Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Hidrobiológica
versión impresa ISSN 0188-8897
Hidrobiológica vol.20 no.3 Ciudad de México ene. 2010
Artículos
Toxic effects of Pseudanabaena tenuis (Cyanobacteria) on the cladocerans Daphnia magna and Ceriodaphnia dubia
Efectos tóxicos de Pseudanabaena tenuis (Cyanobacteria) en los cladóceros Daphnia magna y Ceriodaphnia dubia
Roxana Olvera–Ramírez,1 Carla Centeno–Ramos1 and Fernando Martínez–Jerónimo2
1 Laboratorio de Fisiología Vegetal, Escuela Nacional de Ciencias Biológicas. I.P.N., México, D.F. Carpió esq. Plan de Ayala s/n, Col. Casco de Santo Tomás. C.P. 11340 Miguel Hidalgo, México, D.F. México.
2 Laboratorio de Hidrobiología Experimental. Escuela Nacional de Ciencias Biológicas. I.P.N., México, D.F. E –mail: fjeroni@ipn.mx.
Recibido: 15 de febrero de 2010
Aceptado: 3 de noviembre de 2010
ABSTRACT
Some cyanobacteria can produce toxins that affect the aquatic biota and represent a human health risk. The cyanobacterium Pseudanabaena tenuis was isolated from the Valle de Bravo dam, and cultured in the laboratory under controlled conditions. We determined the acute toxic effects and performed a chronic (consumption) test in the cladocerans Daphnia magna (a reference test organism) and Ceriodaphnia dubia (a cosmopolitan species). To determine acute toxicity, three exposure ways were assayed: a) cell–free culture medium, b) crude cell extracts of the cyanobacterium after lysing, and c) aqueous extracts of P. tenuis phycobiliproteins. On the other hand, both cladocerans were fed P. tenuis, assessing the effects on survival and reproduction. For comparison, a control culture of both cladocerans was fed the green microalga Pseudokirchneriella subcapitata. Exposure to the cell–free culture medium did not produce any mortality in either cladoceran, but the aqueous and crude extracts generated acute toxicity. D. magna and C. dubia were negatively affected when fed P. tenuis, since their survival, total progeny, average number of neonates per clutch, and the number of clutches decreased. C. dubia was more sensitive than D. magna, both in the acute toxicity tests and to the effects of P. tenuis consumption. Although most of the blooms around the world are dominated by cyanobacteria of the genus Microcystis, attention should be given to other species, such as P. tenuis, because, frequently, it is not recognized that smaller cyanobacteria could exceed the larger species in terms of biomass, and produce noxious biological effects.
Key words: Cyanobacteria, eutrophication, cyanotoxins, cladoceran, zooplankton.
RESUMEN
Algunas cianobacterias producen toxinas que afectan la biota acuática y representan un riesgo para la salud humana. La cianobacteria Pseudanabaena tenuis fue aislada del embalse Valle de Bravo y cultivada en el laboratorio. Se determinaron los efectos tóxicos agudos (por exposición) y se evaluó la toxicidad crónica (por consumo), empleando los cladóceros Daphnia magna (organismo de referencia) y Ceriodaphnia dubia (especie cosmopolita). Para determinar la toxicidad aguda se ensayaron tres formas de exposición: a) medio de cultivo libre de células, b) extractos crudos de la cianobacteria después del lisado de las células, y c) extractos acuosos de las ficobiliproteínas. Por otra parte, ambos cladóceros fueron alimentados con P. tenuis, evaluando los efectos sobre la sobrevivencia y la reproducción; como comparación se utilizaron cultivos control alimentados con la microalga verde Pseudokirchneriella subcapitata. La exposición al medio de cultivo libre de células no produjo mortalidad en ninguno de los cladóceros, pero los extractos crudos y los extractos acuosos generaron toxicidad aguda. Adicionalmente la sobrevivencia y la reproducción de ambos cladóceros se afectó negativamente cuando se alimentaron con P. tenuis. C. dubia fue más sensible que D. magna, tanto en las pruebas de toxicidad aguda como en las pruebas de consumo con P. tenuis. Aunque en la mayoría de los florecimientos registrados en todo el mundo domina la cianobacteria Microcystis, se debe poner atención a especies como P. tenuis, que pueden ser importantes en términos de biomasa y de los efectos biológicos que pudieran generar.
Palabras clave: Cianobacteria, eutroficación, cianotoxinas, cladóceros, zooplancton.
INTRODUCTION
Cyanobacteria are cosmopolitan organisms distributed in terrestrial, fresh and marine water environments, and are very common in phytoplankton communities. Under specific environmental conditions, such as high water column stability, high temperature, and low N:P ratio, massive growth of some bloom–forming species is fostered (Roset et al., 2001). These blooms are frequent in euth–rophicated water bodies worldwide, representing an increasing ecotoxicological problem in fresh and marine water environments (Vardaka et al., 2005; Barbosa et al., 2006). Cyanobacterial blooms represent serious problems for water quality, including pH alterations, reduction of dissolved oxygen, foam production, foul smell, and unpleasant taste of the water (Codd, 2000).
During blooms, the structure of the zooplanktonic community is altered (Nagle & Paul, 1999; Nogueira et al., 2006), because some cyanobacteria are able to produce a large variety of bioactive metabolites (Burja et al., 2001), among them, potent toxins, known as cyanotoxins, are released into the environment once the bloom has developed (Carmichael, 1994). These toxins are classified, depending on their effects on mammals, in neurotoxins, hepatotoxins, and dermatotoxins. The most common cyanotoxins are microcystins, cylindrospermopsins, anatoxins, lyngbyatoxins, nodularins, and saxitoxins (Codd, 2000; Ouellette & Wilhelm, 2003; van Apeldoorn, 2007).
In case of excessive cyanobacterial growth, such as bloom formation, these organisms are responsible for diseases and mortality in both humans and animals (van Apeldoorn et al., 2007). Microcystis, Anabaena, Oscillatoria, Aphanizomenon, and Cylindrospermopsis are the most common cyanobacterial genera giving rise to blooms (Moustaka–Gouni et al., 2006).
In aquatic systems, blooms of cyanobacteria can affect the organisms inhabiting them (Barbosa et al., 2006). The toxic effect is exerted through direct exposure to the metabolites released into the water column during the collapse of these blooms (Carmichael, 1994; Moustaka–Gouni et al., 2006; Leflaive & Ten–Hage, 2007), or cyanobacteria can also be ingested by the zooplankton, producing negative effects on its survival and development.
In particular, filter–feeding cladocerans are potential consumers of planktonic cyanobacteria; cladocerans are fundamental in the planktonic structure of freshwater systems, and play an important role in the transfer of energy, stability, productivity, and increase diversity of trophic chains (Ghadouani et al., 2004).
The objective of this study was to evaluate the effect of the metabolites present in the cyanobacterium Pseudanabaena tenuis Kopee on two zooplankton organisms, Daphnia magna Straus, 1820 (international and national reference species for toxicological standards) and Ceriodaphnia dubia (Richard, 1894) (a cosmopolitan species widely distributed in Mexico, Alonso, 1996), by simulating the lysis of a massive bloom and by direct ingestion of cyanobacterial cells. This study is relevant, because attention on the nociceptive effects of blooms is focused usually on Microcystis and on the production of microcystins, without considering other poorly–known cyanobacteria that could be present in blooms. Other cyanobacteria different from Microcystis could represent a risk for environmental and human health, because they can produce cyanotoxins and metabolites with noxious biological activity, besides they can become the dominant taxon, as has been seasonally observed in some dams that supply drinking water to Mexico City.
MATERIAL AND METHODS
The filamentous cyanobacterium Pseudanabaena tenuis Koppe 1924 was isolated from a bloom in the "Valle de Bravo" dam in the state of Mexico (19° 21' 30" N and 100° 11' 00" W), during a sampling performed in January 2005 (Carrera–Ramírez, 2005). This strain was deposited in the Laboratorio de Fisiología Vegetal of the Escuela Nacional de Ciencias Biológicas (ENCB). The cladocerans D. magna and C. dubia were obtained from the cladocerans collection of the Laboratorio de Hidrobiología Experimental of ENCB; in particular, C. dubia was isolated from the "Valle de Bravo" dam 8 years ago and has been maintained under controlled conditions since that time.
Pseudanabaena tenuis was cultured in BG–11 medium (Rippka, 1988), incubated at constant temperature (25 °C), under continuous aeration, and fixed illumination provided by "daylight" fluorescent lamps (54 pmol photons m–2 s–1), with a 12:12 photoperiod (light:darkness). Cultures in the exponential growth phase (ca. 15 days of propagation) were used in all experiments. It should be noted that, because of the high content of phycoerythrin in P. tenuis, the color of these cultures was brown, different from the blue–green color frequently observed for most cyano–bacterial cultures.
Daphnia magna and C. dubia were cultured in reconstituted hard water (160–180 mg L–1 as CaCO3; U.S. Environmental Protection Agency, 2002), at 25 °C with a 16:8 photoperiod (light:darkness). Both species were fed the microalga Pseudokirchneriella subcapitata (Korshikov) Hindák (Chlorophyceae) at a 1,300,000 cells mL–1 concentration. Controlled batches of reproducers of known age were established to obtain neonates (offspring of less than 24 h of age) that were used as test organisms in all the experiments.
Acute Toxicity Assays.To assess the toxic effects of different exposure ways on both cladocerans, we performed three types of acute toxicity assays (48 h): a) using the culture medium in which P. tenuis had been grown, but free of biomass, which was completely separated by filtration at the end of the exponential growth phase; b) using the crude extract of the cell content obtained by cellular lysis; and c) using the aqueous extract of phycobiliproteins from P. tenuis, obtained from crude extracts.
The purpose of the first way of exposure was to determine whether the exudates or the extracellular metabolites from whole cells could cause mortality; the second condition would emulate the effect of the release of cellular contents as a consequence of the collapse of the bloom; whereas the third condition was aimed at evaluating the possible toxic effects of phycobiliproteins, typical pigments from cyanobacteria, which are also released during cellular lysis.
For the cell–free medium, the culture was pre–filtered with Wathman N° 3 paper and then through microfiltration using a 0.45–pm pore nitrocellulose membrane mesh.
To attain cell lysis, 127 mg of cultured biomass (wet weight) was subjected to three continuous freezing (–20 °C) and thawing (room temperature) cycles (Kós et al., 1995; Chorus & Bartram, 1999); cells rupture was confirmed through observation of fresh samples under an optical microscope. Separation of the crude extract from the cell debris was achieved by filtration through nitrocellulose membranes (0.45 pm).
For phycobiliproteins extraction (phycoerythrin, phycocyanin, and allophycocyanin), 127 mg of biomass (wet weight) were resuspended in 100 mL of sodium phosphate buffer (0.1 M, pH 7) and cell rupture was performed as described above. Then, 1% streptomycin sulfate (w/v) was added to precipitate cell debris containing chlorophyll (Bermejo et al., 2002) and to obtain an extract mainly composed by phycobiliproteins; this extract was maintained for 24 h at 4 °C and centrifuged at 3,500 rpm during 45 min at 4 °C. The final concentration of the aqueous extract corresponded to 1.27 mg of wet P. tenuis biomass per milliliter. The concentration of phycobiliproteins present in the concentrated aqueous extract and in the used dilution was determined by absorbance measures at 565, 620, and 650 nm in a Lambda 19–UV/ VIS/NIR Perkin Elmer spectrophotometer, according to the procedure established by Bermejo et al. (2002).
To determine acute toxicity in the three conditions, we applied the test procedure established by the U. S. Enviromental Protection Agency (2002). Reconstituted hard–water was used as dilution water. Mortality was recorded at 24 and 48 h, and with the 48 h results, we determined the median lethal concentration (LC50) by the probit method (Stephan, 1977).
Chronic (Consumption) Test. Daphnia magna and C. dubia were fed three P. tenuis concentrations (dry weight): 2 (Pt1), 4 (Pt2), and 8 mg L–1 (Pt3) during 23 days. We assessed the effects on survival and on the main reproductive parameters (total progeny, mean neonates per clutch, number of clutches, age at first reproduction, and inter–clutch time) of both cladocerans. Results were compared with those obtained in a control series fed the green microalgae Pseudokirchneriella subcapitata (reference diet) at the same concentrations (Ps1, Ps2, and Ps3). To quantify the P. tenuis diet, it was necessary to develop an absorbance–dry weight calibration curve, because the size of the filaments is variable and, hence, it is difficult to quantify in a Neubauer chamber. Additionally, the size of the filaments (trichomes) was determined measuring 100 filaments with a micrometric ruler under a phase contrast microscope (40X).
Chronic consumption assays were performed in individually cultured organisms, in 100–mL containers with 80 mL of test volume, using reconstituted hard water as culture medium. Each experiment had ten replicates. Experiments were started with neonates and, during 23 days, observations were made on the survival and reproduction of organisms of all replicates. Once reproduction had started, the progeny was separated, counted, and discarded. The culture medium and food were exchanged every 48 h. Experiments were performed at constant temperature (25 °C) and with a 16:8 (light:darkness) photoperiod in an environmental chamber.
At the end of the experiment, surviving D. magna and C. dubia females were measured to assess possible effects of the food on their size.
To know the protein supply of the used diets, we determined the amount of total soluble protein of P. tenuis and P. subcapitata by the Bradford method (Bradford, 1976).
The Kaplan–Meier function was used to determine significant differences in the survival of organisms subjected to the diverse treatments, using chi square as statistical test. Kruskal–Wallis test was used to establish the presence of possible differences in the total number of neonates, total number of clutches, age at first reproduction, and inter–clutch time. The average clutch size and the size of the adult females at the end of the assay were compared with a one–way analysis of variance (ANOVA). Finally, Student's t–test was used to compare the amount of total protein between the cyanobacteria and the green microalgae. Tukey's multiple comparison test (post hoc comparisons) was used for all parametric analyses to determine the groups with significant differences (p < 0.05). Statistica ver. 7.0 software was used for all analyses.
RESULTS
Acute Toxicity Tests. No lethal effects (48 h) were recorded in any of the assays performed in the cell–free culture medium, and the physical aspect of the organisms was completely normal, as well as their mobility and locomotion capacity.
When the crude extract was evaluated (total cell content), mortality was recorded in both cladocerans. D. magna neonates showed a lower sensitivity than C. dubia, obtaining an average 48–h LC50 of 130.6 mg L–1. For C. dubia, mortality recorded at 48 h of exposure yielded an LC50 of 37.1 mg L–1.
On the other hand, when exposing the organisms to different concentrations of the aqueous phycobiliproteins extract, acute toxic effects were observed in D. magna since the first 24–h exposure in all assayed concentrations; the average 48–h LC50 was 14.1 mg L–1. In C. dubia neonates the percentage of mortality was low at 24 h of exposure and the LC50 at 48 h was 13.7 mg L–1, a similar value to that recorded for D. magna.
Chronic (Consumption) Test. The width of P. tenuis filaments was of 1.9 pm and the length varied from 11.7 pm to 133.2 µm, with a mean of 58.2 µm (± 10.6, p = 0.05). Both cladocerans consumed P. tenuis filaments as confirmed by the reddish color observed in the digestive tract under the microscope, this reddish color is related with the digestion process of the filaments, which allowed for the release of phycoerythrin from cyanobacteria, contrasting with the green color in the control series fed P. subcapitata.
The survival curves for D. magna with the three concentrations tested indicate that despite the mortality observed with treatments Pt1 and Pt3, there was no significant difference between the Pt and Ps diets (χ2 = 5.45, p = 0.36; Fig. 1a). For C. dubia, survival of females fed P. subcapitata was higher, and in organisms fed Ps1 no mortality occurred during the experimental period. Organisms fed P. tenuis showed a higher mortality starting at day 5, and the lowest survival at the end of the assay was recorded in those fed Pt3 (10%), with significant statistical differences between the Pt and Ps diets (χ2 = 17.5; p = 0.003; Fig. 1b).
For D. magna, the highest average value of total progeny was recorded with treatment Ps2 (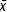 = 189 neonates), whereas the lowest was recorded with Pt1 (
= 189 neonates), whereas the lowest was recorded with Pt1 (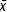 = 37 neonates; Fig. 2). The largest clutch size was recorded with treatments Ps2 and Ps3 (
= 37 neonates; Fig. 2). The largest clutch size was recorded with treatments Ps2 and Ps3 (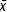 = 27 and 26 neonates, respectively), and the lowest clutch size was observed with treatment Pt2 (
= 27 and 26 neonates, respectively), and the lowest clutch size was observed with treatment Pt2 (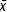 = 8 neonates; Fig. 2). Age at first reproduction ranged from 9.5 to 10.8 days in females fed P. tenuis, which was higher than that recorded in organisms fed microalgae (approximately 7 days; Fig. 2). The diet affected also the number of clutches per female, corresponding the highest value to females fed P. subcapitata(5–6 clutches), whereas females fed cyanobacteria had an average of 3.8 clutches. The inter–clutch time ranged from 1.4 to 1.8 days with both diets. An additional effect observed in D. magna females fed P. tenuis was the presence of dead or affected neonates, as well as abortions (premature release of embryos).
= 8 neonates; Fig. 2). Age at first reproduction ranged from 9.5 to 10.8 days in females fed P. tenuis, which was higher than that recorded in organisms fed microalgae (approximately 7 days; Fig. 2). The diet affected also the number of clutches per female, corresponding the highest value to females fed P. subcapitata(5–6 clutches), whereas females fed cyanobacteria had an average of 3.8 clutches. The inter–clutch time ranged from 1.4 to 1.8 days with both diets. An additional effect observed in D. magna females fed P. tenuis was the presence of dead or affected neonates, as well as abortions (premature release of embryos).
The body size of D. magna females at the end of the assay was significantly larger in those fed microalgae (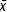 = 5.5 mm) than in females fed P. tenuis(
= 5.5 mm) than in females fed P. tenuis(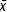 = 4.4 mm) (F=25.7, p< 0.05).
= 4.4 mm) (F=25.7, p< 0.05).
Regarding C. dubia, significant differences were found in all reproductive parameters (total progeny, size of clutch, age at first reproduction, and number of clutches) between those fed cya–nobacteria and those fed microalgae (Fig. 3). However, the inter–clutch time was similar with all treatments (between 1.5 and 1.6 days), except for treatment Pt1 that showed a higher value (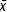 = 2.35 days; Fig. 3). The body size of C. dubia adults was higher in organisms fed P. subcapitata (
= 2.35 days; Fig. 3). The body size of C. dubia adults was higher in organisms fed P. subcapitata (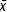 = 1.03 mm), as compared to those fed P. tenuis (
= 1.03 mm), as compared to those fed P. tenuis (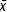 = 0.83 mm), revealing statistically significant differences between both diets (F = 17.0, p < 0.05).
= 0.83 mm), revealing statistically significant differences between both diets (F = 17.0, p < 0.05).
The amount of total soluble protein was slightly higher in P. tenuis than in P. subcapitata (0.8191 µg mL–1 and 0.7299 µg mL–1, respectively). Student's t–test revealed that these amounts differ significantly (t=–3.10, p < 0.006), meaning that cyanobacteria represent a better source of protein than microalgae.
DISCUSSION
We determined that P. tenuis contains intracellular compounds that generate acute toxicity in D. magna and C. dubia neonates; however these biomolecules were not released to the medium in active growing cultures. This is a relevant fact because the Valle de Bravo dam, from which these cyanobacteria were isolated, supplies drinking water to Mexico City, and hosts diverse recreational activities (Ramírez et al., 2004). Unfortunately, toxicological studies on cyanobacteria have focused mainly on the effect of the most common cyanotoxins, i.e., microcystins, ignoring the impact exerted by other bioactive compounds that have not been studied because they are not toxic for humans (Leflaive & TenHage, 2007).
Phycobiliproteins have been attributed antioxidant, antitumor, anti–inflammatory, and neuroprotective pharmacological properties (Sekar & Chandramohan, 2007). Allophycocyanin purified from Spirulina platensis inhibited the enterovirus 71, inducing apoptosis at concentrations that were not toxic for the host cells (Shih et al., 2003). Liu et al. (2000) demonstrated that the phycocyanin extracted also from S. platensis inhibits growth of human leukemia K562 cells. Other studies demonstrated that phycoerythryn inhibits growth of hepatic carcinoma cells (Huang et al., 2002).
However, we determined in the acute toxicity assays that the crude extract was less toxic than the aqueous extract with phycobiliproteins to both cladocerans. It is remarkable that when exposed to the crude extract, C. dubia neonates were more sensitive than D. magna neonates, but both cladocerans showed a similar, higher sensitivity to the aqueous extract of phycobiliproteins. Concerning this result, it is possible that remnants of the streptomycin sulfate could modify the toxic effects of phycobiliproteins, because this antibiotic was not eliminated, since the procedure to purify the phycobiliproteins extract does not consider this situation. To this respect, Isidori et al. (2005) have documented that this antibiotic produces moderately toxic effects on rotifers and cladocerans, but Taub et al. (1983) reported variable effects, depending on the species of cladoceran, because, in some cases, the effects stimulated population growth. Despite this situation, we have evidences that phycobilins can exert toxic effects in D. magna and C. dubia (unpublished data), as was confirmed in the consumption assays.
Among the few studies on the toxicity of the Pseudanabaena genus is that of Oudra et al. (2002), who evaluated the toxic effect of several cyanobacterial strains (among them, Pseudanabaena mucicola (Naumann et Huber–Pestalozzi) Schwabe) through bio–assays in mice. These authors found that P. mucicola produces microcystins at a concentration of 19 µg g–1, an amount much lower than that recorded for the Microcystis strain they used (600 µg g–1), but surprisingly P. mucicola was more toxic (LD50 = 28 mg kg–1) than Microcystis (LD50= 33 mg kg–1), suggesting that the toxicity of P. mucicola is not only due to the presence of cyanotoxins but also to other intracellular metabolites that exert a toxic effect, as could be inferred from our results.
When cladocerans were fed P. tenuis, survival, growth, and reproduction decreased, which agrees with other studies performed on the interaction of cyanobacteria–zooplankton, which demonstrated that the exposure to cyanobacterial extracts, purified toxins, or cells (as feeding source) exerts negative effects on herbivorous organisms (Nogueira et al., 2006). This can be explained by the fact that cyanobacteria are deficient in essential nutrients, many species have a colonial morphology or are filamentous, making their consumption difficult (Porter & McDonough, 1984), aside that several species produce toxic metabolites (including cyanotoxins).
The nutritional value of cyanobacteria for the zooplankton is a controversial issue, since some studies have demonstrated that they are a low nutritional quality food, mainly due to the lack of some essential nutrients needed for growth (DeMott, 1999; Ghadouani et al., 2004; Nogueira et al., 2006). However, other studies have demonstrated that some zooplankton species develop adequately when fed non–toxic cyanobacterial strains (Ferrão–Filho et al., 2000; Kurmayer, 2001).
It has been argued that the filamentous morphology of cyanobacteria could interfere with the filtration mechanisms of the zooplankton; however, in this work we observed that both cladocerans were able to consume the filaments of P. tenuis.
Wilson et al. (2006) suggest that the filaments of some cyanobacteria reduce fecundity in large cladocerans (such as Daphnia), as compared to small cladocerans (such as Ceriodaphnia), this could explain the decrease in the predominance of large cladocerans in water bodies where blooms develop. Notwithstanding, we observed that C. dubia (the smaller species) was the most affected in both survival and reproductive parameters.
Our results suggest that the negative effects on survival and reproduction of D. magna and C. dubia were caused by the toxic metabolites or biomolecules released by cell rupture during digestion, and not by the nutritional deficiency or the difficulty in ingesting the filaments (Okumura et al., 2007). The protein content of P. tenuis was slightly higher than that of P. subcapitata and its average size was similar to that of large size Chlorophycean frequently used as food for cladocerans, such as Ankistrodesmus falcatus (45 pm length). The presence of dead offspring, as well as the abortions in D. magna fed P. tenuis can be considered as responses to the toxic effect of cyanobacteria. Notwithstanding, C. dubia was most affected, as shown by the fact that most females did not reproduce and many did not survive.
Studies on P. tenuis metabolites and their interaction with the zooplankton are scarce. Frequently, it is ignored that small species of cyanobacteria could exceed the larger species in terms of their biomass and biological activity, and, therefore, the major role they play in the ecosystems dynamics is missed (Acinas et al., 2009). In this study, we found evidences of the negative effect on the reproduction and survival of the studied cladocerans, although it is necessary to continue the study on P. tenuis metabolites and their effects on aquatic systems used as water sources for human consumption. The presence of P. tenuis in Valle de Bravo dam, which is a source for water supply for Mexico City, has also been reported previously (Carrera–Ramírez, 2005), and its toxigenic potential to the aquatic biota is now confirmed with this study.
Because of the poisoning incidents with animals and humans, the effects of many cyanotoxins have been investigated mainly in mammals, documenting increases in their effects on target cells and organs; whereas in herbivorous zooplankton the available information is still insufficient. Hence, it is important to make more detailed studies on all bioactive compounds with toxic characteristics produced by cyanobacteria, to better evaluate their effects on aquatic biota. This study evidences the negative effect that the exposure to metabolites and the consumption of these cyanobacteria exert on important zooplankton organisms, such as cladocerans.
ACKNOWLEDGMENTS
C. Centeno–Ramos thanks Consejo Nacional de Ciencia y Tecnología (CONACYT) for the fellowship to perform graduate studies. F. Martínez–Jerónimo and R. Olvera–Ramírez thank the Instituto Politécnico Nacional (IPN) and, particularly, the Sistema de Estímulo al Desempeño de los Investigadores and the Comisión de Operación y Fomento de Actividades Académicas del IPN for the support received to perform this study. We also thank Ms. Ingrid Mascher for editorial assistance with the English version, and two anonymous reviewers who helped to improve the manuscript.
REFERENCES
Acinas, S. G., T. H. A. Haverkamp, J. Huisman & L. J. Stal. 2009. Phenotypic and genetic diversification of Pseudanabaena spp. (Cyanobacteria). The International Society for Microbial Ecology Journal 3: 31–46. [ Links ]
Alonso, M. 1996. Crustácea Brachiopoda. Fauna Ibérica Vol. 7. Museo Nacional de Ciencias Naturales, Madrid, pp. 186–188. [ Links ]
Barbosa, R., E. Silva, N. F. Verani, K. 0. Nonaka & 0. Rocha. 2006. Toxicity of a cyanobacterial bloom in Barra Bonita Reservoir (Middle Tiete River, São Paulo, Brazil). Ecotoxicology and Environmental Safety 64: 163–170. [ Links ]
Bermejo, R. R., J. M. Alvarez, F.G. Acién & E. Molina. 2002. Recovery of pure β–phycoerythrin from the microalgae Porphyridium cruentum. Journal of Biotechnology 93: 73–85. [ Links ]
Bradford, M. M. 1976. A Rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of proteindye binding. Analytical Biochemistry 72: 248–254. [ Links ]
Burja, A. M., B. Banaings, E. Abou–Mansour, J. G. Burgess & P. C. Wright. 2001. Marine cyanobacteria a prolific source of natural products. Tetrahedron 57: 9347–9377. [ Links ]
Carrera–Ramírez, A. 2005. Evaluación de las cianotoxinas de Pseudanabaena tenuis con Daphnia magna. Tesis de Licenciatura en Biología. Escuela Nacional de Ciencias Biológicas. IPN. Mexico. 72 p. [ Links ]
Carmichael, W. W. 1994. The Toxins of Cyanobacteria. Scientific American 270: 78–86. [ Links ]
Chorus, I. & J. Bartram. 1999. Toxic cyanobacteria in water. A guide to their public health, consequences, monitoring and management, E. & F. N. Spon, Londres, 416 p. [ Links ]
Codd, G. A. 2000. Cyanobacterial toxins, the perception of water quality, and the prioritization of eutrophication control. Ecological Engineering 16: 51–60. [ Links ]
DeMott, W. R. 1999. Foraging strategies and growth inhibition in five daphnids feeding on mixtures of a toxic cyanobacterium and green alga. Freshwater Biology 42: 263–274. [ Links ]
Ferrão–Filho, A. S., S. M. F. 0. Azevedo & W. DeMott. 2000. Effects of toxic and non toxic cyanobacteria on the life history of tropical and temperate cladocerans. Freshwater Biology 45: 1–19. [ Links ]
Ghadouani, A., B. Pinel–Alloul, K. Plath, G. A. Codd & W. Lampert. 2004. Effects of Microcystis aeruginosa and purified microcystin–LR on the feeding behavior of Daphnia pulicaria. Limnology and Oceanography 49: 666–679. [ Links ]
Huang, B., G. C. Wang, G. K. Zeng & Z. G. Li. 2002. The experimental research of R–phycoerythrin subunits on cancer treatment: a new photosensitizer in PDT. Cancer Biotherapy and Radiopharmaceuticals 17(1): 35–42. [ Links ]
Isidori, M., M. Lavorgna, A. Nardelli, L. Pasacrella & A. Parella. 2005. Toxic and genotoxic evaluation of six antibiotics on non–target organisms. Science of the Total Environment 346: 87–98. [ Links ]
Kós, P., G. Gorzó, G. Surányi & G. Borbély. 1995. Simple and efficient method for isolation and measurement of cyanobacterial hepatotoxins by plant tests (Sinapis alba L.). Analytical Biochemistry 225: 49–53. [ Links ]
Kurmayer, R. 2001. Competitive ability of Daphnia under dominance of non–toxic filamentous cyanobacteria. Hydrobiologia 442: 279–289. [ Links ]
Leflaive, J. & L. Ten–Hage. 2007. Algal and cyanobacterial secondary metabolites in freshwaters: a comparison of allelopathic compounds and toxins. Freshwater Biology 52: 199–204. [ Links ]
Liu, Y., Xu L., N. Cheng, L. Lin & C. Zhang. 2000. Inhibitory effect of phycocyanin from Spirulina platensis on the growth of human leukemia K562 cells. Journal of Applied Phycology 12: 125–130. [ Links ]
Moustaka–Gouni, M., E. Vardaka, E. Michaloudi, K. A. Kormas, E. Tryfon, H. Mihalatou, S. Gkelis & T. Lanaras. 2006. Plankton food web structure in a eutrophic polymictic lake with a history of toxic cyanobacterial blooms. Limnology and Oceanography 51: 715–727. [ Links ]
Nagle, D. G. & V. Paul. 1999. Production of secondary metabolites by filamentous tropical marine cyanobacteria: ecological functions of the compounds. Journal of Phycology 35: 1412–1421. [ Links ]
Nogueira, I. C. G., A. Lobo–da–Cunha & V. M. Vasconcelos. 2006. Effects of Cylindrospermopsis raciborskii and Aphanizomenon ovalisporum (Cyanobacteria) ingestion on Daphnia magna midgut and associated diverticula epithelium. Aquatic Toxicology 80: 194–203. [ Links ]
Okumura, D. T., R. B. Sotero–Santos R. A. Takenaka & 0. Rocha. 2007. Evaluation of cyanobacteria toxicity in tropical reservoirs using crude extracts bioassay with cladocerans. Ecotoxicology 16: 263–270. [ Links ]
Oudra, B., M. Loudiki, V. Vasconcelos, B. Sabour, B. Sbiyyaa, K. Oufdou & N. Mezrioui. 2002. Detection and quantification of microcystins from cyanobacteria strains isolated from reservoirs and ponds in Morocco. Environmental Toxicology 17: 32–39. [ Links ]
Ouellette, A. & S. W. Wilhelm. 2003. Toxic cyanobacteria: the evolving molecular toolbox. Frontiers in Ecology and Environment 7: 359–366. [ Links ]
Porter, K. G., & R. McDonough. 1984. The energetic cost of response to blue–green algal filaments by cladocerans. Limnology and Oceanography 29: 365–369. [ Links ]
Ramírez, R., E. Martínez, M. D. Martínez & C. Eslava. 2004. Cianobacterias, microorganismos del fitoplancton y su relación con la salud humana. In: Rosas I., A. Cravioto and E. Ezcurra (Eds.). Microbiología Básica, INE–SEMARNAT, México, pp. 83–106. [ Links ]
Rippka, R. 1988. Isolation and purification of cyanobacteria. In: Packer L. & Glazer, A. (Eds.). Methods in Enzimology Vol. 167 Cyanobacteria, Academic Press Inc., San Diego, pp. 3–27. [ Links ]
Roset, J., S. Aguayo & M. J. Muñoz–Mejía. 2001. Detección de cianobacterias y sus toxinas. Una revisión. Revista de Toxicología 18: 65–71. [ Links ]
Sekar, S. & M. Chandramohan. 2007. Phycobiliproteins as a commodity: trends in applied research, patents and commercialization. Journal of Applied Phycology 20 (2): 113–136. [ Links ]
Shih, S., Tsai, K. & Y. Li. 2003. Inhibition of enterovirus 71–induced apoptosis by allophycocyanin isolated from a blue–green alga Spirulina platensis. Journal of Medical Virology 70: 119–125. [ Links ]
Stephan, C. E. 1977. Methods for calculating an LC50. In: Mayer F. L., J. L. Hamelink (Eds.). American Society for Testing and Materials (ASTM). Aquatic toxicology and hazard evaluation, pp. 65–84, ASTM 534, Philadelphia, Pennsylvania. [ Links ]
Taub, F. B., P. L. Read, A. C. Kindig, M. C. Harrass, H. J. Hartman, L. L. Conquest, F. J. Hardy & P. T. Munro. 1983. Demonstration of the ecological effects of streptomycin and malathion on synthetic aquatic microcosms. In: Bishop, W. E., R. D. Cardwell & B. B. Heidolph (Eds.), Aquatic toxicology and hazard assessment: Sixth symposium. ASTM STP 802: 5–25. [ Links ]
U. S. Enviromental Protection Agency. 2002. Methods for measuring the acute toxicity of effluents and receiving waters to freshwater and marine organisms. 5a. Ed. EPA–821–R–02–012. Office of Water. Washington, D. C. 266 p. [ Links ]
van Apeldoorn, M. E. , H. P. van Egmond, G. J. A. Speijers. & G. J. I. Bakker. 2007. Review. Toxins in cyanobacteria. Molecular Nutrition & Food Research 51: 7–60. [ Links ]
Vardaka, E., M. Moustaka–Gouni, C. M. Cook & T. Lañaras. 2005. Cyanobacterial blooms and water quality in Greek waterbodies. Journal of Applied Phycology 17: 391–401. [ Links ]
Wilson, A., 0. Sarnelle & A. Tillmanns. 2006. Effects of cyanobacterial toxicity and morphology on the population growth of freshwater zooplankton: Meta–analyses of laboratory experiments. Limnology and Oceanography 51: 1915–1924. [ Links ]














