Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Hidrobiológica
versão impressa ISSN 0188-8897
Hidrobiológica vol.20 no.3 Ciudad de México Jan. 2010
Artículos
Pathogenic vibrios in the oyster Crassostrea virginica in the lagoon system of Mandinga, Veracruz, Mexico
Vibrios patogénicos en el ostión Crassostrea virginica en el sistema lagunar de Mandinga, Veracruz, México
Christian Reyes–Velázquez,1 María del Refugio Castañeda–Chávez,1 Cesáreo Landeros–Sánchez,2 Itzel Galaviz–Villa,1 Fabiola Lango–Reynoso,1,3 Martha M. Minguez–Rodríguez1 and Iourii Nikolskii–Gavrilov4
1 Instituto Tecnológico de Boca del Río, km 12 Carretera Veracruz–Córdoba, Boca del Río, Veracruz, C.P. 94290, México.
2 Colegio de Postgraduados, Campus Veracruz (COLPOS), Km 88.5 Carretera Federal Xalapa–Veracruz, vía Paso de Ovejas entre Paso San Juan y Puente Jula, Tepetates, Veracruz, C.P. 91700.
3 Estancia Sabática Vinculada al Fortalecimiento de la Calidad del Posgrado Nacional CONACYT 2008.
4 Colegio de Postgraduados, Campus Montecillo (COLPOS), Km 36.5 Carretera México–Texcoco, Montecillo, Mpio. de Texcoco, Edo. de México, C.P. 56230 México. E–mail: clandero@colpos.mx.
Recibido: 20 de diciembre de 2009
Aceptado: 30 de noviembre de 2010
ABSTRACT
Oyster consumption has been associated to the transmission of pathogenic bacteria, including those from the genus Vibrio. The objective of this investigation was to determine the concentrations of Vibrio parahaemolyticus and V. alginolyticus in Crassostrea virginica from the lagoon system of Mandinga, Veracruz, and their relationships with salinity and water temperature. Only the periods of greater production and consumption of oysters were examined: the dry and rainy seasons during 2008. Four sampling sites were selected, from which three samples were collected per site per season, resulting in a total of 24 samples. Each sample consisted of 30 oysters of commercial size that were subsequently analyzed using serial dilution. Biochemical analysis of the resulting bacterial colonies revealed concentrations of V. parahaemolyticus and V. alginolyticus ranged from < 3 to 150 MPN/g. A positive correlation was observed between temperature and the concentration of V. parahaemolyticus (r = 0.69, p < 0.05), while the correlation with salinity was negative (r = –0.68, p < 0.05). No significant correlations between the concentration of V. alginolyticus and water temperature and salinity were observed for the rainy season. The main contribution of this paper is to establish safe areas and periods for oyster extraction.
Key words: Vibrio parahaemolyticus, Vibrio alginolyticus, Crassostrea virginica, Mandinga Lagoon, Mexico.
RESUMEN
El consumo de ostión ha sido asociado con la transmisión de bacterias patógenas, entre las que se encuentran las bacterias del género Vibrio. El objetivo de esta investigación fue determinar las concentraciones de Vibrio parahaemolyticus y V. alginolyticus en Crassostrea virginica del sistema lagunar de Mandinga, Veracruz, y su relación con la salinidad y temperatura del agua. Se consideraron las épocas de mayor producción y consumo de ostión, esto es, secas y lluvias de 2008. Se seleccionaron cuatro sitios de muestreo y se tomaron 3 muestras por sitio por época, resultando un total de 24 muestras. Cada muestra consistió de 30 organismos de talla comercial que se analizaron mediante la técnica de diluciones seriadas. Se hizo una caracterización bioquímica de las colonias encontradas para determinar las concentraciones de V. parahaemolyticus y V. alginolyticus en NMP/g, cuyos valores fueron de < 3 a 150 NMP/g. Se observó una correlación positiva entre la temperatura y la concentración de V. parahaemolyticus (r = 0.69, p < 0.05), mientras que la correlación de ésta ultima y la salinidad fue negativa (r = –0.68, p < 0.05). No se pudo encontrar una correlación entre la concentración de V. alginolyticus y la temperatura y salinidad del agua en la época de lluvias. El principal aporte de este trabajo fue el establecer zonas y períodos seguros de extracción de ostión.
Palabras clave: Vibrio parahaemolyticus, Vibrio alginolyticus, Crassostrea virginica, Laguna Mandinga, México.
INTRODUCTION
The Gulf of Mexico is considered one of the most important coastal zones in the world, and encompasses major fishing activities including that for the American oyster, Crassostrea virginica (Gmelin, 1791) (Baqueiro–Cárdenas et al., 2007). Given that the soft body of the oyster is consumed whole, and either raw or lightly cooked, it is generally classified as a high–risk food (Daniels et al., 1998; Fukushima & Seki, 2004). Oyster consumption is associated with the transmission of pathogenic bacteria such as those in the genus Vibrio. This genus contains more than 70 species, of which 11 are known to be human pathogens such as V. parahaemolyticus Fujino and V. alginolyticus Miyamoto (Chakraborty et al., 1997; Thompson et al., 2004). V. alginolyticus (Fujino et al. 1951), like other species of Vibrio, can produce infections in the ears and open wounds through contact with water containing this species (Parveen et al., 2008). V. parahaemolyticus (Miyamoto et al. 1961) is one of the more pathogenic species and can be found in a proportion of up to 100 times more in cupped oysters than in the aquatic environment, and is associated with gastroenteritis, wound infection, and septicemia (Croci et al., 2002; Cabrera–García et al., 2004).
The genus Vibrio is a group of Gram–negative, halophilic bacteria occurring naturally in estuarine environments. The species are distributed worldwide in sea water and are associated with the resident aquatic organisms. Their presence is independent of anthropogenic pollution, but is dependent on salinity, temperature and organic matter (Hervio–Heath et al., 2002). Their concentration in the aquatic environment and in foods of marine origin is a function of the geographic and hydrographic conditions in the area, and varies according to the time of year and location within the lagoon systems (Sousa et al., 2004). Vibrio are the principal bacteria causing sickness and death as a consequence of consuming contaminated shellfish.
Based on this information and considering that Mexico is the sixth largest producer of oysters globally (FAO, 2006), the objective of the present study was to determine the concentrations of V. parahaemolyticus and V. alginolyticus in C. virginica from the lagoon system of Mandinga, Veracruz, and their relationships with water temperature and salinity during times of greater oyster production and consumption.
MATERIALS AND METHODS
Study Area. The Mandinga lagoon system is located on the coast of the Gulf of Mexico in the central part of the state of Veracruz (19° 00’ to 19° 06’ N, and 96° 02’ to 96° 06’ W), and covers an area of 3,250 ha (Fig. 1).

Sample collection and recording of water temperature and salinity. Samples and recorded data were collected during May (the dry season), and July (the rainy season) of 2008. These seasons are considered to be the highest for the production and consumption of oysters in the region. The sampling sites were located in permanent oyster harvesting areas of the Mandinga lagoon system in Veracruz, Mexico (SEFIPLAN, 1999). Four sampling sites were selected and three samples were taken per season, for a total of 24 samples. Each sample consisted of 30 oysters of commercial size (7 ± 3 cm) that were cleaned, and packaged for transportation according to specifications provided in the Norma Oficial Mexicana NOM–109–SSA1–1994 (DOF, 1994). In each sampling site, water temperature and salinity were measured using a combined probe (Model YSI–6600 V2, YSI Inc., Yellow Springs, Ohio, USA) (DOF, 1994; Castañeda et al., 2005).
Determination of Vibrio parahaemolyticus and V. alginolyticus. In a sterile laboratory area, oysters were opened to obtain the entire visceral mass and the intervalvar liquid which were blended and stored in sterile glass measuring cups (Norma Oficial Mexicana NOM–031–SSA1–1993; DOF, (1993)) and the mixtures were processed in a time no longer than 1 hour, at laboratory conditions, as established by Dileep et al., 2003.
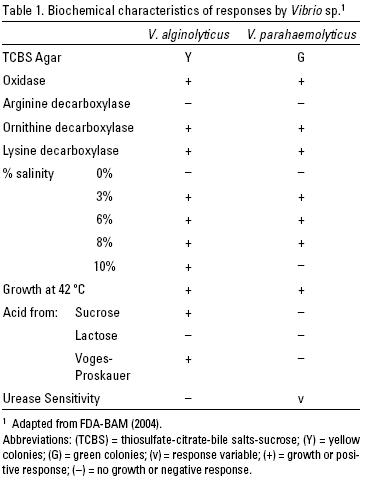
The technique used for the determination of Vibrio spp. was based on the methodology developed by the USDA Food and Drug Administration (FDA–BAM, 2004). A 25 g subsample was removed from each mixture of visceral mass and intervalvar water, and the subsample homogenized for 2 minutes in a sterile food processor (Waring, Hartford, Connecticut, USA) with the addition of 225 ml of alkaline peptone water (APW) enriched with 3 % sodium chloride. Subsequently, a series of three hexadecimal dilutions was performed on each one of the homogenized mixtures (1:100, 1:1000 y 1:10000), which were then incubated at 35 °C for 6–8 hours. An aliquot for inoculation was extracted from each dilution and was applied, using cross–streaking, to the selective Thiosulfate–Citrate–Bile Salts–Sucrose (TCBS) agar (Merck®, Alemania) in Petri dishes and incubated at 35 ºC for 24–48 hours (Olafsen et al., 1993).
Yellow (sucrose positive) (typical of V. alginolyticus) and green (sucrose negative) (typical of V. parahaemolyticus) colonies were selected from the TCBS plates. Petri dishes containing a nonselective medium (trytone casein and salt, T1N3, Merck®, Germany) were then inoculated with samples of these colonies in order to promote growth of the selected bacteria. These plates were incubated for a period of 18 to 24 hours at 35 ºC. Samples were taken from the colonies produced and placed on indicator strips of Bactident oxidase (Merck®, Germany) to test for oxidase. If a violet color appeared, the reaction to the oxidase was considered positive, or when there was no observed color change, the reaction was considered negative.
A small sample was taken from the colonies considered positive for oxidase, and inoculated into media for the biochemical testing of mobility, decarboxylation of ornithine, lysine and arginine, acidity or fermentation of lactose and sucrose, salinity tolerance, the Voges–Proskauer reaction, urea metabolism, and growth at 42 ºC. The results were obtained using the biochemical profile described in Table 1, and were expressed as the Most Probable Number per gram of sample (MPN/g). The latter was determined from dilutions.
Statistical analysis. Data were analyzed using the software Statistica v7.0 (Statsoft, Inc., Tulsa, Oklahoma, USA). A nonparametric Kruskal–Wallis test was used to look for significant differences (P < 0.05) between seasons and sampling sites. Concentrations of Vibrio sp. were correlated with water temperature and salinity.
RESULTS
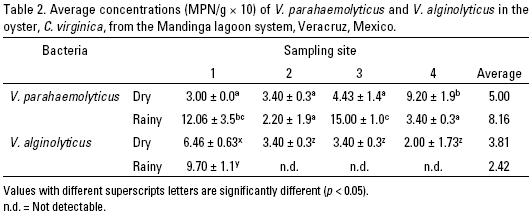
Average concentrations of V. parahaemolyticus and V. alginolyticus in C. virginica by season of production and consumption and by sampling site in the Mandinga lagoon system are presented in Table 2. Results from the Kruskal–Wallis test between seasons and sampling sites are shown in Figures 2 and 3. A possible trend of a correlation between average concentrations of V. parahaemolyticus and water temperature and salinity is shown in Figures 4 and 5.
DISCUSSION
Various studies exist on the capacity for oysters to filter and accumulate bacteria directly from the water, especially marine bacteria such as V. parahaemolyticus and V. alginolyticus which are pathogenic to humans and the latter which also is known to affect larval fish and crustaceans (Barbieri et al., 1999; Parveen et al., 2008). The results from the present study in the Mandinga lagoon system indicate that the American oyster, C. virginica, is a natural reservoir of bacteria such as V. parahaemolyticus and V. alginolyticus (Table 2), which is also confirmed by Di pinto et al. (2006).
Vibrio parahaemolyticus. This bacteria species has been little studied in bivalve molluscs from Mexico and in the world in general. However, when this bacterium is detected and determined to be the causative agent of gastrointestinal illness in humans by the consumption of raw fish and shellfish, the studies of this organism begin to escalate. The presence and concentration of this bacterial species are not regulated by the mexican regulations. The presence of V. parahaemolyticus has been reported in C. virginica and other shellfish (Cabrera–García et al., 2004; Cabanillas–Beltrán et al., 2006), but no studies have been reported in Mexico that evaluate the concentration by unit weight in bivalve molluscs. Countries such as China, Japan, Taiwan, Malaysia, Italy, and the United States of America have reported concentrations of V. parahaemolyticus in bivalve molluscs greater than 1100 MPN/g (Alam et al., 2003; Bilung et al., 2005; Di Pinto et al., 2006; Yi–Chen & Chengchu, 2007; Parveen et al., 2008).
In this study, V. parahaemolyticus was detected among seasons and sampling sites with concentrations that varied between 3 and 150 MPN/g (Table 2). Significant differences were detected between sampling seasons (p < 0.05), with the highest concentration of V. parahaemolyticus during the rainy season (Figure 2). The highest concentrations were found in sites 1 and 3, which coincided with the high temperatures and lower salinities (Table 3). The observed concentrations may also be produced by seasonal variation of the V. parahaemolyticus population by "hibernating" in the sediment or in partnership with the marine fauna, followed by population growth from runoff into the lagoon having high concentrations of organic matter and nutrients. This latter process not only promotes the growth of these bacteria, it eutrophies the Mandinga lagoon system (Aguilar–Ibarra et al., 2006).

The distribution, dynamics and occurrence of populations of V. parahaemolyticus also are influenced by gradients of environmental factors such as temperature, salinity, nutrients, and by biological factors such as the abundance and predation by dinoflagellates and other hosts (Alam et al., 2003; Thompson & Polz, 2006).
In spite of the few available data, the correlation between water temperature and concentrations of V. parahaemolyticus was significant and positive (r = 0.69, P < 0.05), while the correlation between bacterial concentration and salinity was significant and negative (r = –0.68, P < 0.05) (Figures 4, 5). Similar results have been reported by DePaola et al. (1990) and Parveen et al. (2008) who studied the incidence of V. parahaemolyticus in C. virginica along the Atlantic coast of the United States of America.
Thus, we confirmed that temperature is the factor that primarily determines the distribution and abundance of V. parahaemolyticus in lagoon systems of the Gulf of Mexico where the oyster C. virginica is abundant, and the minimum water temperature is 11.6 ºC (Cabrera–García et al., 2004). Also, we have further confirmed that increased salinity results in a reduced concentration of V. parahaemolyticus in C. virginica (Table 3) (Cook et al., 2002; DePaola et al., 2003).
Vibrio alginolyticus. V. alginolyticus was present during the dry season in all four sampling sites, but was only present in site 1 during the rainy season. During the dry season, the salinity in site 1 was 35 o/oo, with a concentration of V. alginolyticus of 6.46 MPN/g. During the rainy season, the salinity decreased to 20 o/oo and the bacterial concentration increased to 9.70 MPN/g (Table 2). This trend in salinity and bacterial concentration is similar to that for V. parahaemolyticus where the bacterial concentration declined with increasing salinity.
The lack of detection of V. alginolyticus in sampling sites 2, 3, and 4 (Figure 3) can be explained by the low primary productivity in Redonda Lagoon in the Mandinga lagoon system during the rainy season where sampling sites 2 and 3 were located. It is likely that a similar condition may have occurred in the mouth of Mandinga Grande Lagoon where site 4 was located in this lagoon system (Arreguin, 1978). The low productivity may be the result of a short time of residence of nutrients in the lagoon systems during the rainy season (Gutiérrez–Mendieta et al., 2006).
Similar conditions can cause V. alginolyticus to emerge from stasis, but it cannot be cultivated at this time of year. This is due to unfavorable conditions such as competition between bacteria for nutrients, space, and light (Avendaño–Herrera et al., 2005; Albertini et al., 2006).
This species was not correlated with temperature, and due to the seasonal fluctuations its occurrence, does not appear to be dependent on temperature in tropical areas (Molitoris et al., 1985). However, to corroborate this assumption, it would be necessary to have field data that span a minimum period of three years. Such efforts would better define trends in bacterial concentrations relative to the environmental conditions and co–occurring environmental management actions.
ACKNOWLEDGEMENTS
We thank Consejo Nacional de Ciencia y Tecnología (CONACYT) for assistance through the program Estancia Sabática Vinculada al Fortalecimiento de la Calidad del Postgrado Nacional 2008 to Dr. Fabiola Lango Reynoso in the Colegio de Postgraduados, Campus Veracruz. We also thank the Instituto Tecnológico de Boca del Rio and the Colegio de Postgraduados, Campus Veracruz, for support during the preparation of this article and in the framework of the Acuerdo General de Colaboracion Academica held between the two institutions since October 2006.
REFERENCES
Aguilar–Ibarra, A., S. Villanueva–Fragoso, P. Guzmán–Ama & A. Vázquez–Botello. 2006. La contaminación del agua como una externalidad para la producción pesquera y acuícola. In: Guzmán, P. & C. D. Fuentes (Eds.). Pesca, acuacultura e investigación en México. CEDRSSA, México, pp. 109–116. [ Links ]
Albertini, M. C., A. Accorsi, L. Teodori, L. Pierfelici, F. Uguccioni, M. B. Rocchi, S. Burattini & B. Citterio. 2006. Use of multiparameter analysis for Vibrio alginolyticus viable but nonculturable state determination. Cytometry A 69: 260–265. [ Links ]
Alam, M. J., S. Miyoshi & S. Shinoda. 2003. Studies on pathogenic Vibrio parahaemolyticus during a warm weather season in the Seto Inland Sea, Japan. Environmental Microbiology 5: 706–710. [ Links ]
Arreguin, S. F. 1978. Contribución al conocimiento del la hidrobiología de las lagunas de Mandinga, Ver., México. In: Resúmenes VI Congreso Nacional de Oceanografía. México. [ Links ]
Avendaño–Herrera, R., M. Lody & C. E. Riquelme. 2005. Producción de substancias inhibitorias entre bacterias de biopelículas en substratos marinos. Revista de Biología Marina y Oceanografía 40: 117–125. [ Links ]
Baqueiro–Cárdenas, E. R., A. D. Aldana, M. L. Sevilla & E. P. F. Rodríguez. 2007. Variations in the reproductive cycle of the Oyster Crassostrea virginica (Gmelin, 1791), Pueblo Viejo Lagoon, Veracruz, México. Transitional Water Bulletin 2: 37–46. [ Links ]
Barbieri, E., L. Falzano & C. Fiorentini. 1999. Occurrence, diversity and pathogenicity of halophilic Vibrio spp. and non–01 Vibrio cholerae from estuarine waters along the Italian Adriatic Coast. Applied Environmental Microbiology 65: 2748–53. [ Links ]
Bilung, L. M., R. Son, A. R. Bahaman, R. A. Rahim, S. Napis, M. W. Clemente, G. B. Tanil & M. Nishibuchi. 2005. Detection of Vibrio parahaemolyticus in cockle (Anadara granosa) by PCR. FEMS Microbiology Letters 252: 85–88. [ Links ]
Cabanillas–Beltrán, H., E. LLausás–Magaña, R. Romero, A. Espinoza, A. García–Gasca, M. Nishibuchi, M. Ishibashi & B. Gómez–Gil. 2006. Out–breakof gastroenteritis caused by the pandemic Vibrio parahaemolyticus O3:K6 in Mexico. FEMS Microbiology Letters 265: 76–80. [ Links ]
Cabrera–García, M. A., C. Vásquez–Salinas & E. Quiñonez–Ramirez. 2004. Serologic and molecular characterization of Vibrio parahaemolyticus strains isolated from seawater and fish products of the Gulf of Mexico. Applied and Environmental Microbiology 70: 6401–6406. [ Links ]
Castañeda, M., S. Pardio, B. Orrantia & F. Lango. 2005. Influence of water temperature and salinity on seasonal occurrences of Vibrio cholerae and enteric bacteria in oyster–producing areas of Veracruz, México. Marine Pollution Bulletin 50: 1641–1648. [ Links ]
Chakraborty, S., G. B. Nair & S. Shinoda. 1997. Pathogenic vibrios in the natural aquatic environment. Reviews of Environmental Health 12: 63–80. [ Links ]
Cook, D. W., J. C. Bowers & A. De Paola. 2002. Density of total and pathogenic (tdh+) Vibrio parahaemolyticus in Atlantic and Gulf coast molluscan shellfish at harvest. Journal of Food Protection 65: 1873–1880. [ Links ]
Croci, L., E. Suffredini, L. Cozzi & L. Toti. 2002. Effects of depuration of molluscs experimentally contaminated with Escherichia coli, Vibrio cholerae O1 and Vibrio parahaemolyticus. Journal of Applied Microbiology 92: 460–465. [ Links ]
Daniels, C., C. Vindurampulle & R. Morona. 1998. Overexpression and topology of the Shigella flexneri O–antigen polymerase (Rfc/Wzy). Molecular Microbiology 28: 1211–1222. [ Links ]
DePaola, A., J. Bowers, J. Wells & D. Cook. 2003. Seasonal abundance of total and pathogenic Vibrio parahaemolyticus in Alabama oysters. Applied Environmental Microbiology 69: 1521–1526. [ Links ]
DePaola, A., L. H. Hopkins, J. T. Peeler, B. Wentz & R. M. McPhearson. 1990. Incidence of Vibrio parahaemolyticus in U.S. coastal waters and oysters. Applied Environmental Microbiology 56: 2299–2302. [ Links ]
Dileep, V., H. S. Kumar, Y. Kumar, M. Nishibuchi & I. Karunasaga. 2003. Application of polymerase chain reaction for detection of Vibrio parahaemolyticus associated with tropical seafoods and coastal environment. Letter of Applied Microbiology 36: 423–427. [ Links ]
Di Pinto, A., G. Ciccarese, M. Fontanarosa, V. Terio & G. M. Tantillo. 2006. Detection of Vibrio alginolyticus and Vibrio parahaemolyticus in shellfish samples using collagenase–targeted multiplex–PCR. Journal of food safety 26: 150–159. [ Links ]
DOF. (Diario Oficial de la Federación). 1993. NOM–031–SSA1–1993. Bienes y servicios. Productos de la pesca. Moluscos bivalvos frescos–refrigerados y congelados. Especificaciones sanitarias. México. [ Links ]
DOF. (Diario Oficial de la Federación). 1994. NOM–109–SSA1–1994. Bienes y servicios. Procedimientos para la toma, manejo y transporte de muestras de alimentos para su análisis microbiológico. México. [ Links ]
FAO (Food and Agriculture Organization). 2006. Fisheries Topics: Statistics. Estadísticas e información. FAO Fisheries and Aquaculture Department. On line: http://www.fao.org/fishery/topic/2017/es [ Links ]
FDA–BAM (Food and Drug Administration–Bacteriological Analytical Manual). 2004. Vibrio. Bacteriological Analytical Manual. On Line: http://www.fda.gov/Food/ScienceResearch/LaboratoryMethods/BacteriologicalAnalyticalManualBAM/ucm070830.htm. [ Links ]
Fukushima, H. & R. Seki. 2004. Ecology of V. vulnificus and Vibrio parahaemolyticus in brackish environments of the Sada River in Shimane Prefecture, Japan. FEMS Microbiology Ecology 48: 221–229. [ Links ]
Gutiérrez–Mendieta, F. J., F. Varona–Cordero & F. Contreras. 2006. Caracterización estacional de las condiciones físico–químicas y de productividad primaria fitoplanctónica de dos lagunas costeras tropicales del estado de Chiapas, México. Hidrobiológica 16: 137–146. [ Links ]
Hervio–Heath, D., R. R. Colwell, A. Derrien, A. Robert–Pillot,J. M. Fournier & M. Pommepuy. 2002. Occurrence of pathogenic vibrios in coastal areas of France. Journal of applied microbiology 92: 1123–1135. [ Links ]
Molitoris, E., S. W. Joseph, M. I. Krichevsky, W. Sindhuhardja & R. R. Colwell. 1985. Characterization and distribution of Vibrio alginolyticus and Vibrio parahaemolyticus isolated in Indonesia. Applied and Environmental Microbiology 50: 1388–1394. [ Links ]
Olafsen, J. A., H.V Mikkelsen, H. M. Giaever & G. H. Hansen. 1993. Indigenous bacteria in hemolymph and tissues of marine bivalves at low temperatures. Applied and Environmental Microbiology 59: 1848–1854. [ Links ]
Parveen, S., K. A. Hettiarachchi, J. C. Bowers, J. L. Jones, M. L. Tamplin, R. McKay, W. Beatty, K. Brohawn, L. V. Dasilva & A. Depaola. 2008. Seasonal distribution of total and pathogenic Vibrio parahaemolyticus in Chesapeake Bay oysters and waters. International journal of food microbiology 128: 354–61. [ Links ]
SEFIPLAN. 1999. Programa Veracruzano de Finanzas Públicas 1999–2004. On line: http://www.sefiplan.gov.mx/menuframes/provefipu/pvfpu–prin.htm [ Links ]
Sousa, 0. V., R. H. Vieira, F. G. DeMenezes, C. M. DosReis, & E. Hofer. 2004. Detection of Vibrio parahaemolyticus and Vibrio cholerae in oyster, Crassostrea rhizophorae, collected from a natural nursery in the Coco river estuary, Ortaleza, Ceara, Brazil. Revista do Instituto de Medicina Tropical de São Paulo 46: 59–62. [ Links ]
Thompson, F., T. Lida & J. Swings. 2004. Biodiversity of vibrios. Microbiology and Molecular Biology Reviews 68: 403–31. [ Links ]
Thompson, J. R. & M. Polz. 2006. Dynamics of Vibrio populations and their role in environmental nutrient cycling. In: Thompson, F. L., B. Austin & J. Swing (Eds.). The Biology of Vibrios. ASM Press, Washington, D.C, pp. 190–203. [ Links ]
Yi–Cheng, S. & L. Chengchu. 2007. Vibrio parahaemolyticus: A concern of seafood safety. Food Microbiology 24: 549–558. [ Links ]














