Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Hidrobiológica
versión impresa ISSN 0188-8897
Hidrobiológica vol.20 no.3 Ciudad de México ene. 2010
Artículos
Phylogenetic relationships among five marine Catfish species (Pisces: Ariidae) from Mexico
Relaciones filogenéticas entre cinco especies de bagres (Pisces: Ariidae) de México
Guadalupe Tenorio–Colín,1,2 Uriel Rodríguez–Estrada,3 Manuel Uribe–Alcocer4 and Píndaro Díaz–Jaimes4
1 Universidad del Mar. Ciudad Universitaria Km. 1.5 Carretera a Zipolite s/n. Puerto Ángel, Oaxaca 70902, México.
2 Doctorado en Ciencias Biológicas de la Universidad Autónoma Metropolitana, México.
3 Universidad Católica de Temuco. Escuela de Acuacultura, Laboratorio de Nutrición Acuícola. Avenida Rudecindo Ortega 02950, Campus Norte, Temuco, Chile.
4 Instituto de Ciencias del Mar y Limnología, Universidad Nacional Autónoma de México, Apdo. Postal 70–305, México, D. F. 04510, México. E–mail: tenorio@angel.umar.mx.
Recibido: 13 de mayo de 2010
Aceptado: 06 de diciembre de 2010
ABSTRACT
The systematics of the marine catfish of the family Ariidae is controversial because at the present time the number of species and genera in the family, or their relationships, remain uncertain. Phylogenetic relationships among five representative species of marine catfish of the family Ariidae from both the Pacific and the Atlantic coasts of Mexico were assessed by the analysis of the variability in 21 alloenzymatic loci, and by the comparison of the electrophoretic patterns of whole muscle proteins. Interspecific genetic divergence levels obtained by both electrophoretic methods showed a clear separation among the genera Cathorops, Bagre and Ariopsis, as well as in the studied species of Cathorops and Ariopsis, with Bagre marinus showing a greater genetic similarity with the Ariopsis group. Finally, our results contribute to the definition of the presence of this species in the coasts of the Gulf of Mexico.
Key words: Phylogeny, catfish, Ariidae, electrophoresis, isoenzymes.
RESUMEN
La sistemática de los bagres marinos pertenecientes a la familia Ariidae es controversial, porque hasta la actualidad, no se conoce con exactitud el número de especies y géneros existentes en la familia, ni las relaciones que se establecen entre ellos. En el presente trabajo se estudiaron las relaciones filogenéticas entre cinco especies representativas de bagres marinos de la familia Ariidae, de ambas costas del Pacífico y del Atlántico Mexicano. El mencionado análisis se llevó a cabo mediante el análisis de la variabilidad en 21 loci aloenzimáticos y a través de la comparación de patrones electroforéticos de proteínas totales de músculo. Los niveles de divergencia interespecífica obtenidos por ambos métodos electroforéticos mostraron una clara separación entre los géneros Cathorops, Bagre y Ariopsis, así como entre las especies estudiadas de Cathorops y Ariopsis con Bagre marinus, mostrando una mayor similitud genética con el grupo Ariopsis. Los resultados obtenidos en este estudio contribuyen al establecimiento de la presencia de estas especies a lo largo de las costas del Golfo de México.
Palabras clave: Filogenia, bagres, Ariidae, electroforesis, isoenzimas.
INTRODUCTION
The family Ariidae (Bleeker, 1862) includes catfish distributed mainly in the warm and tropical waters of both, marine and estuarine habitats. Castro–Aguirre et al. (1999) considered the family Ariidae to be a taxonomic assemblage of 14 genera with approximately 120 species, some of them complex and difficult to identify because of their morphological similarity while Betancur (2003) stated that at least 150 species have been recognised. The systematics of the Ariidae has not been clear in the definition of the species and genera, especially those of the Atlantic (Wheeler & Baddokwaya, 1981; Higuchi, 1982), which has brought about the occurrence and persistence of frequent synonyms (e.g. Meek, 1904; Regan, 1906–1908; Reséndez–Medina, 1983; Fuentes–Mata et al., 1989; Mayden et al., 1992; Kobelkowsky & Castillo–Rivera, 1995). According to Marceniuk & Menezes (2007) the main problems in recognising species identity and monophyletic taxa are due to the wide geographical distribution of the group and the overall similarity in the external morphology of its species, together with a lack of adequate specimens in museum collections.
Thus, it is important to test some of the hypotheses on the taxonomy of the group with genetic methods, including for instance, the proposals of Taylor & Menezes (1978) and Kailola & Bussing (1995), as well as Castro–Aguirre et al. (1999) and Kobelkowsky & Castillo–Rivera (1995) on the separation of the catfish of the genus Ariopsis 2ill (1861) from those of the Arius Valenciennes (1840) group, based on the geography of their distribution. According to this hypothesis, the genus Ariopsis (2ill, 1861) is found north of the Equator while the genus Arius is found in the southern hemisphere.
Morphological studies have been carried out to contribute to the understanding of the systematics of the catfish of this family. Kobelkowsky & Castillo–Rivera (1995) examined the number of pharyngeal teeth in Cathorops melanopus (Günther, 1864), Ariopsis felis (Linnaesus, 1766) and Bagre marinus (Mitchill, 1815), and found that differences help identify these species. Cathorops melanopus present one pair of teeth, Ariopsis felis two pairs and Bagre marinus three pairs.
Recent studies on the morphometry of some ariid fish have provided clear evidences of the monophyly of the group (Betancur, 2003; Acero & Betancur, 2007; Betancur et al., 2004).
The karyotypes of several species of this family have been made looking for clues of their phyletic relationships. Nevertheless, the analysis of chromosome evolution reflected in chromosome numbers and formulas have shown a remarkable karyotype stability not useful to make phylogenetic inferences about the group (Le 2rande, 1980; Rishi et al., 1983; Fitzsimmons et al., 1988; García–Molina & Uribe–Alcocer, 1989; 2omes et al., 1990; 2omes et al., 1992; 2omes et al., 1994; Uribe–Alcocer & Díaz–Jaimes, 2000). Other studies of genetic characters based on protein electrophoresis have been highly effective to detect variability and to evaluate interspecific relationships among different fish groups (Avise, 1994; Ruiz–Carús & Uribe–Alcocer, 2003). Another genetic study, based on the sequences of a 2922 pb fragment of the mitochondrial genome (cytochrome b, ATP synthase 8 and 6, 12S and 16S), and on a 978 pb nuclear fragment (activating gene of the recombination 2), provided a robust phylogenetic analysis of this group by maximum parsimony (Betancur, 2003).
Betancur (2009a) reported new molecular phylogenies of the New World ariids and galeichthyins, and proposed an inclusive phylogeny of the ariid taxa, providing a framework for future comparative studies and classifications by the comparison of the mitochondrial sequences (cytochrome b, ATP synthase 8 and 6, 12S and 16S ~3 kb) and a nuclear marker (rag2, ~1 kb) of specimens representing several biogeographic provinces from the wide distribution area of the catfish group. Betancur (2009b) also reported molecular data of further species to deepen in the phylogenies of the Ariinae, and to infer historical and biogeographical scenarios in the evolution of this group.
The purpose of this work was to evaluate the extent of genetic divergence among five ariid catfish species: Ariopsis felis (Linnaeus, 1766), Bagre marinus (Mitchill, 1815) and Cathorops aguadulce (Meek, 1904) of the Gulf of Mexico, and Cathorops fuerthii (Steindachner, 1877) and Ariopsis guatemalensis (Günther, 1864) of the Pacific coast, through the analysis of electrophoretic patterns of whole muscle protein extracts in polyacrilamide gels and the electrophoretic analysis of 21 alloenzymatic loci, to contribute to the understanding of the systematics of this group. Genetic studies based on protein electrophoresis have proved to be highly effective to detect variability and to evaluate relationships at the species level for different fish groups (Avise, 1994; Ruiz–Carús & Uribe–Alcocer, 2003), including cryptic species (Lima et al., 2005).
MATERIAL AND METHODS
Specimens of Cathorops fuerthii (n = 11) and Ariopsis guatemalensis (n = 60) from the Mexican Pacific coast were collected by cast net in Laguna de Tres Palos, in the Mexican state of 2uer–rero. Ten additional specimens of the first species were also collected further north, in the state of Sinaloa. In the southeastern Gulf of Mexico off Campeche, 40 specimens of Ariopsis felis and 11 of Bagre marinus were collected with a dredge net during the oceanographic campaign "Impacto I", aboard the R/V Justo Sierra (UNAM). Eighty additional specimens of Cathorops aguadulce were collected from several lagoons of the state of Veracruz: Alvarado, Sontecomapan, La Antigua and La Mancha. A specimen of the family Heptapteridae (2ill, 1861), Rhamdia guatemalensis (Günther, 1864), was used as an external reference. Specimens of Ariopsis were identified following the taxonomic keys of Castro–Aguirre (1978), Kailola & Bussing (1995), and those of Cathorops, following the keys of Castro–Aguirre et al. (1999) and Taylor & Menezes (1978).
Whole muscle proteins. Samples of whole muscle protein were prepared by homogenising approximately 1 g of muscle tissue in 750 µl of Tris (Sigma T–1503) – Glycine (Sigma 2–6761), pH 8.9 buffer followed by centrifugation at 4,000 rpm for 10 min. Supernatant fraction was kept at –20 °C until the electrophoretic run. Electrophoresis was carried out in 10% polyacrilamide gels (Sigma A–8887 and M–2022) (Fehrnstrbm & Moberg, 1977). A discontinuous electrophoretic buffer system was used with 0.04M Tris (Sigma–T–1503) –0.052M 2lycine (Sigma–2–6761) at a pH of 8.9 for the cathode, and 0.1M Tris (T–1503) –1N HCl (50 ml), pH 8.1 for the anode. The run was made at 30 mÅ for 2 hours. The general protein stain used was Comassie blue (Sigma B–0630) (Ferguson, 1980).
Whole muscle protein band patterns were analysed qualitatively by determining their presence/absence in the gels, and quantitatively by determining the absolute frequencies of the resolved bands. Ferguson's (1980) similarity coefficient, that considers the number of shared bands between two species relative to the total number of bands resolved, was computed. A matrix of presence/absence of electromorphs was produced in order to construct a dendrogram following the UP2MA method, as implemented in the Jump (JMP) version 3.1.2 programme (Sall et al., 1989–1995). Every species was considered as an Operative Taxonomic Unit (OTU), and the resolved electromorphs as a double state character, that is, as binary data that denote the presence or absence of bands (Crisci & López–Armengol, 1983). A dendrogram was constructed later for a cluster analysis, where the average taxonomic distance was inversely proportional to the similarity coefficient, considering the value 0 as taxonomic distance zero and the value 1 as the maximum taxonomic distance.
Allozymes. Samples of liver and muscle tissue were frozen with liquid nitrogen for transportation to the Laboratory of Genetics, Instituto de Ciencias del Mar y Limnología, Mexico City. Extracts were obtained by grinding tissues on ice in 750 µl of homogenising solution consisting of 0.001M Trizma base (Sigma T–1503), 0.0001M EDTA (Sigma ED2SS) and 0.00005M NADP (Sigma N–3886) at a pH of 7.
Electrophoretic assays were conducted in horizontal 10.5% (weight/volume) starch gels (Sigma Chemicals, St. Louis, Mo, Cat. S–4501). The buffer solutions used were: 0.10M Tris – 0.10M Ma–late – 0.01M EDTA (Sigma T–1503, Sigma M–0375, Sigma–ED2SS), for enzymes isocitrate dehydrogenase (IDH EC. 1.1.1.42), phosphogluconate dehydrogenase (6P2D; EC. 1.1.1.44), lactate dehydrogenase (LDH; EC. 1.1.1.27), and glycerol phosphate dehydrogenase (a–2PD; EC. 1.1.1.8); 0.223M Tris – 0.086M Citrate I (Sigma T–1503, Sigma C–7129), for Aspartate aminotransferase (AAT; EC. 2.6.1.1), esterases (EST; EC. 3.1.1.1), malate dehydrogenase (MDH; EC. 1.1.1.37), and the malic enzyme (ME; EC 1.1.1.40); and the buffer 0.025 M Tris – 0.192M Glycine (Sigma T–503, Sigma 2–6761), for alcohol dehydrogenase (ADH; EC. 1.1.1.1), gluconate dehydrogenase (2LUD; EC. 1.4.1.3), leucil–glycil–glycine (L22; EC. 3.4.11.4), leucyl alanine (LA; EC. 3.4.11.1), leucyl–proline (PAP; EC. 3.4.11.5), superoxid dismutase (SOD; EC.1.15.1.1), glucose phosphate isomerase (2PI; EC. 5.3.1.9.), and adenylate kinase (AK; EC. 2.7.4.3).
Once the electrophoretic assay was concluded, each gel was cut into five or six thin slices that were stained following the histochemical methods of Selander et al. (1971) and Harris & Hopkinson (1976). The slices were incubated at 37°C for 15 min or until clear bands appeared on the gels.
The interpretation of the bands followed Utter (1986), and the loci and allele nomenclature followed the guidelines proposed by Shaklee et al. (1989).
The programme TFP2A (Miller, 1997) was used to compute allelic frequencies, the mean observed and expected heterozygosity for every locus, and the percentage of polymorphic loci under the criterion of 95% of frequency for the most common allele. Genetic similarities and identities between species were computed following the procedure of Reynolds et al. (1983). Finally, using the Phylip 3.67 programme and the method of Neighbor Joining, a resampling of the data base was carried out by "Bootstrap" (5000 iterations), and a dendrogram was obtained (consensus tree) for the relationship of the five studied species (Felsenstein, 1985), with the length of the branches indicated by brackets.
RESULTS
Whole muscle proteins. Figure 1 shows the electrophoretic patterns found in the whole muscle protein runs of the five catfish species studied.
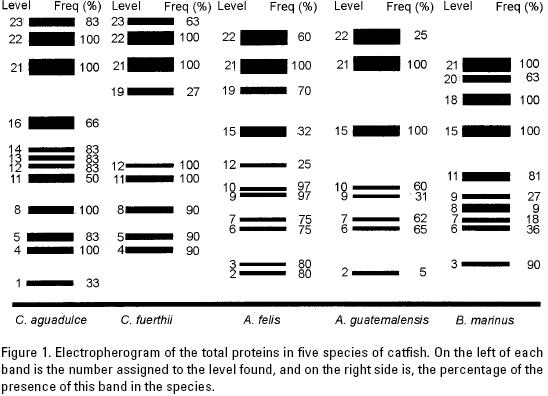
A total of 22 different bands were identified for the five ariid species studied, and three additional bands were detected in R. guatemalensis (Figure 1). Twelve bands were found in specimens of Cathorops aguadulce, nine in Cathorops fuerthii, 11 in Ariopsis felis, eight in Ariopsis guatemalensis, 10 in Bagre marinus and only six in the specimen of the outside group of Rhamdia guatemalensis. At the intrageneric level, C. aguadulce shared eight bands (66.6%) with C. fuerthii, and A. felis also shared eight bands (88.9%) with A. guatemalensis.
At the species level, C. aguadulce showed the highest number of bands with 12 (54.55%) out of the 22 bands resolved, whereas B. marinus presented the lowest number of bands with nine (40%). Only band 18 of B. marinus was diagnostic for this species, as it was exclusive and present with a 100% frequency. The presence of other bands may be highly indicative of other species, as in the case of bands 13, 14 and 16 that may indicate the presence of C. aguadulce, the only species where they occur, although with frequencies below 100%. Likewise, at the intergeneric level, the joint presence of some bands (5 and 23), although not at frequencies of 100%, could nonetheless be diagnostic for the identification of the genus Cathorops, as well as band 2 that is found exclusively in the genus Ariopsis.
Intrageneric comparisons show that the two species of the genus Cathorops share eight of the 12 bands present in this genus (4, 5, 8, 11, 12, 21, 22 and 23), representing 66.6%. The two analysed species of the genus Ariopsis showed a greater similarity (88.8%) by sharing eight of the nine bands resolved (2, 6, 7, 9, 10, 15, 21 and 22). Bands 21 and 22 are shared by the genera Cathorops and Ariopsis, but they consistently appear together only in Cathorops with frequencies of 100%, while lower values for band 22 are found in the genus Ariopsis. When comparing the genus Cathorops and Bagre marinus, only three bands (8, 11 and 21) are shared, whereas the genus Ariopsis and Bagre marinus share five bands (6, 7, 9, 15 and 21) indicating that there is a greater affinity in this latter case.
Ferguson's identity coefficients, shown in table 1, were obtained by computing the number of shared bands between two taxa relative to the total number of bands, in order to evaluate their proximity. It is important to point out that the average taxonomic distance is inversely proportional to the identity coefficient, and that when one increases, the other one decreases proportionally. Values range from one to zero. Estimations close to one indicate no differences, whereas values tending to zero indicate larger differences. In this study, the coefficients presented a range of values of 0 to 0.66, the highest corresponding to the comparison between the species of the genus Cathorops (0.66). The species of Ariopsis had a coefficient of 0.63. The similarity coefficient between Bagre marinus and A. guatemalensis had values of 0.6 and 0.54 when compared with A. felis, indicating a closer proximity between these genera. Smaller similarity values, from 0.16 to 0.36, were recorded for the intergeneric comparisons, particularly in the comparison among the species of the genera Cathorops and Ariopsis (Table 1).
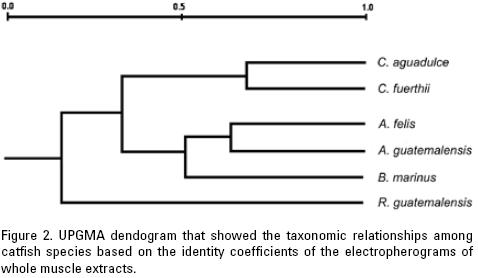
The dendrogram based on these coefficients is shown in figure 2, and illustrates a clear separation between the genus Catho–rops and the cluster of the Ariopsis species and Bagre marinus.
Allozymes. Of the 21 resolved loci, 20 were polymorphic in C. aguadulce (95.2%), three in C. fuerthii (14.3%), 14 in A. felis (66.6%), 15 in A. guatemalensis (71.4%) and 11 in B. marinus (52.3%). No monomorphic locus was found in any of the analysed species. A summary of genetic variability found in the 21 enzyme loci analysed in the five studied species of marine catfish is shown in table 2.

The lowest level of variability was recorded for C. fuerthii with an expected average heterozygosity (He) of 0.070, and the highest level was recorded for Ariopsis felis (He = 0.213), followed closely by B. marinus (He = 0.193) (Table 2). The genetic distances of Reynolds et al. (1983) (Table 1 over the diagonal) ranged from 0.340, for the comparison between A. felis and A. guatemalensis, to 0.920 between C. fuerthii and Rhamdia guatemalensis. The Neighbor Joining analysis used to cluster species according to the genetic distances of Reynolds et al. (1983) showed that the species of the genus Ariopsis cluster together, and that with Bagre marinus they form a node that is clearly separated from the species of the genus Cathorops (Figure 3). The support of the branches obtained by the bootstrap was of 93.18% for the A. felis and A. guatemalensis branch, and of 68.72% for the species of Cathorops, supporting the hypothesis that the genus Ariopsis forms a node close to B. marinus, while the genus Cathorops is clearly separated from the latter.

DISCUSSION
The lowest level of genetic variability was recorded for C. fuerthii, within the range of the values reported by Suzuki & Phan (1990) for the ariid fish of Brazil, in which the levels of expected heterozygosis vary from 0.0018 in Netuma barba (Lacépéde, 1803) to 0.0887 in C. Spixii (Agassiz, 1829). The differences found may be due to the fact that these authors report only six polymorphic loci, while in this study polymorphism was detected in 17 alloenzymatic loci for the five analysed species. The differences could also be due to our larger sample size.
Our findings agree with the results of the morphological comparison of the digestive tract structures of A. felis, B. marinus and C. aguadulce, that indicated that the number and position of pharyngeal teeth, the length of the intestine and the number of secondary walls in the swimming bladders increase gradually in the sequence Cathorops aguadulce – Ariopsis felis – Bagre marinus (Kobelkowsky & Castillo Rivera, 1995). Acero et al. (2005) found differences between the genera Ariopsis and Cathorops with respect to the enormous development of the pelvic fins in the females of Ariopsis, whereas in Cathorops the dimorphism in the pelvic structures, even in mature fish, is less pronounced. However, the differentiation of the shoulder girdle in this genus is more contrasting than in Ariopsis, and the molariform teeth in the palate of Cathorops, an autopomorphy of the genus, are less numerous in the males than in the females (Betancur et al., 2004).
Reproduction in ariids involves the greater development of the pelvic fins of the females to sustain the egg mass at the time of spawning and for hatching for several weeks. A less strong and rigid girdle may be important in the males to facilitate the expansion of the bucco–pharyngeal cavity for oral incubation, and a smaller development of the pharyngeal teeth may be advantageous to avoid damage and mistreating of eggs and larvae (Acero et al., 2005).
The ariid species of the genera Cathorops and Ariopsis studied in the present work, are representative transisthmian species: C. aguadulce and A. felis are found in the Gulf of Mexico, and C. fuerthii and A. guatemalensis in the Pacific Ocean. Different levels of genetic distance appeared when the species of each genus were clustered: 0.478 in the case of Cathorops species and 0.340 in the Ariopsis species. The distance between C. aguadulce and the Ariopsis species is 0.441 for A. felis and 0.444 for A. guatemalensis, while C. fuerthii presented a larger distance with respect to these two species, of 0.714 and 0.760 respectively. These differentiation levels are considered to be related to the time of independent evolutionary divergence, since the concurrent establishment of reproductive isolation between the congeneric species should coincide with the emergence of the Panama isthmus about 3 to 3.5 million years ago (Keigwin, 1978; Coates et al., 1992). Betancur (2003) described this event as vicarious and mentioned that four recent events of transisthmian speciation may be identified in the family, two of which are present in the group ('Arius A). The low values of genetic divergence between 'Arius A' cookei and A. aff. cookei (1.7%), and their distribution patterns found also in other marine fish, suggest a recent connection between the Pacific Ocean and the Atrato river basin. The diversification of the Ariinae lineages seems to have happened for short periods a long time ago, perhaps in the Tethys Sea before its final closure, and could explain the monophyly of the American ariines.
Betancur (2003) suggested the existence of two lineages in the family Ariidae: he proposed the Ariinae, and the Galeichthynae (Acero & Betancur, 2007) as a new subfamily, corroborating the monophyly and validating the genera Ariopsis, Bagre (Cloquet, 1816), Cathorops and Galeichthys (Velenciennes, 1840).
Cathorops aguadulce is largely one species confined to freshwater environments, sporadically present in river mouths (i.e. secondary freshwater fish) (Hubbs & Miller, 1960; Marceniuk, 1997; Castro–Aguirre et al., 1999; Acero, 2002; Acero & Betancur, 2002; Marceniuk & Ferraris, 2003) It is a species probably related to C. melanopus (Marceniuk, 1997), implying a common freshwater origin, although the examination of samples of this species, in a phylogenetic context, is needed to test this proposal (Betancur, 2003).
Although Betancur (2003) found that discrepancies between molecular and morphological data of the genus Cathorops could imply a morphological convergence, this theory has been tested and the monophyly of the group has been established. Marceniuk (1997) made a taxonomic revision of several species of Cathorops of both coasts of the American continent, based on discriminate methods with morphometric data, and suggested that osteological synapomorphies confirm the monophyly of the genus.
The studies of Betancur et al. (2004), Marceniuk (2007), Acero & Betancur (2007), Marceniuk & Betancur (2008) and Betancur (2009a) coincided in the results obtained so far, and support and confirm the monophyly of the ariids. Nevertheless, this monophyly, based and validated through previous morphological studies, is not congruent with molecular phylogenies and has been challenged (Betancur, 2009b).
According to Betancur (2003, 2010), the restriction to freshwater environments of some of the studied species involves at least three events of secondary invasion of neotropical ariines, and implies a reversal to the primitive conditions of the Otophysi. The confinement of Potamarius (Hubbs & Miller, 1960), C. aguadulce / C. melanopus and other marine fish to the basins of the river Usumacinta and Izabal, can be explained by shared histories or particular ecological conditions, such as high ionic concentrations and an ecological unsaturation of primary ichthyofauna that favoured the invasion of these systems (Betancur, 2003).
Several authors have pointed out that most species of Cathorops and Ariopsis present anadromous trends, and occur in both marine and fresh water environments entering the lower and even the higher areas of the rivers (Cervigón, 1991; Marceniuk, 1997; McEachran & Fechhelm, 1998; Lucas & Baras, 2001; Acero, 2002; Robertson & Allen, 2002; Betancur, 2003).
A similar pattern of differentiation has been found for the genus Centropomus (Lacépéde, 1802) of the eastern tropical Pacific. The species C. robalito (Jordan y 2ilbert, 1882), with oceanic habits and a seemingly larger larval dispersal capacity, has been found to be homogenous, while the coastal and fresh or brackish water–dependent species C. viridis (Lockington, 1877) and C. medius (Günther, 1864), that are confined mainly to estuaries and coastal lagoons, revealed some population structure (Díaz–Jaimes et al., 2007).
The dendrograms derived from the 21 allozymatic loci showed the same topology as the dendrogram built with the total proteins of muscle tissue, evaluated by means of the similarity coefficient of Ferguson (1980), corroborating the consistency of these methods in this study, and their likely usefulness for the study of phylogenetic relationships among these fish groups. Likewise, the general topology of the trees obtained by Betancur (2003), by maximum parsimony achieved through mitochondrial DNA (cytochrome b, ATP synthase 8 and 6, 12S and 16S), nuclear fragment (activating gene of the recombination 2) and morphometrics, agree with our results in clustering the Cathorops species apart from the Ariopsis species. The genus Bagre is also separated from these two genera, but is closer to Ariopsis. Similarly, Kobelkowsky & Castillo–Rivera (1995) and Taylor & Menezes (1978) concurred in the acknowledgement of clear cut anatomical differences among these three genera, as mentioned above and as found in our study.
ACKNOWLEDGMENTS
This study was part of the project for the Doctorate Program in Biological Sciences of the Universidad Autónoma Metropolitana, plantel Iztapalpa, a program within the PNCP (Programa Nacional de Posgrados de Calidad del CONACYT, No: 0307–O). The first author received a scholarship awarded by CONACYT (No.83532). The valuable help of Alma Delia Hernández Pérez, Ma. de Lourdes Barbosa Saldaña and Edson Sandoval Castellanos, as well as the observations and support of José Ángel Ronsón Paulín, Pedro Cervantes Hernández and Derek Brockett are gratefully acknowledged. The valuable comments of two anonymous reviewers are also gratefully acknowledged.
REFERENCES
Acero, P. A. 2002. Ariidae. In: K. E. Carpenter (Ed.). The living marine resources of the Western Central Atlantic. Vol. II. FAO, Roma, pp. 831–852. [ Links ]
Acero, P. A. & R. Betancur–R. 2002. Arius cookei, a new species of ariid catfish from the tropical American Pacific. Aqua Journal of Ichthyology and Aquatic Biology 5 (4): 133–138. [ Links ]
Acero, P. A. & R. Betancur–R. 2007. Monophyly, affinities, and subfamilial clades of sea catfishes (Siluriformes: Ariidae). Ichthyological Exploration of Freshwaters 18 (2): 133–143. [ Links ]
Acero, P. A., R. Betancur–R, A. F. Polanco & N. Chaparro. 2005. Diferenciación sexual temprana a nivel óseo en dos géneros de bagres marinos (Pisces: Ariidae) del Caribe. Memoria de la Fundación La Salle de Ciencias Naturales 163: 37–43. [ Links ]
Avise, J. C. 1994. Molecular Markers, Natural History and Evolution. Chapman and Hall, New York. 511 p. [ Links ]
Betancur R, R. 2003. Filogenia de los bagres marinos (Siluriformes: Ariidae) del Nuevo Mundo. Tesis de Maestría en Ciencias, Universidad Nacional de Colombia–INVEMAR, Bogotá. 121 p. [ Links ]
Betancur R, R. 2009a. Systematics and evolutionary history of sea catfishes (Siluriformes: Ariidae). Ph.D. Dissertation, Auburn University, Auburn. 200 p. [ Links ]
Betancur R, R. 2009b. Molecular phylogenetics and evolutionary history of ariid catfishes revisted: a comprehensive sampling. Evolutionary Biology 9: 175. [ Links ] Betancur–R, R. 2010. Molecular phylogenetics supports multiple evolutionary transitions from marine to freshwater habitats in ariid catfishes. Molecular Phylogenetics and Evolution 55 (1): 249–258. [ Links ]
Betancur R, R., A. Acero & L. M. Mejía–Ladino. 2004. Sistemática filogenética preliminar de algunos bagres marinos (Siluriformes: Ariidae) neotropicales [Preliminary phylogenetic analysis of some Neotropical sea catfishes (Siluriformes: Ariidae)]. Memoria de la Fundación La Salle de Ciencias Naturales 158: 61–85. [ Links ]
Castro–Aguirre, J. L. 1978. Catálogo sistemático de los peces marinos que penetran a las aguas continentales de México con aspectos zoo–geográficos y ecológicos. Instituto Nacional de la Pesca. México. Serie Científica 19: 1–298. [ Links ]
Castro–Aguirre, J. L., H. S. Espinosa & J. J. Schmitter–Soto. 1999. Ictiofauna Estuarino–Lagunar y Vicaria de México. Colección Textos Politécnicos. Serie Biotecnologías. Ed. Limusa. México, D.F. pp. 140-158, 521–532. [ Links ]
Cervigón, F. 1991. Los peces marinos de Venezuela. Segunda edición. Vol. 1. Fundación Científica Los Roques, Caracas. 423 p. [ Links ]
Coates, A. G., J. B. Jackson, L. S. Collins, T. M. Cranin, H. J. Dowset, L. M. Bybell, P. Jung & J. 0. Obando. 1992. Closure of the isthmus of Panama. The near–shore marine record of Costa Rica and Western Panama. Geological Society of America. Bulletin 104: 814–828. [ Links ]
Crisci, J. V. & M. F. López–A. 1983. Introducción a la teoría y práctica de la taxonomía numérica. Secretaría General de la Organización de Estados Americanos. Programa Regional de Desarrollo Científico y Tecnológico. Washington, D. C. 132 p. [ Links ]
Díaz–Jaimes, P., E. Sandoval–Castellanos & M. Uribe–Alcoccer. 2007. Comparative population structure of three snook species (Centropomidae) from the eastern central Pacific. Icthyological Research 54 (4): 380–387. [ Links ]
Fehrntröm, H. & U. Moberg. 1977. SDS Conventional Polyacrylamide Gel Electrophoresis with LKB 2117 Multiphor Application LKB Produkter Note 306, Sweden. 15 p. [ Links ]
Felsenstein, J. 1985. Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39: 783–791. [ Links ]
Ferguson, A. 1980. Biochemical systematic and evolution. John Wiley and Sons, New York. 194 p. [ Links ]
Fitzsimmons, J. M., W. H. LeGrande & J. W. Korth. 1988. Karyology of the marine catfish Bagre marinus (Ariidae) with an analysis of chromosome number among siluriform fishes. Japanese Journal of Ichthyology 35: 189–193. [ Links ]
Fuentes–Mata, P., H. Espinosa–Pérez & J. Luna–Wiarco. 1989. Nuevos registros de peces en la laguna de Sontecomapan, Veracruz, México. Anales del Instituto de Biología. Universidad Nacional Autónoma de México. Serie. Zoología 60 (2): 257–262. [ Links ]
García–Molina, F. & M. Uribe–Alcocer. 1989. Análisis cromosómico del Bagre Marino Arius felis (Ariidae: Siluriformes) de la Región de la Laguna de Términos Campeche. Anales del Instituto de Ciencias del Mar y Limnología. Universidad Nacional Autónoma de México 16: 69–74. [ Links ]
Gomes, V., V. N. Phan & M. J. A. C. R. Passos. 1990. The karyotype of a marine catfish Bagre bagre from Brazil. Japanese Journal of Ichthyology 37: 321–323. [ Links ]
Gomes, V., V. N. Phan. & M. J. A. C. R. Passos. 1992. The karyotype of Cathorops sp. a marine catfish from Brazil. Boletim do Instituto Oceanografico. São Paulo 40: 87–91. [ Links ]
Gomes, V., V. N. Phan & M. J. A. C. R. Passos. 1994. Karyotypes of three species of marine catfishes from Brazil. Boletim do Instituto Oceanografico. São Paulo 42: 55–61. [ Links ]
Harris, H. A. & D. A. Hopkinson. 1976. Handbook of enzyme electrophoresis in human genetics. American Elsevier Publishing Company, Inc. New York. 306 p. [ Links ]
Higuchi, H. 1982. Estudo osteologico do bagres marinhos do litoral sul do Brasil (Osteichthyes: Siluroidei: Ariidae). Dissertagao do maestrado. Universidade do São Paulo. Instituto de Biociencias, Brasil. 135 p. [ Links ]
Hubbs, C. L. & R. R. Miller. 1960. Potamarius, a new genus of ariid catfishes from the fresh waters of Middle America. Copeia 1960(2): 101-112. [ Links ]
Kailola, P. J. & W. A. Bussing. 1995. Ariidae. In: Fischer, F., W. Krupp, F. Schneider, C. Sommer, K. E. Carpenter & ZV. Niem (Eds.). Guía FAO para identificación de Especies para los Fines de la Pesca, Pacífico Centro–Oriental. Vol. II, Parte 1. FAO, Roma, pp. 860–886. [ Links ]
Keigwin, L. D. 1978. Pliocene closing of the Isthmus of Panama based on biostratigraphic evidence from nearby Pacific and Caribbean sea cores. Geology 6: 630–634. [ Links ]
Kobelkowsky, D. A. & M. Castillo–Rivera. 1995. Sistema digestivo y alimentación de los bagres (Pisces: Ariidae) del Golfo de México. Hidrobiológica 5 (1–2): 95–103. [ Links ]
LeGrande, W. H. 1980. The chromosome complement of Arius felis (Siluriformes: Ariidae). Japanese Journal of Ichthyology 27: 82–84. [ Links ]
Lima, D., J. E. P. Freitas, M. E. Araujo & A. M. Sole–Cava. 2005. Genetic detection of cryptic species in the frillfin goby Bathygobius soporator. Journal of Experimental Marine Biology and Ecology 320 (2): 211–223. [ Links ]
Lucas, M. C. & E. Baras. 2001. Migration of freshwater fishes. Blackwell Science, Oxford. 420 p. [ Links ]
Marceniuk, A. 1997. ReviSão sistematica do género (Osteichthyes: Siluriformes, Ariidae). Dissertagao do maestrado. Universidade do São Paulo, Brasil. 315 p. [ Links ]
Marceniuk, A. P. 2007. Description of Cathorops manglarensis, a new species from the Colombian Pacific, with redescription of Cathorops multiradiatus (Siluriformes; Ariidae). Zootaxa 1529: 33–48. [ Links ]
Marceniuk, A. P. & R. Betancur–R. 2008. Revision of the species of the genus Cathorops (Siluriformes: Ariidae) from Mesoamerica and the Central American Caribbean, with description of three new species. Neotropical Ichthyology 6 (1): 25–44. [ Links ]
Marceniuk, A. P. & C. J. Ferraris. 2003. Ariidae. In: Reis, R. E., S. O. Kullander & C. J. Ferraris (Eds.). Checklist of the Freshwater Fishes of South and Central America. Edipucrs, Porto Alegre, Brazil, pp. 447–455. [ Links ]
Marceniuk, A. P. & N. A. Menezes. 2007. Systematics of the family Ariidae (Ostariophysi Siluriformes), with a redefinition of the genera. Zootaxia 1416: 1–126. [ Links ]
McEachran J. D. & J. D. Fechhelm. 1998. Fishes of the Gulf of México. University of Texas Press, Texas. Vol. 1: 1–1112. [ Links ]
Mayden, R. L., B. M. Burr, L. M. Page & R. R. Miller. 1992. The native freshwater fishes of North America, In: R. L. Mayden (Ed.). Systematics, Historical Ecology and North American Freshwater fishes. Stanford University Press, Stanford, California pp. 827–863. [ Links ]
Meek, S. E. 1904. The Fresh Water Fishes of Mexico North of The Isthmus of Tehuantepec. Publication Field Columbian Museum Zoological Series 5: 1–252. [ Links ]
Miller, M. P. 1997. Tools for population genetic analyses (TFPGA) version 1.3. A windows program for the analysis of allozyme and molecular population genetic data. [ Links ]
Regan, C. T. 1906–1908. Pisces. In: Biologia Centrali Americana 8: 1–201. [ Links ]
Reséndez–Medina, A. 1983. Hidrología e ictiofauna de la laguna de Zontecomapan, Veracruz, México. Anales del Instituto de Biología. Universidad Nacional Autónoma de México 53. Serie Zoología (1): 385–417. [ Links ]
Reynolds, J. B., B. S. Weir & C. C. Cockerham. 1983. Estimation of the concestry coefficient: basis for a short–term genetic distance. Genetics 105: 767–779. [ Links ]
Rishi, K. K., J. Singh & M. S. Haoban. 1983. Karyological study on a marine catfish Arius dussumieri (Val.) (Ariidae: Siluriformes). Chromosome Information Service 34: 7–9. [ Links ]
Robertson, D. R. & G. R. Allen. 2002. Shore fishes of the tropical eastern Pacific: an information system. Smithsonian Tropical Research Institute, Balboa, Panamá. CD. [ Links ]
Ruiz–Carus, R. & M. Uribe–Alcocer. 2003. Phylogenetic assessment of Eucinostomus gula, Eugerres plumieri and Diapterus auratus (Gerreidae: Pisces) based on allozyme and mtDNA analyses. Caribbean Journal of Science 39 (1): 109–115. [ Links ]
Sall, J., K. Hecht, M. D. Tilley & R. Potter. 1989–1995. JMP Statistics Made Visual, versión 3.1.2. SAS Institute Inc. [ Links ]
Selander, R. K., M. H. Smith, J. Y. Yang, E. Johnson & J. B. Gentry. 1971. Biochemical polymorphism and systematics in the genus Peromyscus I, variation in the old field mouse (Peromyscuspolionatus). Studies in Genetics 6: 49–90. [ Links ]
Shaklee, J. B., F. W Allendorf, D. C. Morizot & G. S. Whitt. 1989. Genetic nomenclature for protein coding loci fish: Proposed guidelines. American Fisheries Society Transaction 118: 218–227. [ Links ]
Suzuki, H. & V. N. Phan. 1990. Electrophoretic study on intraspecific variation and interspecific relationship of marine catfishes (Siluriformes: Ariidae) of Cananeia (São Paulo, Brazil) 2. Isozymes of skeletical muscle. Boletim do Instituto Oceanográfico. São Paulo 38 (4): 43–55. [ Links ]
Taylor, W. R. & N. A. Menezes. 1978. Family Ariidae. In: W. Fischer (Ed.). Western Central Atlantic (fishing area 31) FAO species identification sheets for fishery purposes. Rome, Vol. 1. FAO, Roma. [ Links ]
Uribe–Alcocer, M. & P. Díaz–Jaimes. 2000. Fish chromosomes as biomarkers of genotoxic damage and proposal for the use of tropical catfish species for short–term screening of genotoxic agents. In: Butter–Worth F. M., A. Gunatilaka & M. E. Gonsebatt (Eds.) Biomonitors and Biomarkers as Indicator of Environmental Change. Vol. II. Plenum Press, NY. pp. 361–390. [ Links ]
Utter, F. M. 1986. Validity of electrophoresis in identifying fish populations structures. In: D. Hedgecock (Ed.). Workshop on identify in fish subpopulations. California Sea Grant collection. pp. 14–19. [ Links ]
Wheeler, A. & A. Baddokwaya. 1981. The generic nomenclature of the marine catfishes usually referred to the genus Arius (Osteichthyes: Siluriformes). Journal of Natural History 15 (5): 769–773. [ Links ]














