Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Hidrobiológica
versão impressa ISSN 0188-8897
Hidrobiológica vol.22 no.1 Ciudad de México Jan./Abr. 2012
Artículos
Intensive culture of Litopenaeus vannamei without water exchange and with an artificial substrate
Cultivo intensivo de Litopenaeus vannamei sin recambio de agua y con un sustrato artificial
Juan Manuel Audelo–Naranjo,1 Luis Rafael Martínez–Córdova,2 Silvia Gómez–Jiménez3 and Domenico Voltolina4
1 Universidad Autónoma de Sinaloa, Facultad de Ciencias del Mar, Paseo Claussen s/n, Col. Los Pinos, P.O. Box 610, Mazatlán, Sinaloa. 82000. México
2 Universidad de Sonora, Departamento de Investigaciones Científicas y Tecnológicas, Rosales y Blvd. Luis Encinas s/n, P.O. Box 1819, Hermosillo, Sonora. 83000. México
3 Centro de Investigación en Alimentación y Desarrollo, km 0.6 Carretera a La Victoria, Hermosillo, Sonora. 83304. México
4 Centro de Investigaciones Biológicas del Noroeste, Laboratorio UAS–CIBNOR, P.O. Box 1132, Mazatlán, Sinaloa. 82000. México e–mail: voltolin04@cibnor.mx.
Recibido: 10 de marzo de 2011.
Aceptado: 1 de agosto de 2011.
ABSTRACT
Aiming to determine the effect of the periphyton growing on artificial substrates, juveniles (3 g initial weight and 440 g m–3 stocking biomass) of the whiteleg shrimp Litopenaeus vannamei (Boone, 1931) , were grown during 32 days in eight 1 m3 cylindrical tanks with 3.7 m2 of total submerged surface. Two culture treatments (with and without artificial substrate or control) were tested with four replicates each. Artificial substrate (AquamatsTM) provided an additional surface area of 7.2 m2. The mean dissolved ammonium (NH4+) and ammonia (NH3) concentrations for the Aquamats group were 39 and 22% lower than the respective values obtained for the control cultures. The artificial substrate stimulated nutrient recycling among the biological components (shrimp, biofilm, bottom microfauna, etc.) since mean shrimp biomass yield was 13% higher for the Aquamats group, and it contained a significantly higher percentage of the total nitrogen and phosphorus inputs than the control treatment. The protein content of shrimp cultured with Aquamats was 21.4% higher than that obtained for the control group, which is explained by the higher availability (and diversity) of the natural food of the periphyton. In view of these results, the use of closed cultures added this artificial substrate seems a viable alternative for shrimp culture.
Key words: Shrimp production, closed culture, artificial substrate, nutrient budget, periphyton.
RESUMEN
Con el fin de verificar el efecto del perifiton presente en sustratos artificiales sobre la calidad del agua y el reciclaje de nutrientes, se cultivaron en sistemas cerrados durante 32 días juveniles de camarón blanco Litopenaeus vannamei (Boone, 1931) (peso inicial: 3 g y biomasa inicial de 440 g m–3) en ocho estanques cilíndricos de 1 m3 y 3.7 m2 de superficie sumergida total. Se utilizaron dos tratamientos (con o sin sustrato artificial), cada uno con cuatro repeticiones. En las repeticiones de uno de los tratamientos se adicionaron 7.2 m2 de sustrato artificial (AquamatsTM). Los niveles medios de amonio (NH4+) y de amoniaco (NH3) en los cultivos con Aquamats fueron equivalentes al 39% y al 22% respectivamente, de los valores registrados en los cultivos control. El tratamiento con sustrato artificial favoreció el reciclaje de nutrientes, ya que la biomasa final fue 13% superior al control, y su contenido de nitrógeno y fósforo representó porcentajes significativamente mayores de los respectivos ingresos totales. El mayor nivel de proteína en la biomasa de camarón (21.4%) en los tratamientos con sustrato adicional, se explica por la mayor disponibilidad de alimento natural representado por el perifiton. En vista de estos resultados, el uso de cultivos cerrados y del sustrato artificial AquamatsTM, parece una alternativa viable para el cultivo de camarón.
Palabras clave: Producción de camarón, cultivos cerrados, sustratos artificiales, balance de nutrientes, perifiton.
INTRODUCTION
The continuous growth and tendency to intensification of shrimp culture face several challenges: some are related to its impacts on the natural environment, such as the destruction of mangrove forests and the eutrophication of coastal areas (Naylor et al., 2000; Piedrahita, 2003), while others are due to the limits to intensification, mainly caused by the large amounts of costly formulated feed elaborated with animal protein, needed for intensive culture (Chamberlain, 1995; Sorgeloos, 2001). Additionally, formulated feed is responsible of water quality and pond bottom deterioration, due to the animal excretion, and sedimentation and lixiviation of uneaten food and feces (Burford & Williams, 2001; Avnimelech & Ritvo, 2003). This problem is magnified in closed intensive cultures, because food assimilation efficiency decreases in ponds with a high standing biomass (Martin et al., 1998; Zaki et al., 2004).
Thus, intensification of aquaculture is self–limiting, not only because of the high cost of formulated feed, but also for its poor utilization, which causes deterioration of the pond environment and poor growth, or generates even higher costs, because of the need to increase water exchange rates. This adds to the poor perception of aquaculture by the stakeholders, because it is perceived as an environmental threat (Tacon & Forster, 2003).
There are several techniques which allow the reduction of this threat, maintaining at the same time the water quality within acceptable levels (reviewed by Crab et al., 2007). Most are designed to remove waste products from the culture but with added costs because of the need of additional space for waste removal in settling ponds (van Rijn, 1996; Hargreaves, 2006) or through mechanical filters, generally followed by fine solid removal and foam fractionation, UV or ozone treatment and removal of dissolved organic waste in different types of biological filters (Greiner & Timmons, 1998; Malone & Beecher, 2000; Gutiérrez–Wing & Malone, 2006; Timmons et al., 2006a, b; Crab et al., 2007).
However, there are other alternatives based on the utilization of the dissolved waste products within the culture system —by autotrophic bacteria and algae—, or through direct heterotrophic conversion of organic and inorganic nitrogen species into microbial biomass (Ebeling et al., 2006; Linares & Sundbáck, 2006), which improve water quality, and at the same time, the microbial biomass becomes an important direct or indirect source of natural feed for the farmed organisms (Nunes & Parsons, 2000; Burford et al., 2004). It is achieved either using active suspension ponds —where strong aeration and mixing lead to the formation and growth of microbial flocs in the water column—, or through the addition to the pond of submerged substrates, which serve for the promotion of growth of mixed microalgae–bacteria mats (periphyton) (Avnimelech, 2006; Crab et al., 2007).
In this study, we evaluated the effects of one artificial substrate on water quality, and on survival, individual growth, biomass production and food conversion efficiency of the white shrimp Litopenaeus vannamei (Boone, 1931), cultured in mesocosm under intensive conditions with zero water exchange.
MATERIALS AND METHODS
The experiment was performed between October 3 and November 4, 2008, using eight 1000 l cylindrical high density polyethylene tanks (water depth: 0.9 m, bottom surface: 1.1 m2, submerged surface of the walls: 3.7 m2), located on the grounds of a commercial shrimp farm close to the Urías estuary (Mazatlán, Sinaloa, Mexico).
A 10–cm–deep layer of the superficial (upper 5 cm) bottom sediment of an operating pond of the farm, untreated and previously homogenized in a concrete mixer, was added to each tank. The tanks were filled with 1 m3 of 300–μm filtered estuary water and, after one week, the juveniles (mean weight: 3.0 ± 0.2 g) were stocked at 133 shrimp m–2 tank–1, equivalent to 440 g m–3 of stocking biomass.
One day before the experiment started, four tanks were provided with 7.2 m2 of artificial substrate (AquamatsTM, Meridian Applied Technology Systems, Calverton, Maryland, USA), which had been previously submerged in an operating shrimp pond during 5 days to allow the formation of the biofilm (Burford et al., 2004). Then, AquamatsTM colonized with microorganisms were placed vertically in a circular arrangement at a distance of about 10 cm from the tank walls, increasing by 150% the surface area available to the shrimps as substrate.
Shrimp were fed twice daily (08:00 y 18:00 hours) with 35% protein pelletized commercial food (Camaronina 35, Agribrands Purina Mexico®, Cuautitlán, Estado de México), supplied in feeding trays (36 cm in diameter) used for adjusting the daily food ration according to the apparent consumption observed on the feeding trays (Clifford, 1997). The culture units were kept with zero water exchange, but replacing weekly the water lost by evaporation (estimated average loss, close to 4.8% of the total volume). Aeration (3–5 l min–1; >1–mm air bubbles) was provided to all units with an air blower (Sweetwater 1 HP, Aquatic Eco–System, Inc., FL, USA) to keep adequate dissolved oxygen concentration, avoid thermal stratification and promote renovation of the water in contact with all submerged surfaces.
Temperature and dissolved oxygen were measured in each unit twice daily (08:00 and 18:00 hours) using an oxygen meter (YSI model 57, Yellow Springs, OH, USA). Salinity and pH were determined at 12:00 with an Atago S/Mill–E refractometer (Atago Co., Ltd., Tokio, Japan) and a portable field pH meter (Hanna HI 98150, Hanna Instruments, Woonsocket, RI, USA), respectively.
The concentrations of the dissolved nitrogen (N) and phosphorus (P) species (N–NO3–, N–NO2–, N–NH4+, dissolved organic N, P–PO43–, dissolved non reactive P) and of particulate N and P were determined with three replicates, by sampling 250 ml of the each tank. All water samples were filtered through Whatman GF–C filters, and the concentrations of dissolved N–NO3–, N–NO2–, N–NH4+, P–PO43– and dissolved organic N were determined as in Strickland and Parsons (1972). Non reactive phosphorus was measured as in Rosales–Hoz (1979), and the concentration of unionized ammonia (NH3) was calculated according to Spotte and Adams (1983).
The organic nitrogen and total phosphorus contents of the particles retained on the filters were determined as described by Holm–Hansen (1968) and Solorzano and Sharp (1980), respectively.
The initial and final organic nitrogen content of the sediment and the accompanying meiofauna (determined in triplicate samples after addition to each of the mesocosms, and of composite samples of the sediment obtained at the end of the experiment from the center and sides of each tank), shrimp biomass and the periphyton present on the artificial substrates (obtained scraping the substrate with a scalpel: Burford et al., 2004) were determined with the Kjeldahl method (AOAC, 2005). This method was also used to determine the organic N of shrimp feed. The total phosphorus content was determined as dissolved reactive phosphate (Strickland & Parsons, 1972) after digestion, according to Jackson (1982).
The N and P budgets were calculated as:
Σ of the inputs = Σ of the outputs + losses not considered
Where:
Σ inputs: initial nutrient (N or P) content of the sediment and periphyton + amount supplied to each tank with shrimp feed + sum of the dissolved and particulate species of the relevant nutrient determined in the water used for tank filling and for the weekly water replacements + nutrient content of the shrimp stocked in each tank, and
Σ outputs: nutrient content of the shrimp biomass harvested + sum of the dissolved and particulate species determined in the water discharged + content of the sediment and periphyton at the end of the experiment.
The final survival and biomass yield were determined at the end of the experiment by counting and weighing the surviving shrimp of each tank respectively. The increases in weight followed an almost linear trend (R2 = 0.985 ± 0.042 for the regression calculated with weights in grams, vs 0.971 ± 0.009 with ln–transformed data). Therefore, the daily specific growth rate (SGR) was calculated with the equation:
SGR = (FW – IW)/t
Where: IW and FW = mean initial and final individual wet weights, respectively; t = duration (in days) of the experiment.
The mass yield and the economic feed conversion ratios, FCR and ECR respectively, were calculated for each tank as suggested by Lawrence y Houston (1993) using the next two equations:


Where: BY = biomass yield, IB = initial biomass and FS = total feed supplied, MV = market value of the biomass harvested and FC = cost of the feed supplied (average 2009 prices: 32 Mexican pesos kg–1 of head–on 9 to 10 g shrimp and 14.2 pesos kg–1 of formulated feed).
The mean protein content of the shrimp muscle was determined in triplicate (one sample of 10 shrimp for each tank) for each treatment, and the total concentration of proteins in the hemolymph, were determined at the end of the experiment using the Kjeldhal method (AOAC, 2005) and the bicinchoninic acid technique (Smith et al., 1985), respectively.
The mean values of temperature, dissolved oxygen, pH, salinity and dissolved nutrient concentrations were compared using paired t tests or the equivalent Wilcoxon's tests when the data were not normal or homoscedastic (Kolmogorov–Smirnov and Fisher's F tests).
The mean values of final yields, survival, individual weights, SGR, and the feed and economic conversion ratios were compared using t or Mann Whitney's tests, after arcsine square root transformation in the case of final survival. In all cases, the level of significance was α = 0.05 (Zar, 1999).
RESULTS
The NH4+ and NH3 mean concentrations for the artificial substrate culture group were significantly lower (p < 0.05) compared with the control culture. There were no significant differences (p > 0.05) between the mean values for the rest of the water characteristics tested (Table 1).

The AquamatsTM culture obtained a mean final biomass yield of 1302 ± 16 g and survival above 81%. Both were significantly higher (p < 0.05) than the control group (1144 ± 106 g and 75% survival, respectively). The culture of shrimp without artificial substrate resulted in lower individual mean weights and specific growth rates, as well as significantly higher feed conversion efficiencies (Table 2).

The protein content of the hemolymph for both culture treatments was close to 50 mg ml–1, but the muscle protein was higher (p < 0.05) in the culture with Aquamats (22.7 ± 1.3%) than in the control culture (18.7 ± 1.1%).
The nutrient balances are summarized in Table 3. The shrimp feed was the most important nitrogen input (78.4–79.6%) and the initial shrimp biomass represented between 16.4 and 16.9%. The nitrogen of water and sediment, as well as the initial biofilm on the Aquamats added in the experimental cultures (between 1.6 and 1.9%), were the least important contributions to the total N inputs.
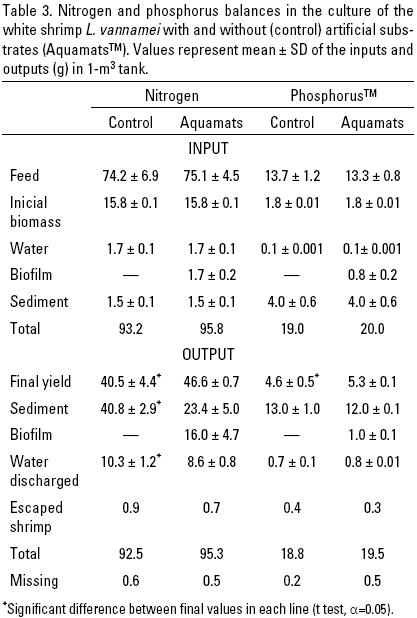
At harvest, the nitrogen content of the shrimp biomass for the control culture was 40.5 g (43.4% of the total nitrogen inputs).
This was significantly lower (p < 0.05) than the 46.6 g of N (48.7% of the inputs) contained in the shrimp harvested in the cultures with artificial substrate. The N content of sediment and biofilm (23.4 ± 5.0 and 16.0 ± 5.7 g, 24.4 and 16.6%, respectively) for AquamatsTM was similar to the amount determined in the sediment of the control culture (40.8 ± 2.9 g: 43.8% of the total nitrogen inputs).
The mean total P input ranged between 19 and 20 g, close to 25% of this amount was harvested as shrimp biomass; as in the case of N, the amount recovered was significantly higher (p < 0.05) in the tanks provided with AquamatsTM, in which an additional 5.2% was recycled as food still available in the biofilm.
Less than 4% of the P input was discharged at harvest, and between 60.3 and 68.8% of the P inputs remained stored in the sediments.
DISCUSSION
Although the shrimp groups were maintained in closed culture systems, the ammonia concentrations of both treatments remained below the safety levels for white shrimp at the size used in our experiment (6.5 mg NH4+ l–1: Frías–Espericueta et al., 1999; Frías–Espericueta & Paez–Osuna, 2001), and the mean concentrations of NH3 were lower than 50% of the value which is likely to impair shrimp growth (0.45 mg NH3–N l–1: Wickins, 1976). For the control culture, this is partially explained by the biological activity of the biofilm present on the tank walls, which give a higher submerged surface to water volume ratios of the culture containers, in comparison to commercial ponds.
However, the biofilm of the additional submerged substrate maintained the mean ammonium (NH4+) and ammonia (NH3) concentrations close to 39% and 22% of the values obtained in the control tanks, and allowed an effective nutrient recycling, because the bacteria and periphyton present on the AquamatsTM maintained an adequate water quality and were an additional food source for the shrimps in culture (Thompson et al., 2002; Avnimelech, 2006). This also improved the quality of the bottom environment, because a sizeable percentage of the nitrogenous wastes was recycled into shrimp biomass, rather than accumulated in the tank sediment.
There were no differences in the protein levels of the shrimp hemolymph between the culture groups, which were within the normal range for post–molt white shrimp (Racotta & Palacios, 1998; Sreenivasa–Rao et al., 2008). This could indicate that there was no undue stress on the cultured organisms in spite of the lack of water exchange, which seems to confirm the positive effect of the high submerged surface to water volume ratios of the culture containers used in this experiment.
However, in agreement with the results obtained in shrimp cultures by other authors (Burford et al., 2004; Fernandes da Silva et al., 2008; Khatoon et al., 2009), the significantly higher protein contents of the shrimp muscle for the culture with artificial substrate confirm the good quality of the protein–rich natural food which is associated to the biofilm of these substrates.
Our results confirm that the addition of submerged substrate may have several beneficial effects for shrimp culture. Some refer to production costs, because they maintain good water quality, and therefore permit low or zero water exchange rates (Milstein, 2005). Additionally, they significantly improve the shrimp growth and survival, and allow an important decrease of the mass and economic feed conversion ratios (Azim et al., 2004, 2005).
One additional advantage in comparison to the traditional open culture systems, refers to the low amount of nutrients discharged to the surrounding environment, which is one of the several problems faced at present by the aquaculture industry worldwide. The obvious advantage in comparison to other treatment techniques for closed cultures, such as external ponds, biofiltration and solids removal systems, is the lower initial and operating cost, with the added advantage of a better utilization of the shrimp feed (Crab et al., 2007; Avnimelech, 2009).
ACKNOWLEDGMENTS
In partial fulfillment for a Ph. D. degree at Centro de Investigaciones Biológicas del Noroeste of the first author, who acknowledges the support of CONACYT and PROMEP scholarships. V. Núñez, O. Zamudio, B. Mejía and José Madero of the Shrimp and Fish Culture Academic Group, and E. Romero–Beltrán (CRIP Mazatlán) helped with field and analytical work.
REFERENCES
Association of Analitigal Communities (AOAC). 2005. Standard methods for the examination of water and wastewater. 21st Ed. American Public Health Association (APHA), American Water Works Association (AWWA), Water Environment Federation (WEF). Washington, D.C. 1368 p. [ Links ]
Avnimelech, Y. 2006. Bio–filters: The need for a new comprehensive approach. Aquacultural Engineering 34: 172–178. [ Links ]
Avnimelech, Y. 2009. Biofloc technology: a practical guide book. The World Aquaculture Society, Baton Rouge. 182 p. [ Links ]
Avnimelech, Y. & G. Ritvo. 2003. Shrimp and fish pond soils: processes and management. Aquaculture 220: 549–567. [ Links ]
Azim, M. E., M. M. Rahaman, M. A. Wahab, T. Asaeda, D. C. Little & M. C. J. Verdegem. 2004. Periphyton based pond polyculture system: a bio–economic comparison of on–farm and on–station trials. Aquaculture 242: 381–396. [ Links ]
Azim, M. E., M. Verdegem, A. van Dam & M. Beveridge. 2005. Periphyton: ecology, exploitation and management. CABI Publishing, Wallingford. 352 p. [ Links ]
Burford, M. A. & K. C. Williams. 2001. The fate of nitrogenous waste from shrimp feeding. Aquaculture 198: 79–93. [ Links ]
Burford, M. A., M. J. Sellars, S. J. Arnold, P. J. Crocos & N. P. Preston. 2004. Contribution of natural biota associated with substrates to the nutritional requirements of the post–larval shrimp, P. esculentus (Haswell) in high–density rearing systems. Aquaculture Research 35: 508–515. [ Links ]
Chamberlain, G. W. 1995. Frontiers in shrimp nutrition research. In: Browdy C. L. & J. S. Hopkins (Eds.). Swimming through troubled water. The World Aquaculture Society. Baton Rouge. pp. 108–117. [ Links ]
Clifford, H. C. 1997. Manual de operación para el manejo de SuperShrimp en estanques. División de Servicios Técnicos. Super Shrimp, S.A. de C.V., Mazatlán. 105 p. [ Links ]
Crab, R., Y. Avnimelech, T. Defoirdt, P. Bossier & W. Verstraete. 2007. Nitrogen removal techniques in aquaculture for a sustainable production. Aquaculture 270: 1–14. [ Links ]
Ebeling, J. M., M. B. Timmons & J. J. Bisogni. 2006. Engineering analysis of the stoichiometry of photoautotrophic, autotrophic and heterotrophic removal of ammonia–nitrogen in aquaculture systems. Aquaculture 257: 346–358. [ Links ]
Fernandes da Silva, C., E. Ballester, J. Monserrat, L. Geracitano, W. J. Wasielesky & P. C. Abreu. 2008. Contribution of microorganisms to the biofilm nutritional quality: protein and lipid contents. Aquaculture Nutrition 14: 507–514. [ Links ]
Frías–Espericueta, M. G. & F. Páez–Osuna. 2001. Toxicidad de los compuestos del nitrógeno en camarones. In: Páez–Osuna, F. (Ed.). Camaronicultura y medio ambiente. Universidad Nacional Autónoma de México, Instituto de Ciencias del Mar y Limnología y El Colegio de Sinaloa, México, D.F., pp. 224–242. [ Links ]
Frías–Espericueta, M. G., M. Harfush–Melendez, J. I. Osuna–López & F. Páez–Osuna. 1999. Acute toxicity of ammonia to juvenile shrimp Penaeus vannamei Boone. Bulletin of Environmental Contamination and Toxicology 62: 646–652. [ Links ]
Greiner, A. D. & M. B. Timmons. 1998. Evaluation of the nitrification rates of microbead and trickling filters in an intensive recirculating tilapia production facility. Aquacultural Engineering 18: 189–200. [ Links ]
Gutiérrez–Wing, M. T. & R. F. Malone. 2006. Biological filters in aquaculture: trends and research directions for freshwater and marine applications. Aquacultural Engineering 34: 163–171. [ Links ]
Hargreaves, J. A. 2006. Photosynthetic suspended–growth systems in aquaculture. Aquacultural Engineering 34: 344–363. [ Links ]
Holm–Hansen, O. 1968. Determination of particulate organic nitrogen. Limnology and Oceanography 13: 175–178. [ Links ]
Jackson, M. L. 1982. Análisis químico de suelos. Ediciones Omega, S.A., Barcelona. 55 p. [ Links ]
Khatoon, H., S. Banerjee, F. M. Yusoff & M. Shariff. 2009. Evaluation of indigenous marine periphytic Amphora, Navicula and Cymbella grown on substrate as feed supplement in Penaeus monodon postlarval hatchery system. Aquaculture Nutrition 15: 186–193. [ Links ]
Lawrence, A. L. & D. M. Houston. 1993. Nutritional response of juvenile Penaeus setiferus and Penaeus vannamei to different quality feeds in presence and absence of natural productivity. In: Collie M. R. & J. P. Mcvey (Eds.). Proceedings of the XX U.S.–Japan Symposium on Aquaculture Nutrition. National Oceanic and Atmospheric Administration, Silver Spring, pp. 113–124. [ Links ]
Linares, F. & K. Sundbàck. 2006. Uptake of dissolved free amino acids (DFAA) by microphytobenthic communities. Aquatic Microbial Ecology 42: 175–186. [ Links ]
Malone, R. F. & L. E. Beecher. 2000. Use of floating bead filters to recondition recirculating waters in warmwater aquaculture production systems. Aquacultural Engineering 22: 57–73. [ Links ]
Martin, J. L. M., Y. Veran, O. Guelorget & D. Pham. 1998. Shrimp rearing: stocking density, growth, impact on sediment, waste output and their relationships studied through the nitrogen budget in rearing ponds. Aquaculture 164: 135–149. [ Links ]
Milstein, A. 2005. Effects of periphyton on water quality. In: Azim, M. E., M. Verdegem, A. van Dam & M. Beveridge. (Eds.). Periphyton–ecology, exploitation and management. CABI Publishing, Wallingford. pp. 179–190. [ Links ]
Naylor, R. L., R. J. Goldburg, J. H. Primavera, N. Kautsky, M. C. M. Beveridge, J. Clay, C. Folke, J. Lubchenco, H. Mooney & M. Troell. 2000. Effect of aquaculture on world fish supplies. Nature 405: 1017–1024. [ Links ]
Nunes, A. J. P. & G. J. Parsons. 2000. Effects of the southern brown shrimp, Penaeus subtilis, predation and artificial feeding on the population dynamics of benthic polychaetes in tropical pond enclosures. Aqua–culture 183:125–147. [ Links ]
Piedrahita, R. H. 2003. Reducing the potential environmental impact of tank aquaculture effluents through intensification and recirculation. Aquaculture 226: 35–44. [ Links ]
Racotta, I. S. & E. Palacios. 1998. Hemolymph metabolic variables in response to experimental manipulation stress and serotonin injection in Penaeus vannamei. Journal of the World Aquaculture Society 29: 351–356. [ Links ]
Rosales–Hoz, L. 1979. Manual de laboratorio de oceanografía química. UNAM–Instituto de Ciencia del Mar y Limnología. México, D.F. 178 p. [ Links ]
Smith, P. K., R. I. Krohn, G.T. Hermanson, A. K. Mallia, F. H. Gartner, M. D. Provenzano, E. K. Fujimoto, N. M. Goeke, B. J. Olson & D. C. Klenk. 1985. Measurement of protein using bicinchoninic acid. Analytical Biochemistry 150: 76–85. [ Links ]
Solorzano, L. & J. Sharp. 1980. Determination of total dissolved phosphorus and particulate phosphorus in natural waters. Limnology and Oceanography 25: 754–758. [ Links ]
Sorgeloos, P. 2001.Technologies for sustainable aquaculture development, Plenary Lecture II. In: Subasinghe, R. P., P. Bueno, M. J. Philips, C. Hough, S. E. McGladdery & J. R. Arthur (Eds.). Aquaculture in the third millennium. Technical Proceedings of the Conference on Aquaculture in the Third Millennium Bangkok, Thailand, NACA, Bangkok. pp. 23–28. [ Links ]
Spotte, S. & G. Adams. 1983. Estimation of the allowable upper limit of ammonia in saline waters. Marine Ecology Progress Series 10: 207–210. [ Links ]
Sreenivasa–Rao, M., B. Rajitha, E. Pavitra & N. Anjaneyulu. 2008. Changes of copper and protein profiles in hepatopancreas and hemolymph tissues during different molt stages of white shrimp, L. vannamei (Boone, 1931). Biotechnology 7: 153–156. [ Links ]
Strickland, J. & J. R. Parsons. 1972. A practical handbook of seawater analysis. Fisheries Research Board of Canada Bulletin 169: 1–310. [ Links ]
Tacon, A. G. T. & I. P Forster. 2003. Aquafeeds and the environment: policy implications. Aquaculture 226: 181–189. [ Links ]
Thompson, F. L., P. C. Abreu & W. Wasielesky. 2002. Importance of biofilm for water quality and nourishment in intensive shrimp culture. Aquaculture 203: 263–278. [ Links ]
Timmons, M. B., J. L. Holder & J. M. Ebeling. 2006a. Application of microbead biological filters. Aquacultural Engineering 34: 332–343. [ Links ]
Timmons, N., M. B. Timmons & J. M. Ebeling. 2006b. Recirculating Aquaculture System (RAS) technologies – Part 2. Aquaculture Magazine (September/October 2006): 32–39. [ Links ]
van Rijn, J. 1996. The potential for integrated biological treatment systems in recirculating fish culture–a review. Aquaculture 139: 181–201. [ Links ]
Wickins, J. F. 1976. The tolerance of warm–water prawns to recirculated water. Aquaculture 9: 19–37. [ Links ]
Zaki, M. A., A. A. Nour, M. M. Abdel–Rahim & T. M. Srour. 2004. Effect of stocking density on survival, growth, feed utilization and production of marine shrimp Penaeus semisulcatus in earthen ponds. Egyptian Journal of Aquatic Research 30(B): 429–442. [ Links ]
Zar, J. H. 1999. Biostatistical Analysis. 4th Ed. Prentice Hall, New Jersey. 663 p. [ Links ]














