B., J. Chervinski & H. Tagari. 1971. Studies on carp nutrition III. Experiments on the effect on fish yields of dietary protein source and concentration. Bamidgeh 23: 11-37. [ Links ]
Jiménez-Martínez, L. D., C. A. Álvarez-González, W. M. Contreras-Sánchez, G. Márquez-Couturier, L. Arias-Rodríguez & J. A. Almeida-Madrigal. 2009. Evaluation of larval growth and survival in Mexican mojarra, Cichlasoma urophthalmus and bay snook, Petenia splendida under different initial stocking densities. Journal of the World Aquaculture Society 40: 753-761. [ Links ]
Jussilaa, J. 1997. Physiological responses of astacid and parastacid crayfishes (Crustacea: Decapoda) to conditions of intensive culture. Kuopio University Publications C. Natural and Environmental Sciences. Perth, Western Australia. 140 p. [ Links ]
Kang, W. K., J. K. Yong & M. C. Se. 2005. Optimum dietary protein levels and protein to energy ratios in olive flounder Paralichthys olivaceus. Journal of the World Aquaculture Society 36: 165-178. [ Links ]
Kim, L.O. & S.M. Lee. 2005. Effects of the dietary protein and lipid levels on growth and body composition of bagrid catfish, Pseudobagrus fulvidraco. Aquaculture 2: 323-329. [ Links ]
Kim, J. D., S.P. Lall & J.E. Milley. 2001. Dietary protein requirements of juvenile haddock (Melanogrammus aeglefinus L.). Aquaculture Research 32: 1-7. [ Links ]
Kim, K.W., Y. J. Kang, S. M. Choi, X. Wang, Y. H. Choi, S. C. Bai, J. Y. Jo & J. Y. Lee. 2005. Optimum dietary protein levels and protein to energy ratios in olive flounder Paralichthys olivaceus. Journal of the World Aquaculture Society 36: 165-178. [ Links ]
Kim, K. W., X. J. Wang & S. C. Bai. 2002. Optimum dietary protein level for maximum growth of juvenile olive flounder Paralichthys olivaceus (Temminck et Schlegel). Aquaculture Research 33: 673-679. [ Links ]
Koppe, W. & A. Roem. 1998. Variación del perfil de aminoácido en harina de pescado. En Profundidad 243: 18-19. [ Links ]
Lee, J. K., S. H. Cho, S. U. Park, K. D. Kim & S. M. Lee. 2003 Dietary protein requirement for young turbot (Scophthamus maximus L.). Aquaculture Nutrition 9: 283-286. [ Links ]
Lee, S. M., S. H. Cho & K. D. Kim. 2000. Effects of dietary protein and energy levels on growth and body composition of juvenile flounder Paralichthys olivaceus. Journal of the World Aquaculture Society 31: 306-315. [ Links ]
Lovell, R. T. 1998. Nutrition and Feeding of Fish. Van Nostrand Reinhold, New York. 284 p. [ Links ]
Luo, Z., Y. J. Liu, K. S. Mai, L. X. Tian, & X. Y. Tan. 2004. Optimal dietary protein requirement of grouper Epinephelus coioides juveniles fed isoenergetic diets in floating net cages. Aquaculture Nutrition 10: 247-252. [ Links ]
Martínez, M. J. L. 2004. Desarrollo embrionario y larval de la mojarra tenguayaca Petenia splendida. Tesis de Licenciatura. División Académica de Ciencias Biológicas, Universidad Juárez Autónoma de Tabasco, México. 72 p. [ Links ]
Martínez-Palacios, C. A., M. Harfush-Melendez & C. Chavez-Sanchez. 1996. The optimum dietary protein level for the Mexican cichlid Cichlasoma urophthalmus (Günther): a comparison of estimates derived from experiments using fixed-rate feeding and satiation feeding. Aquaculture Nutrition 2: 11-20. [ Links ]
Menghe, H. L., B. M. Bruce, H. R. Edwin & G. B. Brian. 2003. Effect of dietary protein concentration and stocking density on production characteristics of pond-raised channel catfish Ictalurus puntactus. Journal of the World Aquaculture Society 34: 147-155. [ Links ]
Meyer, G. & F. D. Machado. 2004. Protein requirement of jundia fingerlings Rhamdia quelen at two dietary energy concentrations. Aquaculture 240: 331-343. [ Links ]
Mohanty, S. S. & K. Samantaray. 1996. Effect of varying levels of dietary protein on growth performance and feed conversion efficiency of snakehead Channa striata fry. Aquaculture Nutrition 2: 89-94. [ Links ]
Moon, L. H., K. C. Cho, J. E. Lee & S. G. Yang. 2001. Dietary protein requirement of juvenile giant croaker Nibea japonica Temminck & Schlegel. Aquaculture Research 32: 112-118. [ Links ]
Myklestad, S. & A. Haug. 1972. Production carbohydrates by the marine diatom Chaetoceros affini var. wille (Gran) Husted: I. Effect of the concentration of nutrients in the culture medium. Journal of Experimental Marine Biology and Ecology 9: 125-136. [ Links ]
NRC (National Research Council). 1993. Nutrient Requirements of Fish. National Academy Press, Washington, D.C., USA. 128 p. [ Links ]
Navas, J. 1997. Efecto del contenido lipídico de las dietas administradas a adultos de lubina (Dicentrachus labrax L.) sobre el proceso reproductor y sobre la calidad y composición de los huevos. In: Memoria Departamento de Biología Animal de la Universidad de Valencia. España. 67 p. [ Links ]
Ng, W. K, S. C. Soon & R. Hashim. 2001. The dietary protein requirement of a bagrid catfish Mystus nemurus (Curvier & Valenciennes), determined using semipurified diets of varying protein level. Aquaculture Nutrition 7: 45-51. [ Links ]
Ng, N. K., P. K. Lim & P. L. Boey. 2003. Dietary lipid and palm oil source affects growth, fatty acid composition and muscle α-tocopherol concentration of African catfish Clarias gariepinus. Aquaculture 215: 229-243. [ Links ]
Olvera-Novoa, M. A. & E. Gasca-Leyva. 1996. The dietary protein requirements of Cichlasoma synspilum Hubbs, 1953 (Piscis: Cichlidae) fry. Aquaculture Research 27: 167-173. [ Links ]
Pandian, T. J. & S. G. Sheela. 1995. Hormonal induction of sex reversal in fish. Aquaculture 138: 1-22. [ Links ]
Peresa, H. & A. Oliva-Teles. 2005. The effect of dietary protein replacement by crystaline amino acid on growth and nitrogen utilization of turbot Scophthalmus maximus juveniles. Aquaculture 250: 755-764. [ Links ]
Real-Ehuan, G. 2003. Masculinización de crías de mojarra castarrica Cichlasoma urophthalmus mediante la administración de 17-α Metiltestosterona, Tesis de Licenciatura. División Académica de Ciencias Biológicas, Universidad Juárez Autónoma de Tabasco, México. 57 p. [ Links ]
Reséndez, M. A. & V. M. L. Salvadores. 1983. Contribución al conocimiento de la biología del pejelagarto Lepisosteus tropicus (Gil) y la tenguayaca Petenia splendida Günter del Estado de Tabasco. Biótica 8: 413-426. [ Links ]
Robbins, K. R., H. W. Norton & D. H. Baker. 1979. Estimation of nutrient requirements from growth data. Journal of Nutrition 109: 1710-1714. [ Links ]
Salgado, Z. H., P. E. Maya, H. S. Marañon & E. D. Martínez. 1997. Empleo de hormonas en la producción de peces de ornato como estrategia alternativa en México. In: R.G. Ramírez (Ed.). Memoria V Congreso Nacional de Ictiología. Universidad Autónoma de Sinaloa. Mazatlán, Sinaloa, México. 46 p. [ Links ]
Santiago, L. M. C., J. Jardono, S. G. Jaramillo, A. J. E. Reyes & V. A. Sánchez. 1997. Edad, crecimiento y hábitos alimenticios de Cichlasoma salvini (Günter), Cichlasoma urophthalmus (Günter), Oreochromis niloticus (Linneo) y Petenia splendida (Günter) en la presa Miguel de la Madrid H. "Cerro de Oro" Tuxtepec, Oaxaca. In: R. G. Ramírez (Ed.). Memoria V Congreso Nacional de Ictiología. Universidad Autónoma de Sinaloa. Mazatlán, Sinaloa, México. 23 p. [ Links ]
Schuchardt, D., J. M. Vergara, H. Fernández-Palacios, C. T. Kalinowski, C. M. Hernández-Cruz, M. S. Izquierdo & L. Robaina. 2008. Effects of different dietary protein and lipid levels on growth, feed utilization and body composition of red porgy (Pagrus pagrus) fingerlings. Aquaculture Nutrition 14: 1-9. [ Links ]
Serrano, G. E. 2004. Reemplazo parcial de harina de pescado por harina de lupino blanco (Lupinus albus) en dietas extruidas para trucha arcoiris (Oncorhynchus mykiis): efectos sobre los índices productivos y la composición de ácidos grasos en el músculo. Tesis de Licenciatura. Universidad Católica de Temuco, Chile. 63 p. [ Links ]
Shearer, K. D. 1994. Factor affecting the proximate composition of cultured fishes with emphasis on salmonids. Aquaculture 119: 63-88. [ Links ]
Sheehan, R., P. S. Scott, V. S. Arul, A. R. Kapuscinski & J. E. Seeb. 1999. Better growth in all-female diploid and triploid rainbow trout. Transaction of American Fishery Society 128: 491-498. [ Links ]
Siccardi III, A. J., A. L. Lawurence, D. M. Gatiln III, J. M. Fox, F. L. Castille, M. Perez-Velazquez, M. L. González-Félix. 2006. Digestibilidad aparente de energía, proteína y materia seca de ingredientes utilizados en alimentos balanceados para el camarón blanco del Pacífico Litopenaeus vannamei. In: Cruz Suárez, L. E., D. Ricque Marie, M. Tapia Salazar, M. G. Nieto López, D. A. Villarreal Cavazos, A. C. Puello Cruz & A. García Ortega. Avances en Nutrición Acuícola VIII. VIII Simposio Internacional de Nutrición Acuícola. 5-17 de Noviembre de 2006. San Nicolás de los Garza, N. L. Universidad Autónoma de Nuevo León, Monterrey, Nuevo León, México. pp. 213-237. [ Links ]
Soler, J. M del P., G. H. Rodríguez & D. P. Victoria. 2000. Fundamentos de nutrición y alimentación en acuacultura. Hollman Echeverry Cubillos. Colombia. 342 p. [ Links ]
Strickland, J. D. H. & T. R. Parsons. 1972. A practical handbook of seawater analysis. Fisheries Research Board of Canada, Ottawa, Canada. 311 p. [ Links ]
Swann, D. L., J. R. Riepe, J. D. Stanley, M. E. Griffin & P. B. Brown. 1994. Cage culture of hybrid striped bass in Indiana and evaluation of diets containing three levels of dietary protein. Journal of the World Aquaculture Society 25: 281-288. [ Links ]
Valtierra, V. M. T. & S. J. J. Schmitter. 2000. Hábitos alimentarios de las mojarras (Perciformes: Cichlidae) de la laguna Caobas, Quintana Roo, México. Revista de Biología Tropical 48: 2-3. [ Links ]
Vergara, J. M., H. F. Palacios, L. Robaina, K. Jauncey, M. De La Higuera & M. S. Izquierdo. 1996. The effects of varying dietary protein level on the growth feed efficiency, protein utilization and body composition of gilthead sea bream fry. Fishery Science 62: 620-623. [ Links ]
Vidal, J. M., C. A. Álvarez-González, W. M. Contreras-Sánchez, & U. Hernández-Vidal. 2009. Masculinización del cíclido nativo Tenhuayaca, Petenia splendida (Gunther, 1862), usando nauplios de Artemia como vehículo del esteroide 17-α metiltestosterona. Hidrobiológica 19: 211-216. [ Links ]
Wang, Y., J. L. Guo, K. Li. & P. D. Bureau. 2006. Effects of dietary protein and energy levels on growth, feed utilization and body composition of cuneate drum (Nibea miichthioides). Aquaculture 252: 421-428. [ Links ]
Wilson, R. P. 2002. Amino acids and proteins. In: Halver, J. E., Hardy, R. W. (Eds.). Fish Nutrition, 3rd ed. Academic Press, New York, USA. pp. 143-179. [ Links ]
Zar, J. H. 1996. Biostatical Analysis. Third edition. Prentice Hall, New York, USA. 929 p. [ Links ]
Zhou, J. B., Q. C. Zhou, S. Y. Chi, Q. H. Yang & Ch. W. Liu. 2007. Optimal dietary protein requirement for juvenile ivory shell, Babylonia areolate. Aquaculture 270: 186-192. [ Links ]














