Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Hidrobiológica
versão impressa ISSN 0188-8897
Hidrobiológica vol.22 no.2 Ciudad de México Mai./Ago. 2012
Artículos
Common reed (Phragmites australis) harvest as a control method in a Neotropical wetland in Western México
Cosecha de carrizo (Phragmites austialis) como método de control en un humedal del occidente de México
Yazmín Escutia-Lara,1 Sabina Lara-Cabrera,2 Mariela Gómez-Romero3 and Roberto Lindig-Cisneros3
1 Doctorado institucional en Ciencias Biológicas, Universidad Michoacana de San Nicolás de Hidalgo, Ciudad Universitaria, Morelia, Michoacán, 58030. México.
2 Laboratorio de Sistemática Molecular de Plantas, Facultad de Biología, Universidad Michoacana de San Nicolás de Hidalgo. Ciudad Universitaria, Morelia, Michoacán.
3 Laboratorio de Ecología de Restauración, Centro de investigaciones en Ecosistemas, Universidad Nacional Autónoma de México. Apartado Postal 27, Admón. 3, Santa María, Morelia, Michoacán, 58091. México. E-mail: rlindig@oikos.unam.mx
Recibido: 06 de junio de 2011.
Aceptado: 04 de mayo de 2012.
ABSTRACT
Common reed (Phragmites australis) has invaded wetlands worldwide and displaced native vegetation and wildlife. Control measures include herbicides, but their use can cause negative environmental impacts. An alternative is to harvest aerial biomass. We tested harvest as a control for common reed in Mintzita springs, Michoacán in Western Mexico. Results showed that harvest increased native plant species establishment, and that species richness varied with harvesting method. In plots where reed was completely removed every two months, 9 native species established, the same number as in plots where all reed biomass was removed if at least one stem was 2 m tall at the harvest date. When only reed stems 2m or taller where removed, 6 species established, whereas, in control plots only three species established. Species composition correlated with harvesting method (ANOSIM, R=0.4514, p < 0.01). Harvest reduced resprouting measured as standing biomass (F(3,20) = 27, p < 0.000001). After one year of treatment, full removal plots had the lowest aerial dry biomass (108 ± 15 g) followed by plots with full removal once a reed stem was at least 2m tall (197 ± 81 g), followed by plots where only 2 m or taller stems were removed (593 ± 466 g) and control (3296 ± 232 g). Several reed plants died after the first year of the experiment. Although more trials and long term follow up are needed, our results suggest that harvest can be an efficient control method for reed-infested wetlands in Western Mexico.
Key words: Herbicide, native species, removal, restoration, water supply.
RESUMEN
El carrizo (Phragmites australis) invade humedales a nivel mundial y desplaza a especies nativas. Se controla con herbicidas pero ésto puede causar impactos ambientales negativos. Una alternativa es la cosecha de la biomasa aérea, la cual se probó en los humedales del manantial de la Mintzita, Michoacán, en el occidente de México. La cosecha incrementa el establecimiento de especies nativas, pero la riqueza de especies varía en función del método de cosecha. En parcelas en donde el carrizo se removió completamente cada dos meses, 9 especies nativas se establecieron, el mismo número que en parcelas en donde se removió el carrizo cuando un tallo alcanzara los 2 m de altura. En parcelas en donde sólo se removieron tallos de dos metros de altura o más, se establecieron 6 especies, y sólo tres en las parcelas control. La composición de especies y la capacidad de retoñar del carrizo (F(3,20) = 27, p < 0.000001), se correlacionaron con el método de cosecha. Después de tan sólo un año, las parcelas de remoción total presentaron la menor biomasa (108 ± 15 g) seguida por la remoción total cuando un tallo alcanzara los dos metros de altura (197 ± 81 g), el tratamiento de remoción de tallos > 2 m (593 ± 466 g) y el control (3296 ± 232 g). Aunque se requieren ensayos de largo plazo en sitios invadidos, estos resultados sugieren que la cosecha de carrizo puede ser una medida de control eficiente en humedales invadidos en el occidente de México.
Palabras clave: Especies nativas, fuente de agua, herbicida, remoción, restauración.
INTRODUCTION
Plant invasions displace native species, alter key ecosystem functions and are a main cause of biodiversity decline (Mack etal., 2000). In wetlands, most invasions are fostered by human induced changes in disturbance regimes and nutrient loads (Alpert et al., 2000; D'Antonio, 1993; Thompson etal., 2001). Once established, invasive plant species can alter environmental conditions and the intensity and nature of biotic interactions (D'Antonio & Vitousek, 1992; Goldberg, 1990; Gordon, 1998; Vitousek etal., 1987), resulting in dramatic changes in the composition of native communities (Howard & Goldberg, 2001; Reader& Bonser, 1993).
Common reed (Phragmites australis Cav. Trin ex. SteudJ is a coarse perennial grass with a worldwide distribution. It is common in brackish and freshwater wetlands (Gleason & Cronquist, 1963) especially in disturbed habitats along water bodies (Ailstock etal, 2001Marks et al., 1994; Saltonstall, 2002). It has been suggested that this species turns invasive after human induced changes in disturbance regimes, particularly changes in hydrological and nutrient regimes as well as salinity (Marks et al., 1994).
Our experimental area, the Mintzita wetland complex, is located to the south of the city of Morelia, the capital of the state of Michoacán, México. The dominant species are cattail (Typha domingensis Presl.) and chairmaker's bulrush (Schoenoplectus americanus (Pers.) Volkart ex Schinz et Keller). Two major impacts on the wetlands are nitrogen inputs from the watershed and phosphorus release from fires (Escutia-Lara et al., 2009). In recent years, P. australis has increased its cover in the wetlands, displacing native vegetation and altering hydrological regimes. Elsewhere, after invasion by P. australis, control measures were necessary to reduce its negative effects on natural wetland communities (Ailstock et al., 2001). The Mintzita springs provides water for 300,000 inhabitants of Morelia and conservation and restoration of the area is required, including P. australis control.
Phragmites australis control has been done by applying herbicides, mostly glyphosate, to affected areas (Back & Holomuzki, 2008; Mozdzer et al., 2008; Turner & Warren, 2003), but use of glyphosate can have the undersired environmental impact of causing damage to non target species (Tsiu & Chu, 2007). Furthermore, glyphosate, is no longer considered completely safe for humans (Williams et al., 2000), because in recent studies it has been suggested that long term exposure to some formulations might pose risks to human health (Romano et al., 2010). Since there are no official guidelines or norms regarding the use of herbicides in Mexican wetlands, and to avoid potential hazards to human populations, information on alternative reed control methods is needed. This is especially true for wetlands that provide drinking water, such as the Mintzita springs, that provide up to 40% of the water of the city of Morelia. One alternative control measure is the removal of aerial biomass, because eliminating the photosynthetic tissues of the plant can reduce posterior growth and spread of the species (Asaeda & Karunaratne, 2000). In some areas, harvesting during late summer has no effect because reserves have already accumulated in plant tissues (Husak ,1978), whereas harvesting after winter, when the plant has already sprouted, can have a considerable effect because the plant has low reserves (Karunaratne et al., 2004), also continuous harvesting can significantly reduce P. australis biomass (Asaeda & Karunarante, 2000; Cizkova et al., 1996; Rajapakse et al., 2006; Van der Putten et al., 1997). Therefore, it is necessary to determine the optimal harvesting regime depending on regional conditions (Ostendorp, 1995; Rajapake et al., 2006). We compared the effect of four harvesting treatments in an area invaded by P. australis within Mintzita springs on native species establishment, in order to assess its management potential. Harvesting treatments simulated existing practice. Traditionally, stems are used for handcrafts, as well as for other purposes. Usually, only stems 2 m or taller are harvested (Gerritsen et al., 2009), but removal of all stems can also be observed (Lindig-Cisneros, pers. obs.). Therefore, harvest of reed aerial biomass is socially accepted and provides an economic benefit.
MATERIALS AND METHODS
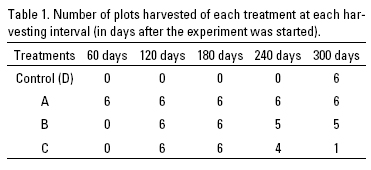
An area invaded by P. australis was selected within the Mintzita wetlands (101°17'47" W, 19°38'43" N) for a harvesting experiment in 2009. The area has been studied for the last 5 years by biannual plant composition sampling of 30, 1 m2 permanent plots across the water depth gradient (Escutia-Lara et al., 2009). In our study site, P. australis grows all year round, although growth peaks during summer and slows from October to February, when some leaves senesce and isolated stems die. Within a P. australis invaded area where the water level did not varied significantly and was between soil level and 10 cm above it, all live and dead stems were removed at the beginning of the 2009 growing season (February), cut stems were not submerged after removal. Four harvesting treatments were chosen: 1) harvesting all stems every 60 days, 2) harvesting only stems that were 2 m or taller every 60 days, 3) when one stem reached 2 m tall harvesting all stems every 60 days, 4) and a no harvesting treatment as control (Table 1). Each treatment had 6 replicates, each in one 1 m2 plot. Plots were placed at random leaving 50 cm between plots. Plant species cover by plot was recorded as percentage every 30 days, by dividing each plot in square decimeters and recording presence-absence in each, for a year and P. australis stem height was also evaluated by measuring all stems in the plots.
Phragmites australis response to the experimental treatments was analyzed by means of ANOVA with R software (R. Development Core Team, 2010), for all tests compliance with assumptions of homogeneity of variances and normality was checked. Species composition as affected by the treatments was tested with ANOSIM on a Bray-Curtis distance matrix (Manly, 2000) using vegan in R (Oksanen et al., 2009). Comparison of species composition in reed-invaded plots with the composition of not-invaded plots within the same water depth area of the wetlands was done using the data of the permanent plots that have been monitored since 2005 (Escutia-Lara et al., 2009) through a similarity analysis with Jaccard's index for presence-absence data using MVSP software (Kovach Computing Services, 1998.)
RESULTS
Sixty days after initial removal of Phragmites australis live and dead stems in february 2009, plots showed vigorous sprouting of this species and a few individuals of other species. After 60 additional days, 4 species were observed (Table 2): Hidrocotyle verticillata Thunb., Typha domingensis and Eupatorium rugosum Kunth., and in one plot an individual of Schoenoplectus america-nus. Throughout the experiment only a few individuals of Hidro-cotyle verticillata, Typha domingensis and Eupatorium rugosum were found in control plots.
lt was during June (120 days after the experiment started), at the beginning of the rainy season, that P. australis height (Fig. 1) as well as cover started to diverge between treatments, for example, only 10 plots had more than 50% cover (Table 2). Also, two new species were recorded in the plots, Galium trifidium L. in 23 plots and Polygonum hidropiperoides Michx. in two plots. ln August (180 days after initial harvest), Schoenoplectus americanus was present in 6 plots and Carex comosa Booth was recorded for the first time in one plot. In the following months more species were recorded and most native species increased their cover, and seasonal changes in cover were also evident (Table 2). Using the October cover data, the month when more species were present at the end of the rainy season, Bray-Curtis distances correlated with removal treatments (ANOSIM, R=0.4514, p=0.0099).

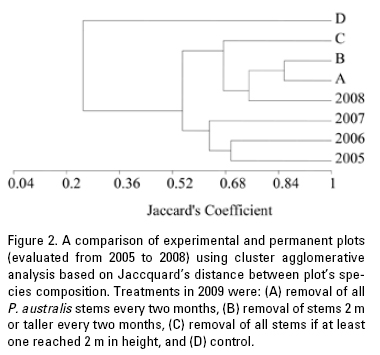
A comparison of experimental and permanent plots (evaluated from 2005 to 2008) using cluster agglomerative analysis based on Jaccquard's distance between plot's species composition shows that removal treatments are closer to permanent plots evaluated in 2008 (Fig. 2). Control plots do not clustered with other treatment or permanent plots evaluated in 2005 and 2006 that are clustered together, because they share 16 of the 21 species present in the study area and are similar to plots evaluated in 2007 because these plots share 12 species of 21 with those of 2005 and 2006. At the end of the experiment, treatment A (complete removal) had 9 species and also treatment B (removal of all stems when at least one reached 2 m in height) being the most similar, and close to treatment C (only stems 2 m high or taller removed) plots that had 6 species .
Trends in species composition were correlated with removal treatments because P. australis became less abundant in harvested treatments, in particular the treatment of full removal every 60 days, and differences were found at the end of the removal experiment. Treatment A, full removal, had the lowest aerial dry biomass of all treatments at the end of the experiment (108 ± 15 g) followed by treatment C, full removal once a stem reached 2 m in height (197 ± 81 g ), treatment B, where only 2 m or higher stems were removed (593 ± 466 g) and finally control (3296 ± 232 g). The differences among treatments were significant (F(3,20) = 92, p < 0.000001). Differences in height among treatments (A = 83 ± 33 cm, B = 225 ± 121 cm, C = 100 ± 80 cm, D = 437 ± 35 cm), were also significant (F(3,20) = 27, p < 0.000001). All P. australis plants died in 3 plots.
DISCUSSION
Harvesting of Phragmites australis reduced sprouting and aerial biomass accumulation as other experiments have shown (Asaeda & Kurunaratne, 2000; Healy et al., 2007; Husak, 1978; Karunaratne et al., 2004; Rajapakse et al., 2006; van der Putten et al., 1997), and native species were able to colonize harvested plots. When comparing, using a dendrogram, native species present in the experimental plots with those of the permanent plots within the adjacent area of the wetland it was noticeable that permanent plots from 2005 to 2007 clustered together. Plots monitored in 2008 clustered with harvest treatments. The Mintzita wetlands have been subjected to increased human disturbance in the last decade changing species composition in our permanent plots (Escutia-Lara et al., 2009). In fact, permanent plots were not evaluated in 2009 because a provoked fire burned them just weeks before the scheduled sampling date. Harvest treatments, in particular full removal (A) and removal of stems 2 m in height or taller (B), clustered with plots evaluated in 2008, the closest sampling date of the permanent plots. Harvesting of all P. australis stems when at least one of them reached 2 m in height (treatment C) was not as close in species composition to permanent plots, probably because the harvest date of a particular plot was not necessarily the same as in treatment A. Therefore, the amounts of biomass removed were more variable, as reflected in the last harvest date, when this treatment was the most variable of all.
For management of P. australis in our study area, several conclusions can be reached. First, there is not optimal season for removal, unlike in other areas where this species is present (Asaeda et al., 2002; Asaeda & Karunaratne, 2000; Bjorndahl, 1985; Gryseels, 1989a; Gryseels, 1989b; Husak, 1978). Second, it is important to note that the area intervened in this study was small, nevertheless, because removal is done manually, damage to the soil would be minimal even in larger projects. Also, in larger project where propagules might no be available planting of desired species might be needed.
Third, although all harvest treatments allow native species to establish, species composition in harvested treatments that are similar to the traditional use of the species (Gerritsen et al., 2009) are more similar to the composition of the natural wetland. Therefore, harvesting can be an efficient control method of this species in infested wetlands in Western Mexico because local human populations already use the resource following a method that can be adapted for control.
ACKNOWLEDGEMENTS
This work was financed by the National Autonomous University of Mexico by grant PAPIIT IN203608 and by CONACYT grant SEP-CONACYT-2008-101335.
REFERENCES
Ailstock, M. S., C. M. Norman & P. J. Bushmann. 2001. Common Reed Phragmites australis: control and effects upon biodiversity in freshwater nontidal wetlands. Restoration Ecology 9: 49-59. [ Links ]
Alpert, P., E. Bone & C. Holzapfel. 2000. Invasiveness, invasibility and the role of environmental stress in the spread of non-native plants. Perspectives in Plant Ecology Evolution and Systematics 3: 52-66 [ Links ]
Asaeda, T. & S. Karunaratne. 2000. Dynamic modeling of the growth of Phragmites australis: model description. Aquatic Botany 67: 301318. [ Links ]
Asaeda, T., L. H. Nam, P. Hietz, N. Tanaka & S. Karunaratne. 2002. Seasonal fluctuations in live and dead biomass of Phragmites australis as described by a growth and decomposition model: implications of duration of aerobic conditions for litter mineralization and sedimentation. Aquatic Botany 73: 223-239. [ Links ]
Back, C. I. & J. R. Holomuzki. 2008. Long-term spread and control of invasive, common reed (Phragmites australis) in Sheldon Marsh, Lake Erie. Ohio Journal of Science 108: 108-112. [ Links ]
Bjorndahl, G. 1985. Influence of winter harvest on stand structure and biomass production of the common reed, Phragmites australis (Cav.) Trin. ex Steud. in Lake Takern, Southern Sweden. Biomass 7: 303319. [ Links ]
Cizkova, H., J. A. Strand & J. Lukavska. 1996. Factors associated with reed decline in an eutrophic fishpond, Roz˘mberk (South Bohmemia, Czech Republic). Folia Geobotanica 31: 111-118. [ Links ]
D'Antonio, C. M. & P. M. Vitousek. 1992. Biological invasions by exotic grasses, the grass/fire cycle, and global change. Annual Review in Ecology and Systematics 23: 63-87. [ Links ]
D'Antonio, C. M. 1993. Mechanisms controlling invasion of coastal plant communities by the alien succulent Carpobrotus edulis. Ecology 74: 83-95. [ Links ]
Escutia-Lara, Y., S. Lara-Cabrera & R. Lindig-Cisneros. 2009. Efecto del fuego y dinámica de las hidrófitas emergentes en el humedal de la Mintzita, Michoacán, México. Revista Mexicana de Biodiversidad 80: 771-778. [ Links ]
Gerritsen, P. R. W., C. Ortiz-Arrona & R. González-Figueroa. 2009. Popular usage, tradition and exploitation of reed: a case study in the south coast of Jalisco, Mexico. Economía, Sociedad y Territorio 9: 185207. [ Links ]
Gleason, H. A. & A. Cronquist. 1963. Manual of vascular plants of northeastern United States and adjacent Canada. D. Van Nostrand Company, New York. 910 p. [ Links ]
Goldberg, D. E. 1990. Components of resource competition in plant communities. In Grace JB, Tilman D. (Eds.) Perspectives on plant competition. Academic Press. San Diego. pp. 27-49. [ Links ]
Gordon, D. R. 1998. Effects of invasive, non-indigenous plant species on ecosystem processes: lessons from Florida. Ecological Applications 8: 975-989. [ Links ]
Gryseels, M. 1989a. Nature management experiments in a derelict reed marsh. I. Effects of winter cutting. Biological Conservation 47: 171193. [ Links ]
Gryseels, M. 1989b. Nature management experiments in a derelict reed marsh. II. Effects of summer mowing. Biological Conservation 48: 85-99. [ Links ]
Healy, M. G., J. Newell & M. Rodgers. 2007. Harvesting effects on biomass and nutrient retention in Phragmites australis in a free-water surface constructed wetland in Western Ireland. Biology an Environment: Procedings ofthe Royal Irish Academy 107B: 139-145. [ Links ]
Howard, T. G. & D. E. Goldberg. 2001. Competitive response hierarchies for germination, growth, and survival and their influence on abundance. Ecology 82: 979-990. [ Links ]
Husak, S. 1978. Control of reed and reed mace stands by cutting. Ecological Studies 28: 404-408. [ Links ]
Karunaratne, S., T. Asaeda & K. Yutani. 2004. Shoot regrowth and age-specific rhizome storage dynamics of Phragmites australis subjected to summer harvesting. Ecological Engineering 22: 99-111. [ Links ]
Kovach Computing Services. 1998. MVSP. Versión 3.01. [ Links ]
Mack, R. N., D. Simberloff, W. M. Lonsdale, H. Evans, M. Clout & F. A. Bazzaz. 2000. Biotic invasions: causes, epidemiology, global consequences and control. Ecological Applications 10: 689-710. [ Links ]
Marks, M., B. Lapin & J. Randall. 1994. Phragmites australis (P. commu-nis): Threats, management, and monitoring. Natural Areas Journal 14: 285-294. [ Links ]
Manly, B. F. J. 2000. Multivariate statistical methods: a primer. Second Edition. Chapman and Hall, CRC Press, Boca Raton, Florida. 224 p. [ Links ]
Mozdzer, T. J., C. J. Hutto, P. A. Clarke & D. P. Field. 2008. Efficacy of imazypar and glyphosate in the control of non-native Phragmites australis. Restoration Ecology 16: 221-224. [ Links ]
Oksanen, J., R. Kindt, P. Legendre, B. O'Hara, G. L. Simpson, P. Solymos, M. Henry, H. Stevens & H. Wagner. 2009. Vegan: Community Ecology Package. R package version 1.15-4. Available in: http://CRAN.R-project.org/package=vegan (downloaded June 2010). [ Links ]
Ostendorp, W. 1995. Effect of management on the mechanical stability of lakeside reeds in Lake Constance-Untersee. Acta Oecologica 16: 277-294. [ Links ]
R. Development Core Team. 2010. R: A language and environment for statistical computing. R Foundation for Statistical Computing. Vienna, Austria. ISBN 3.900051-07-0. [ Links ]
Rajapakse, L., T. Asaeda, D. Williams, R. Roberts & J. Manatunge. 2006. Influence of water depth on the nutrient dynamics of Eleocharis sphacelata and litter accumulation in deep water leading to eutro-phication. Chemical Ecology 22: 47-57. [ Links ]
Reader, R. J. & S. P. Bonser. 1993. Control of plant frequency on an environmental gradient: effects of abiotic variables, neighbours, and predators on Poa pratensisand Poa compressa (Gramineae). Canadian Journal of Botany 71: 592-597. [ Links ]
Romano, R. M., M. A. Romano, M. M. Bernardi, P. V. Furtado & C. A. Oliveira. 2010. Prepubertal exposure to commercial formulation of the herbicide glyphosate alters testosterone levels and testicular morphology. Archives of Toxicology 84: 309-317. [ Links ]
Saltonstall, K. 2002. Cryptic invasion by a non-native genotype of the common reed Phragmites australis, into North America. Proceedings of the National Academy of Sciences 99(4): 2445-2449. [ Links ]
Thompson, K., J. G. Hodgson, J. P. Grime & M. J. W. Burke. 2001. Plant traits and temporal scale: evidence from a 5-year invasion experiment using native species. Journal of Ecology 89: 1054-1060. [ Links ]
Tsui, M. T. K. & L. M. Chu. 2007. Environmental fate and non-target impact of glyphosate-based herbicide (Roundup®) in a subtropical wetland. Chemosphere 71: 439-446. [ Links ]
Turner, R. E. & R. S. Warren. 2003. Valuation of continuous and intermittent Phragmites control. Estuaries 26: 618-623. [ Links ]
Van der Putten, W. H., B. A. M. Peters & M. S. Van der Berg. 1997. Effects of litter on substrate conditions and growth in emergent macrophytes. New Phytologist 135: 527-537. [ Links ]
Vitousek, P. M., L. R. Walke, L. D. Whittacker, D. Mueller-Dombois & P. A. Matson. 1987. Biological invasion by Myrica faga alters ecosystem development in Hawaii. Science 238: 802-804. [ Links ]
Williams, G. M., R. Kroes & i. C. Munro. 2000. Safety evaluation and risk assessment of the herbicide Roundup and its active ingredient, glyphosate, for humans. Regulatory Toxicology and Pharmacology 31: 117-165. [ Links ]














