Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Hidrobiológica
Print version ISSN 0188-8897
Hidrobiológica vol.21 n.2 Ciudad de México May./Aug. 2011
Characterizing spatial and temporal reef fisheries in Chinchorro Bank Biosphere Reserve, northern Mesoamerican Reef System
Caracterización espacial y temporal de la pesquería en la Reserva de la Biosfera Banco Chinchorro, norte del Sistema Arrecifal Mesoamericano
José Manuel Castro–Pérez,1 Gilberto–Acosta González2 and Jesús Ernesto Arias–González2
1 Laboratorio de Ecología, Instituto Tecnológico de Chetumal, Av. Insurgentes Núm. 330, Col. David Gustavo Gutiérrez, Chetumal Quintana Roo, 77013. México.
2 Laboratorio de Ecología de Ecosistemas de Arrecifes Coralinos, Departamento de Recursos del Mar. Centro de Investigación y de Estudios Avanzados IPN, Carretera Antigua a Progreso km 6, Mérida Yucatán, 97310. México. E–mail: posgradoitch@hotmail.com
Recibido: 13 de mayo de 2010.
Aceptado: 22 de julio 2011.
ABSTRACT
The main objective of this paper was to create a baseline for the spatial and temporal characterization of fisheries in the Chinchorro Bank Biosphere Reserve. Monthly records of one of three fishing cooperatives in the area were taken between August 2004 and June 2005. The individual length and weight of each fish species were recorded per boat. Catch per unit effort (CPUE) was calculated as kilograms per fisherman per hour (kg–fisherman–1–hr–1). CPUE values for Epinephelus striatus, Mycteroperca bonaci, Lachnolaimus maximus and Sphyraena barracuda were highest in the "Nortes" (northerly–winds) season due to increased fishing effort and to the fact that they were apparently caught in spawning aggregation sites. Generally, fishing at Chinchorro Bank exerts low to moderate ecological impact because fishing gear restrictions and fisheries are closely linked to the extraction of spiny lobster, a resource with a higher aggregated–value in contrast to reef fisheries.
Key words: Management, reef fisheries, Mexico, Chinchorro Bank, Biosphere reserve.
RESUMEN
El objetivo principal de este artículo fue crear la línea base para la caracterización espacial y temporal de la pesquería en la Reserva de la Biosfera Banco Chinchorro. Registros mensuales de la captura de escama en una de las tres cooperativas en el área de estudio fueron realizados entre agosto de 2004 y junio de 2005. La longitud y el peso de los individuos de las especies de peces fueron registradas por embarcación. La Captura por Unidad de Esfuerzo (CPUE) fue calculada como kilogramo por pescador por hora de pesca (kg–pescador–1–hr–1). Epinephelus striatus, Mycteroperca bonaci, Lachnolaimus maximus y Sphyraena barracuda presentaron los valores más altos de la CPUE en la época de "Nortes", lo cual está asociado al incremento en el esfuerzo de pesca y al hecho de que aparentemente fueron capturados en sus sitios de agregación reproductiva. Generalmente la pesca en Banco Chinchorro representa un impacto ecológico de bajo a moderado debido a las restricciones en los artes de pesca y a que la principal actividad pesquera esta dirigida hacia la langosta, un recurso con un mayor valor agregado que la pesca de escama.
Palabras clave: Manejo, pesquería arrecifal, México, Banco Chinchorro, Reserva de la Biosfera.
INTRODUCTION
Coastal development in the Caribbean poses a major threat to coral reef ecosystems and mangroves. Coastal areas of the Mexican Caribbean are no exception and to avoid coral reef degradation by massive tourism and over–fishing, Mexican federal and state governments have been working to establish marine protected areas in the Caribbean to protect mangroves and coral ecosystems. Chinchorro Bank Biosphere Reserve (CHBBR) is considered to be a priority conservation area. Coral reef systems of CHBBR support many different species, which are the main component of fisheries in the area. These fisheries are generally small–scale, artisanal and multi–specific; however, economic progress, growing coastal tourism and increased population have led to greater competition for fishery resources and possible over–fishing.
Fisheries on the CHBBR are closely linked to the extraction of spiny lobster, Panulirus argus (Latreille, 1804) and queen conch, Strombus gigas (Linnaeus, 1758); however, the reef fisheries operate all year round, including the spawning aggregations of different fish species. Despite their socio–economic and ecological importance, reef fisheries off Chinchorro Bank are poorly documented. Nevertheless, it is known that in the Mesoamerican Reef System (MAR), within which the CHBBR is located, there are 68 commercially important fish species from 31 genera and 16 families (WWF, 2006). The majority of the studies in the MAR have been conducted on the impact of fisheries on the spawning aggregations of different fish species from Serranidae (e.g. Epinephelus striatus Bloch, 1792) and Lutjanidae (e.g. Lutjanus analis Cuvier, 1828) families (Aguilar–Perera & Aguilar–Dávila, 1996; Sala et al., 2001; Aguilar–Perera, 2006; Graham et al., 2008). Recently, several studies on fisheries in Belize have reported signs of fishing down the food web, caused by low lobster and conch catches, resulting in the finfish being increasingly targeted (Gibson & Hoare, 2006; Coleman, 2008). This highlights the need for knowledge on what is being fished and where. Which are the main fishing zones? In which seasons is maximum fishing effort exerted? Which species are being exploited and where are fish spawning aggregations located?
The main objective of this paper is to create a baseline for the spatial and temporal characterization of fisheries in order to improve scientific guidance for managing the CHBBR.
MATERIALS AND METHODS
Chinchorro bank is one of the most important platform reefs of the Caribbean. It was declared a Biosphere Reserve in 1996, making it a marine protected area under Mexican law. The CHBBR is located in southeast Mexico (18°47', 18°23' N; 87°14', 87°27' W, Fig. 1), in the state of Quintana Roo, approximately 39 km offshore from Mahahual (Jordán & Merino, 1987).
The CHBBR is exploited by three fishing cooperatives, but, due to the complexity of simultaneously sampling catches from all three, samples were only taken from the largest one: Langosteros del Caribe. Each of the fishermen of this cooperative operates a boat all around the reef and throughout the year, fishing lobster and finfish. At the end of a day of fishing every fisherman downloads and stores their capture in a "mother" ship. Catches from each fisherman were recorded directly on the mother ship. Records were taken for one week each month between August 2004 and June 2005. All cooperative boats were surveyed daily throughout the week and their entire catch was registered per boat. Each fish species, its abundance and individual length (cm) and weight (g) were recorded. Fish length was measured from the anterior extreme of the head (mouth closed) to the end of the caudal fin (i.e. total length or TL), and weight was measured with a 20 kg–capacity scale. Additional data such as fishing zone, fishing gear, number of fishermen, and fishing time were obtained via an interview with the boat's captain.
Habitat characteristics of principal fishing areas were obtained by recording the benthic coverage via the video transect method (Aronson & Swanson, 1988). Several stations were positioned in different fishing areas around the reef. At each sampling station a geo–referenced (point) 50 m long transect was laid over the substrate to serve as a guide for recording. Video transect recordings were processed by viewing them on a high–definition monitor and freezing the image at determined intervals. A series of 13 points distributed systematically on the screen were overlaid on the frozen frame, and the benthic organisms at these points identified according to morphostructural groups (MSG): scleractinian corals, hydrocorals, octocorals, sponges, algae, and seagrass.
Production and species composition. Catch data were used to extrapolate an estimation of total coral reef production (Y using the equation (Luchavez et al., 1984):

Where C is total recorded catch, d is the number of days sampled (45 days), D is the number of days fished per year (305 days) and A is the reef area where catches were obtained (238 km2). Only 305 days were included in the analysis because no reef fish were caught in July and September (all fishing effort was focused on the spiny lobster), and severe weather (primarily winter storms) occasionally prevented navigation.
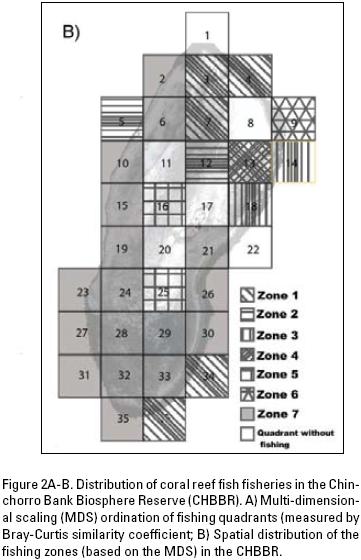
Spatial and temporal arrangement of fisheries. Catch per unit effort (CPUE) was calculated as kilograms per fisherman per hour of catch (kg–fisherman–1 · hr–1). Each fish species was captured by using a specific fishing tool (harpoon or hand line) over the entire period of the study. These data were grouped into 30 fishing quadrants within the reef system because catch per fisherman was not spatially explicit. From a preliminary study, the average fishing area that a fisherman covers to obtain his catch was acquired. Fifteen random fishermen's boats were traced using a GPS to estimate mean fishing area. Quadrant size was based on the approximate fishing area covered by one boat per trip (Fig. 1).
Determination of fishing zones based on the CPUE per quadrant for the ten most commercially important species was carried out with non–parametric multidimensional scaling (MDS) using the Bray–Curtis similarity index (Clarke & Warwick, 2001). This was followed by a similarity percentage analysis (SIMPER; Clarke & Warwick, 2001) to identify species that discriminate between fishing zones. All multivariate techniques were carried out using the PRIMER v6 program. In order to perform a seasonal analysis of fishing effort, the CPUE was calculated for three climatic seasons: rainy (July–October), "Nortes" (northerly–winds) (November–February) and dry (March–June).
A two–way ANOVA was applied without replication (Zar, 1999) in order to determine differences in the CPUE and size of organisms of the commercially important species for each fishing zone and climatic season. Tukey's HSD was used as a post–hoc multiple comparison test (Zar, 1999). Data were tested for normal distribution using the Shapiro–Wilk test (Sokal & Rohlf, 1995) and for homogeneity of variance using Levene's test. The data which did not meet the assumptions for the application of an ANOVA were log transformed (x).
The procedure employed by Marquet et al. (1995) was used to determine the relationship between the CPUE and morphostructural benthic groups. This consisted in determining the slope of the upper limit (maximum CPUE values) of the relationship between CPUE and live substrate cover, which could be an indicator of energy availability in reef systems. This procedure involves dividing the X–axis into intervals of equal width and registering the maximum value of the response variable (CPUE) of the fishing quadrants along the X–axis for each interval. Subsequently, simple linear regressions (r2) and Pearson correlations (r) were used to relate these two variables.
An ANOVA was applied to determine the significance of the relationship between MSG coverage and CPUE values (Zar, 1999).
RESULTS
Production and species composition. Based on annual catch and the presence of three fishing cooperatives in the CHBBR, total production for the entire reef system was estimated to be 0.6579 t–km–2 · yr–1. The reef area covered by the fishing zones used by fishermen was calculated to be 238 km2, considering an 18 m isobath. A total of 37 species from 13 families were identified from 176 fishing trips. Four families were the most representative and corresponded to 87.88% of the total catch (Table 1): Lutjanidae (36.42%), Serranidae (27.01%), Balistidae (14.76%) and Labridae (9.69%). Balistidae, Serranidae and Labridae species were captured using spear, while Sphyraenidae species were captured using hand lines. In the case of the Lutjanidae species, Lutjanus analis and Lutjanus griseus were captured using spear, whereas Lutjanus vivanus and Ocyurus chrysurus were captured using hand lines (Table 1).
Spatial and temporal arrangement of fisheries. The results of the MDS, using the CPUE in the area of the CHBBR, divides the reef system into seven fishing zones (Fig. 2A–B). In zones 1, 2, 4 and 5, quadrants were located within the lagoon with some patch reefs formed mainly by scleractinian corals, octocorals, hydrocorals, sponges, algae, and seagrass, whereas in zone 7 the quadrants were generally covered by scleractinian corals, octocorals, hydrocorals, sponges and algae. Using a 90 % cumulative percentage for different species per zone, the SIMPER indicated hogfish, Lachnolaimus maximus (Walbaum, 1792); E. striatus; black grouper, Mycteroperca bonaci (Poey, 1860); L. analis silk snapper, Lutjanus vivanus (Cuvier, 1828); and yellowtail snapper, Ocyurus chrysurus (Bloch, 1791) to be diagnostic species for different fishing zones (Table 2). Analysis of the CPUE by fishing zone with the ANOVA showed differences between L. analisand L. vivanus the highest values for L. analis were presented in zone 7 (mean = 3.14 ± 3.66 S.D.) in relation to zones 2 (mean = 0.98 ± 1.34 S.D.) and 5 (mean = 0.82 ± 0.83 S.D.), and the highest values for L. vivanus were presented in zone 6 (mean = 1.79 ± 0.53 S.D.) in comparison with zones 3 (mean = 1.65 ± 0.88 S.D.) and 7 (mean = 1.03 ± 0.88 S.D.) (Table 1; Fig. 3). Grey triggerfish, Balistes capriscus (Gmelin, 1789) was caught only in quadrant 32 of zone 7. The size of the organisms captured for each of the species analyzed showed no significant differences between fishing zones, except for O. chrysurus, which presented highest values in zones 7 (mean = 44.87 ± 4.22 S.D.) and 2 (mean = 47.47 ± 3.24 S.D.) as opposed to zone 1 (mean = 35.55 ± 2.65 S.D.) (Table 1; Fig. 3).


The temporal analysis of the CPUE showed significant differences between climatic seasons. The greatest CPUE values for E. striatus, M. bonaci, L. maximus and great barracuda, Sphyraena barracuda (Edwards, 1771) were presented in the "Nortes" season (Table 1; Fig. 4), whereas for L. analis the greatest CPUE value was recorded during the dry season. Balistes capricus was only captured in February, during the "Nortes" season. The temporal analysis of the size of individuals was significantly different for L. analis in the dry season (Table 1; Fig. 4).
The CPUE was significantly related to the cover of scleractinian coral and sponges (p < 0.01), although these variables explained only 37 and 38% of the total variation, respectively (Fig. 5).
DISCUSSION
Production and species composition. Three potential causes of low yield estimates for the CHBBR are: 1) a low number of fishermen due to control by the authorities of the reserve limiting the number of fishermen per cooperative; 2) fishermen focus most of their effort on the spiny lobster fishery; and 3) the use of scuba diving or nets for any fishing activity is prohibited. These factors may also be resulting in the exploitation of fewer species (37 species) compared to Glovers Reef and the MAR region where 57 and 68 species respectively, are normally exploited (WWF, 2006; Coleman, 2008). The annual production estimates made here are different from those reported in Cuba (1.4 t km–2 yr–1) (Claro et al., 1994); and on the Lesser Antilles (4.01 knr2 yr1) (Gobert, 1990). A reef system's production depends on a number of factors (e.g. depth, coral cover, fishing area, methodology, etc.), however fishing effort is by far the most important (Arias–Gonzalez etal., 1994; Costa et al., 2003). The species in the sample are characteristic of the fauna exploited at other sites in the Caribbean and Gulf of Mexico (Munro, 1983; Butler etal., 1993; Claro et al., 1994; Coleman et al., 2000; Schmitter–Soto et al., 2000). Of the 10 species analyzed the great majority correspond to piscivorous or carnivorous species, and none correspond to parrotfish or other herbivorous species, as is the more common in areas of overfishing such as Mexican fringing reefs (pers. obs.) and Belize coral reefs (Gibson & Hoare, 2006; Coleman, 2008) where fishing down the food web is increasingly notable.
Spatial and temporal arrangement of the fishery. In many reef systems in the Atlantic, the variability of the CPUE and the distribution of the size of the reef fish captured are generally related to the characteristics of the substrate of the fishing areas and their depth (e.g. Woff et al., 1999; Gobert, 2000; Costa et al., 2003; Frédou & Ferreira, 2005). However, in this study the greatest variability was due to the effort directed towards fishing spiny lobster and the behaviour of the reproductive activity of certain species of fish. In the fishing zones in the CHBBR, when the lobster starts to become scarce, fishermen mainly direct their effort towards catching larger fish, primarily belonging to Serranidae, Lutjanidae and Sphyraenidae families. These species are characterized by their widespread distribution in reef systems in their adult stage (Hobson, 1973; Meyer etal., 2000; Appeldoorn et al., 2003), which results in them being caught throughout the reef. Zones 3 and 6 stood out due to the catch of L. vivanus; in order to catch this species, fishermen prefer these zones for two reasons: firstly, these zones are closer to the fishing village on Cayo Centro, which reduces operational costs; and second, calcareous terraces characterize the topographic relief of these zones with no obstacles for the use of handlines employed for fishing at the 200 to 300 m isobath. In the particular case of B. capriscus and L. analis, their highest catch occurred in spawning aggregation sites (zone 7). The capture site of B. capricus is located within the reef lagoon (18° 25' 40.2" N; 87° 24' 46.7" W), which is characterized by small reef promontories surrounded by seagrasses and sandy areas within a depth range of 3 to 6 m. The L. analis site is situated on the reef border (18° 23' 52.1" N; 87° 24' 34.2" W) in the windward section at depths that vary between 12 and 14 m, where a complex reef development is evident with abundant colonies of gorgonians. These are the first records of reproduction aggregation sites for these two species in the Mexican Caribbean.
The differences in size of the individuals of O. chrysuruscaptured using a hand line may be due to the fact that this species was fished in different types of habitat. The sites with fish catches of smaller sizes are located in shallow habitats within the reef lagoon, which can serve as a shelter, while larger organisms were fished in deeper areas in the southern zone of the reef lagoon and in the windward region, which present more complex reef structures in the CHBBR (Jordán & Merino, 1987). In various studies this species, in its juvenile state, has been observed to present a very high dependency on shallow habitats (mangroves, seagrasses and small reef structures, whereas the adult organisms have been found in reefs of greater structural complexity in deeper waters (Nagelkerken et al., 2000; Nagelkerken et al., 2002; Mumby et al., 2004; Nagelkerken & van der Velde, 2004a; Nagelkerken & van der Velde, 2004b; Adams et al., 2006).
The fishing activity of the spiny lobster also apparently influenced the differences in fishing effort during the climatic seasons. The start of the lobster fishing season and the greatest fishing effort was in July, corresponding to the rainy season. Between November and February (Nortes) lobster catch became scarce and effort increased towards finfish. After the lobster fishing season from March to June (dry), many boats did not go out to fish because the capture of finfish is not highly profitable.
Throughout the Caribbean Sea, certain species present gregarious habits during their reproductive activity (Aguilar–Perera & Aguilar–Dávila, 1996; García–Cagide & García, 1996; Domeier & Colin, 1997; Crabtree & Bullock, 1998; Aguilar–Perera, 2006; Heyman & Kjerfve, 2008). In this study, exploitation of E. striatus, L. maximus and M. bonaci was shown during their spawning aggregation events, which are presented during the Nortes season. For E. striatus spawning aggregation sites have been reported near the study area during the Nortes season (Aguilar–Perera & Aguilar–Dávila, 1996; Aguilar–Perera, 2006); whereas the spawning seasons of S. barracuda are not known, although, in the study area organisms with mature gonads were found during the "Nortes" season.
Most studies on spawning aggregations of Lutjanus analis in reefs of the Atlantic have found that from May to July; this species presents this gregarious behaviour in specific sites (Domeier et al., 1996; Lindeman etal., 2000; Burton et al., 2005; Graham et al., 2008; Claro et al., 2009). For Balistes capriscus there is little information on its spawning aggregation period, however, it has been reported between November and August (Bernardes & Dias, 2000; Sedberry et al., 2006).
In general, the results suggest that fishing activity in the area of study is directed towards the resource that offers the maximum economic benefit (i.e. lobster) and the minimum fishing effort (i.e. spawning aggregations), which influences the capture of commercially important fish In this context, resource managers at CHBBR are currently locating the spawning areas of these species in order to protect them and thus ensure the supply of recruits. They are also searching for alternative sources of income for fishermen, such as ecotourism, marine fish culture and exploitation of pelagic species, which are all more profitable than direct fishing of aggregations. For example, in an evaluation of the economic impact of fishing the grouper aggregation on Glover's Reef, Belize, Sala et al., (2001) found that ecotourism income from the aggregation was 20 times greater than that generated from extracting the resource.
The positive relationship between CPUE and the cover of scleractinian corals and sponges was due to the combination of deeper habitats in the southern lagoon and the reef system edge, generally located in fishing zone 7, which has the highest structural complexity and consequently greater microhabitat diversity. The benthic organisms that contribute most to the structural complexity in Caribbean reefs are scleractinian corals and sponges (Opresko, 1973; Alcolado & Herrera, 1989; González–Sansón et al., 1997; González, 1999; Jordán–Dahlgren, 2002; Ruiz–Zarate et al., 2003; Caballero et al., 2004). Depth may also be affecting cover in these areas since many studies show higher scleractinian coral and sponge abundances in environments between 6 and 30 m depth (e.g. Goreau, 1959; Huston, 1985; Graus & Macintyre, 1989; Díaz et al., 2000; Valderrama & Zea, 2003).
Management implications. Considering the classification by Russ (1991), the impact of fishing in the CHBBR is low to moderate because: 1) it uses selective equipment (spear gun) and occasionally a hand line; 2) the majority of the species caught are top predators and belong to the snapper–grouper complex, since these species are highly valued in the market; and 3) fishing is the only activity that affects the fish communities and their habitats. However, this investigation proves that the principal species fished are exploited in their spawning aggregation seasons. This is a common occurrence in the Caribbean and a great number of records exist that indicate that this fishing strategy is harmful to the populations since it causes the extirpation of reef fish spawning aggregations (e.g., Sadovy & Eklund, 1999) and changes to the reproductive population structure, such as decreases in mean fish size (e.g., Sadovy, 1994), abundance (e.g., Claro etal., 2001), genetic diversity (e.g., Chapman et al., 1999) and alterations in aggregation sex ratio (e.g., Koenig et al., 1996). For this reason, a strategy for reducing fishing impact would be the seasonal closure of their spawning sites. This measure could be accompanied by catch control policies in the landing locations. Personal observations indicate that fishermen are starting to fish parrot and angel fish and shell them as fillets of grouper or snapper. Finally, although this study presents a general overview of the current state of fishing, it is important to focus studies at the population level for the species of greatest commercial importance and to perform studies at ecosystem level, analyzing indicators that could measure fishing stress within fish communities (Pikitch et al., 2004).
ACKNOWLEDGEMENTS
This research was financially supported by Fondos Mixtos CONACYT (Ref. QROO–2003–C02–13020). The authors thank members of the Langosteros del Caribe Fishing Cooperative and the Chinchorro Bank Biosphere Reserve personnel for their cooperation in the field work.
REFERENCES
Adams, A. J., C. P. Dahlgren, G. T. Kellison, M. S. Kendall, C. A . Layman, J. A. Ley, I. Nagelkerken & J. E. Serafy. 2006. Nursery function of tropical back–reef systems. Marine Ecology Progress Series 318: 287–301. [ Links ]
Aguilar–Perera, A. & W. Aguilar–Dávila. 1996. A spawning aggregation of Nassau grouper Epinephelus striatus (Pisces: Serranidae) in the Mexican Caribbean. Environmental Biology of Fishes 45: 351–361. [ Links ]
Aguilar–Perera, A. 2006. Disappearance of a Nassau grouper spawning aggregation off the southern Mexican Caribbean coast. Marine Ecology Progress Series 327: 289–296. [ Links ]
Alcolado, P. & A. Herrera. 1989. Estructura de las comunidades de esponjas del arrecife de Rincón de Guanabo, Cuba. Reporte de Investigación 68, Instituto de Oceanología. 65 p. [ Links ]
Appeldoorn, R. S., A. Friedlander, J. Sladek Nowlis, P. Ussegilo & A. Mitchell–Chui. 2003. Habitat connectivity on the insular platform of Old Providence–Santa Catalina, Colombia: mechanism, limits and ecological consequences relevant to marine reserve design. Gulf and Caribbean Research 14: 61–77. [ Links ]
Arias–González, J. E., R. Galzin, J. Nielson, R. Mahon & K. Aiken. 1994. Reference area as a factor affecting potential yield estimates of coral reef fishes. NAGA ICLARM Quarterly 17: 37–40. [ Links ]
Aronson, R. B. & D. W. Swanson. 1988. Video survey of coral reefs: uni and multivariate applications. Proceedings of the 8th International Coral Reef Symposium 2: 1441–1446. [ Links ]
Bernardes R. A. & J. F. Dias. 2000. Aspectos da reprodução do peixe–porco, Balistes capriscus (Gmelin) (Actinopterygii, Tetraodontiformes, Balistidae) coletado na costa sul do Estado de São Paulo, Brasil. Revista Brasileira de Zoologia 17 (3): 687–696. [ Links ]
Burton, M. L, K. J. Brennan, R. C. Muñoz & R. O. Jr Parker. 2005. Preliminary evidence of increased spawning aggregations of mutton snapper (Lutjanus analis) at Riley's Hump two years after establishment of the Tortugas South Ecological Reserve. Fisheries Bulletin 103: 404–410. [ Links ]
Butler, J. N., J. Burnett–Herkes, J. A. Barnes & J. A. Ward. 1993. The Bermuda fisheries: a tragedy of the commons averted? Environment 35: 7–33. [ Links ]
Caballero, H., P. P. Chevalier, G. Varona, A. L. Cárdenas, L. Pastor, A. Pérez–Hernández & Y. García. 2004. Componentes más comunes de la fauna del arrecife de coral de la costa oriental de Bahía de Cochinos, Cuba: corales, esponjas, gorgonáceos y peces. Revista de Investigaciones Marinas 25 (1): 37–44. [ Links ]
Chapman, R. W., G. R. Sedberry, C. C. Koenig & B. E. Eleby. 1999. Stock identification of gag, Mycteroperca microlepis, along the southeast coast of the United States. Marine Biotechnology 1: 137–146. [ Links ]
Clarke, K. R. & R. M. Warwick. 2001. Change in marine communities: An approach to statistical analysis and interpretation, 2nd edn. Natural Environment Research Council, Plymouth Marine Laboratory. Plymouth. 172 p. [ Links ]
Claro, R., J. A. Baisre & J. P. García–Arteaga. 1994. Evolución y manejo de los recursos pesqueros. In: Claro, R. (Ed.). Ecología de los peces marinos de Cuba. Instituto de Oceanología Academias de Ciencias de Cuba and Centro de Investigaciones de Quintana Roo, México, pp. 435–456. [ Links ]
Claro, R., J. A . Baisre, K. Lindeman & J. P. García–Arteaga. 2001. Cuban fisheries: historical trends and current status. In: Claro, R., K.C. Lindeman & L.R. Parenti (Eds.). Ecology of the marine Fish of Cuba. Smithsonian Institution, Washington DC, pp. 194–219. [ Links ]
Claro, R., Y. Sadovy, K. C. Lindeman & A. R Garcia–Cagide. 2009. Historical analysis of Cuban commercial fishing effort and the effects of management interventions on important reef fishes from 1960–2005. Fish Research 99 (1): 7–16. [ Links ]
Coleman, F. C., C. C. Koenig, G. R. Huntsman, J. A. Musick, M. Eklund, J. C. McGovern, R. W. Chapman, G. R. Sedberry & C. B. Grimes. 2000. Long–lived reef fishes: the grouper–snapper complex. Fisheries 25: 14–20. [ Links ]
Coleman, R. 2008. Fisheries catch data collection Glover's Reef Marine Reserve. Report for the period August 2004–December 2007. Wildlife Conservation Society, Belize. 16 p. [ Links ]
Costa, P. A. S., A. C. Braga & L. O. F. Rocha. 2003. Reef fisheries in Porto Seguro, eastern Brazilian coast. Fisheries Research 60: 577–583. [ Links ]
Crabtree, R. E. & L. H. Bullock. 1998. Age, growth, and reproduction of black grouper, Mycteroperca bonaci, in Florida waters. Fisheries Bulletin 96: 735–753. [ Links ]
Díaz, J. M., G. Díaz–Pulido & J. A. Sánchez. 2000. Distribution and structure of the southernmost Caribbean coral reefs: Golfo de Urabá, Colombia. Scientia Marina 64 (3): 327–336. [ Links ]
Domeier, M. L. & P. L. Colin. 1997. Tropical reef fish spawning aggregations: defined and reviewed. Bulletin of Marine Science 60 (3): 698-726. [ Links ]
Domeier, M. L., C. Koenig & F. Coleman. 1996. Reproductive biology of gray snapper (Lutjanus griseus) with notes on spawning for other western Atlantic snappers (Lutjanidae). In: Arreguin–Sanchez, F., J.L. Munro, M.C. Balgos & D. Pauly (Eds.). Biology, fisheries and culture of tropical groupers and snappers. ICLARM Manila, Philippines, pp. 189–201. [ Links ]
Frédou, T. & B. P. Ferreira. 2005. Bathymetric trends of northeastern Brazilian snappers (Pisces, Lutjanidae): implications for the reef fishery dynamic. Brazilian Archives of Biology and Technology 48: 787–800. [ Links ]
García–Cagide, A. & T. García. 1996. Reproducción de Mycteroperca bonaci y Mycteroperca venenosa (Pisces: Serranidae) en la plataforma cubana. Revista de Biología Tropical44 (2): 771–780. [ Links ]
Gibson, J. & S. Hoare. 2006. Fisheries catch data collection – Glover's Reef. Wildlife Conservation Society, Belize. 27 p. [ Links ]
Gobert, B. 1990. Production relative des pêcheriescôtières en Martinique. Aquatic Living Resources 3: 181–191. [ Links ]
Gobert, B. 2000. Comparative assessment of multispecies reef fish resources in the Lesser Antilles. Fisheries Research 44: 247–260. [ Links ]
González, P. 1999. Comunidades de esponjas, corales y gorgonias en un arrecife coralino costero de Ciudad de La Habana. Trabajo de Diploma, Universidad de La Habana. 25 p. [ Links ]
González–Sansón, G., E. de la Guardia, C. Aguilar, C. González & M. Ortiz. 1997. Inventario de los componentes más comunes en un arrecife de coral costero de la región noroccidental de Cuba. Revista de Investigaciones Marinas 18 (3): 193–197. [ Links ]
Goreau, T. F. 1959. The ecology of Jamaican coral reefs, 1. Species composition and zonation. Ecology 40: 67–90. [ Links ]
Graham, R. T., R. Cárcamo, K. L. Rhodes, C.M. Roberts & N. Requena. 2008. Historical and contemporary evidence of a mutton snapper (Lutjanus analis Cuvier, 1828) spawning aggregation fishery in decline. Coral Reefs 27: 311–319. [ Links ]
Graus, R. R. & I. G. Macintyre. 1989. The zonation patterns of Caribbean coral reefs as controlled by wave and light energy input, bathymetric setting and reef morphology: computer simulation experiments. Coral Reefs 8: 9–18. [ Links ]
Heyman, W. D. & B. Kjerfve. 2008. Multi–species reef fish spawning aggregations at Gladden Spit, Belize. Bulletin of Marine Science 83 (3): 531–551. [ Links ]
Hobson, E. S. 1973. Diel feeding migrations in tropical reef fishes. Helgol"nder Meeresuntersuchungen 24: 361–370. [ Links ]
Huston, M. A. 1985. Patterns of species diversity on coral reefs. Annual Review of Ecology Systematics 16: 149–177. [ Links ]
Jordán, E. & E. M. Merino. 1987. Chinchorro: morphology and composition of a Caribbean atoll. Atoll Research Bulletin 310: 1–20. [ Links ]
Jordán–Dahlgren, E. 2002. Gorgonian distribution patterns in coral reef environments of the Gulf of México: evidence of sporadic ecological connectivity? Coral Reefs 21: 205–215. [ Links ]
Koenig, C. C., F. C. Coleman, L.A. Collins, Y. Sadovy & P. L. Colin. 1996. Reproduction in gag (Mycteroperca microlepis) (Pisces: Serranidae) in the eastern Gulf of Mexico and the consequences of fishing spawning aggregations. In: Arreguin–Sanchez, F., J. L. Munro, M. C. Balgos & D. Pauly (Eds.). Biology, fisheries and culture of tropical groupers and snappers. ICLARM Manila, Philippines, pp. 307–323. [ Links ]
Lindeman, K. C., R. Pugliese, G. T. Waugh & J. S. Ault. 2000. Developmental patterns within a multispecies reef fishery: management applications for essential fish habitats and protected areas. Bulletin of Marine Science 66 (3): 929–956. [ Links ]
Luchavez, T., J. Luchavez & A. C. Alcalá. 1984. Fish and invertebrate yields of the coral reefs of Selinog Island in the Mindanao Sea and Hulao–Hulao in Panay Gulf, Philippines. Silliman Journal31: 57–71. [ Links ]
Marquet, P. A., S. A. Navarrete & J. C. Castilla. 1995. Body size, population density, and the energetic equivalence rule. The Journal of Animal Ecology 64: 325–332. [ Links ]
Meyer, C. G., K. N. Holland, B. M. Wetherbee & C. G. Lowe. 2000. Movement patterns, habitat utilization, home range size and site fidelity of whitesaddle goatfish, Parupeneus porphyreus in a marine reserve. Environmental Biology of Fishes 53 (3): 235–242. [ Links ]
Mumby, P. J., A. E. Edwards, J. E. Arias–González, K. C. Lindeman, P. G. Blackwell, A. Gall, M. I. Gorczynska, A. R. Harborne, C. L. Pescod, H. Renken, C. C. C. Wabnitz & G. Llewellyn. 2004. Mangroves enhance the biomass of coral reef fish communities in the Caribbean. Nature 427: 533–536. [ Links ]
Munro, J. L. 1983. Coral reef fish and fisheries of the Caribbean Sea. In: Munro, J.L. (Ed.). Caribbean Coral Reef Fisheries. ICLARM Studies and Reviews 7, pp. 1–9. [ Links ]
Nagelkerken I. & G. van der Velde. 2004a. Are Caribbean mangroves important feeding grounds for juvenile reef Wsh from adjacent seagrass beds? Marine Ecology Progress Series 274: 143–151. [ Links ]
Nagelkerken I. & G. van der Velde. 2004b. Relative importance of interlinked mangroves and seagrass beds as feeding habitats for juvenile reef Wsh on a Caribbean island. Marine Ecology Progress Series 274: 153–159. [ Links ]
Nagelkerken, I., C. M. Roberts, G. van der Velde, M. Dorenbosch, M. C. Van Riel, E. Cocheret de la Moriniêre & P. H. Nienhuis. 2002. How important are mangroves and seagrass beds for coral reef fish? Marine Ecology Progress Series 244: 299–305. [ Links ]
Nagelkerken, I., G. van der Velde, M.W. Gorissen, G. J. Meijer, T. van 't Hof & C. den Hartog. 2000. Importance of mangroves, seagrass beds and the shallow coral reef as a nursery for important coral reef fishes, using a visual census technique. Estuarine, Coastal and Shelf 51: 31–44. [ Links ]
Opresko, D. M. 1973. Abundance and distribution of shallow–water gorgonians in the area of Miami, Florida. Bulletin of Marine Science 23 (3): 535–558. [ Links ]
Pikitch, E. K., C. Santora E. A. Babcock, A. Bakun, R. Bonfil, D. 0. Conover, P. Dayton, P. Doukakis, D. Fluharty, B. Heneman, E. D. Houde, J. Link, P. A. Livingston, M. Mangel, M. K. McAllister, J. Pope & K. J. Sainsbury. 2004. Ecosystem–based fishery management. Science 305: 346–347. [ Links ]
Ruiz–Zárate, M. A., R. Hernández–Landa, C. González–Salas, E. Núñez–Lara & J. E. Arias–González. 2003. Condition of coral reef ecosystems in central–southern Quintana Roo (Part 1: stony corals and algae). Atoll Research Bulletin 496: 318–337. [ Links ]
Russ, G. R. 1991. Coral reef fisheries: effects and yields. In: Sale, P.F. (Ed.). The Ecology of Fishes on Coral Reefs. Academic Press, California, pp. 601–635. [ Links ]
Sadovy, Y. & A. M. Eklund. 1999. Synopsis of biological data on the Nassau grouper Epinephelus striatus (Bloch, 1792), and the jewfish, E. itajara (Lichtenstein, 1822). NOAA Technical Report 146. NMFS, FAO Fisheries Synopsis. 157 p. [ Links ]
Sadovy, Y. 1994. Grouper stocks of the Western Central Atlantic: the need for management and management needs. Proceedings of the Gulf and Caribbean Fisheries Institute 43: 42–64. [ Links ]
Sala, E., E. Ballesteros & R. M. Starr. 2001. Rapid Decline of Nassau Grouper Spawning Aggregations in Belize: Fishery Management and Conservation Needs. Fisheries 26: 23–30. [ Links ]
Schmitter–Soto, J. J., L. Vázquez–Yeomans, A. Aguilar–Perera, C. Curiel–Mondragón & J. A. Caballero–Vázquez. 2000. Lista de peces marinos en el Caribe mexicano. Anales del Instituto de Biología, UNAM, Serie Zoología 71: 143–178. [ Links ]
Sedberry, G. R., 0. Pashuk, D. M. Wyanski, J. A. Stephen & P. Weinbach. 2006. Spawning locations of Atlantic reef fishes off the southeastern U.S. Proceedings of the Gulf and Caribbean Fisheries Institute 57: 463–51. [ Links ]
Sokal, R. R. & J. F. Rohlf. 1995. Biometry: The Principles and Practice of Statistics in Biological Research, 3rd edn. W. H. Freeman and Company, New York. 887 p. [ Links ]
Valderrama, D. F. & S. Zea. 2003. Esquemas de distribución de esponjas arrecifales (Porifera) del Noroccidente del Golfo de Urabá, Caribe sur, Colombia. Boletín de Investigaciones Marinas y Costeras 32: 37–56. [ Links ]
Woff, N., R. Gromer–Dunsmore, C. S. Rogers & J. Beets. 1999. Management implications of fish trap effective in adjacent coral reef and gorgonian habitats. Environmental Biology of Fishes 55: 81–90. [ Links ]
World Wild Fund (WWF). 2006. Mejores prácticas de pesca en arrecifes coralinos. Guía para la colecta de información que apoye el Manejo de Pesquerías Basado en Ecosistemas. W.W.F. México/Centroamérica. 81 p. [ Links ]
Zar, J. H. 1999. Biostatistical Analysis, 4th edn. Prentice–Hall, Englewood Cliffs, NJ. 672 p. [ Links ]














