Servicios Personalizados
Revista
Articulo
Indicadores
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Hidrobiológica
versión impresa ISSN 0188-8897
Hidrobiológica vol.20 no.2 Ciudad de México may./ago. 2010
Artículos
Characteristics of population dynamics of Lutjanus guttatus (Pisces: Lutjanidae) in Bufadero Bay, Michoacan, Mexico
Características de la dinámica poblacional de Lutjanus guttatus (Pisces: Lutjanidae) en Bahía Bufadero, Michoacán, México
Marcela Sarabia–Méndez1, Manuel Gallardo–Cabello1, Elaine Espino–Barr2 and Vicente Anislado–Tolentino3
1 Instituto de Ciencias del Mar y Limnología, Universidad Nacional Autónoma de México, Apartado Postal 70–305, México, C. P. 09340 D.F. México.
2 CRIP–Manzanillo, INAPESCA, Playa Ventanas s/n, Manzanillo, Colima, 28200, México.
3 Universidad del Mar–Campus Puerto Ángel. Ciudad Universitaria. Puerto Ángel, San Pedro Pochutla, C. P. 70902. Oaxaca, México. E–mail: elespino@gmail.com.
Recibido: 13 de noviembre de 2009
Aceptado: 28 de abril de 2010
ABSTRACT
Analysis of scales of Lutjanus guttatus (Steindachner, 1869) allowed the identification of three growth rings. Similar data were obtained with kernel density method. Values of the von Bertalanffy's growth equation are: L∞ = 96.60 cm, W∞ = 7,508 g, K = 0.22 years–1 and t0 = –0.10 years. Maximum values of the condition factor occur during February and June and were preceded by two months of high values of the gastric repletion index. Maximum reproductive period was during April and August. Recruitment periods were from November to January and April to June. The recruitment length is 16 cm. The highest values of the hepatosomatic index are during December to June and showed an inversely proportional relation to the gonadosomatic index. First sexual maturity length is 30.63 cm and its longevity of 13.5 years. Forty one percent of L. guttatus individuals captured in Bufadero Bay are sexually immature, therefore we suggest a minimum capture size of 45 cm (age 2.5 years) and a closed season from August to September.
Key words: Life cycle parameters, von Bertalanffy's growth equation, longevity, recruitment, fishery.
RESUMEN
El análisis de las escamas de Lutjanus guttatus (Steindachner, 1869) permitió la identificación de tres anillos de crecimiento. Se observaron datos similares por medio del análisis de los estimadores de densidad por kernel. Los valores de la ecuación de crecimiento de von Bertalanffy calculados por el método no lineal simple son: L∞ = 96.60 cm, W∞ = 7,508 g, K = 0.22 años–1 y t0 = –0.10 años. Los máximos valores del factor de condición ocurren durante febrero y junio y son precedidos en un lapso de dos meses por los mayores valores de los índices de repleción gástrica. Los períodos de máxima reproducción se presentaron durante abril y agosto. Los períodos de reclutamiento comprenden de noviembre a enero y de abril a junio. La talla de reclutamiento es de 16 cm. Los valores mayores del índice hepatosomático se presentaron durante diciembre y junio y mostraron una relación inversamente proporcional a los valores del índice gonadosomático. La talla de primera madurez sexual es de 30.63 cm y la longevidad de 13.5 años. El 41.10 % de la pesca comercial en Bahía Bufadero es de organismos sexualmente inmaduros, por lo que se propone como talla mínima de captura una longitud de 45 cm (2.5 años de edad) y un período de veda de agosto a septiembre.
Palabras clave: Parámetros poblacionales, ecuación de von Bertalanffy, longevidad, reclutamiento, pesquería.
INTRODUCTION
Age determination is one of the most important objectives in the study of fish population dynamics, and from this information it is possible to get to know the population structure by age groups, longevity, recruitment age, sexual maturity and captures. Also, the determination of age allows studying the growth or the biomass increase of the population and the study of mortality or diminution of the biomass.
The method employed more frequently for age determination is growth rings identification in hard structures as: scales, otoliths, vertebrae and spines. The formation of these rings or marks happens periodically, fast growth rings in the period of higher food availability (generally in spring and summer) and slow growth rings in the periods with decrease of food availability (generally autumn and winter) (Gallardo–Cabello et al., 2007). These patterns of growth rings on scales may change because of the oscillation of physical and chemical parameters of oceanic currents, as happens in ENSO years. Fishes inhabiting tropical regions have less marked changes in food availability determined by seasonal periodicity than in cold regions; in these areas rainy and dry periods determine these oscillations in food quality and quantity, which will produce fast growth rings or slow growth rings in hard structures, respectively.
The diminution of growth rates of fishes happens when organisms reach sexual maturation (first to third year of age) and most of the energy goes to the formation of sexual products. It is in this period that the weight growth starts because of the storage of the fatty acids in the liver. These hepatic reserves decrease after spawning period (Espino–Barr et al., 2008).
Studies on age and growth of L. guttatus (Steindachner, 1869) have been carried out by Espino–Barr (1996), who found 14 age classes from scales and an equation Ls = 63.0 [1 – e–0–1(t+0.1)] and by Amezcua et al. (2006) who found 11 classes in the otoliths and its equation Lt = 66.19[1 – e–0.13(t–0.23)].
On this species there are important papers on reproduction analysis: Grimes (1987), Rojas (1997), Arellano et al. (2001), Rojas–Herrera (2001), Piñón (2003), Santamaría et al. (2003a) and Chiappa–Carrara et al. (2004). Studies on feeding and fat reserves indexes have been made by Sheaves (1995), Arellano et al. (2001) and Piñón (2003). Fishing assessment studies have been carried out by Rojo et al. (1999), Cruz–Romero et al. (2000), Santamaría et al. (2003b) and Chiappa–Carrara et al. (2004).
The spotted rose snapper, Lutjanus guttatus has a geographical distribution from the Gulf of California to Ecuador (Allen and Robertson, 1994; Fischer et al., 1995; Castro–Aguirre et al., 1999; Amezcua–Linares, 2008); it has a great commercial importance and is considered a first class species that reaches in the coasts of Jalisco, Colima, Michoacán, Oaxaca, and Guerrero, a price of $40.00 to $50.00 mexican pesos ($3.00 to $4.00 USD) on the beach and $80.00 to $100.00 mexican pesos ($6.00 to $8.00 USD) at the market (prices during 2008). Of the snapper group, L. guttatus is only overcome by L. peru, that reaches a higher commercial value (pers. com. Alejandro Trujillo, president of the Coop. Puerto Viejo). This study gives original information on age, growth, longevity, spawning periods, first sexual maturity length, recruitment periods and length, and condition factor, gastric repletion, gonadoso–matic and hepatosomatic indexes of L. guttatus in Bufadero Bay, Michoacán, that will help design basic norms for the administration and regulation of fishing for this species, whose catches are not correctly registered.
MATERIALS AND METHODS
During 5 days in every other month from August 2005 to June 2006, samplings of 1,579 individuals from the commercial catch were taken in Bufadero Bay, Michoacán (18°04'24"N and 102°45'18" W). Of each individual total (Lt) and standard length (Ls), height (H), total (W) and eviscerated (We) weight were measured. Liver, gonad and stomach were weighed in situ, and preserved in 10% formaldehyde and 90% sea water and taken to the lab. Sex of 930 individuals was determined; the other 649 were immature. For age study scales were obtained from 984 individuals. The sample size was calculated with the formula described by Daniel (1991).
Around 10 scales were taken from the area under the left pectoral fin, below the lateral line (Ehrhardt, 1981; Holden & Raitt 1975, Ruiz–Durá et al., 1970) and stored in dry labeled envelopes. Following the method described by Holden and Raitt (1975), scales were washed to clean them of any tissue stuck to them. Later four scales were put in between two slides, sealing them with adhesive tape and labeled. Reading of the scales was carried out with the help of a transparency projector Kodak Ektagraphic with a 127 mm lens (that increases the size of the scale 13.4 times), and measured from the focus to the farthest border as its length, and as the width, between the longest lateral borders. Lines or rings of the scales were observed independently by two different people and the results compared.
Determination of the marginal increment was carried out according to Lai and Liu (1979) in order to determine the date in which the mark is formed and to validate its periodicity. In order to compare and validate the observations of growth rings on scales, indirect methods were used: Petersen (1892), Bhattacharya (1967), and kernel density method (KDE), to determine the components of polymodal curves with monthly and annual periodicity (Salgado–Ugarte, 1992 y 2002).
The constants L∞, K and t0 of von Bertalanffy's (1938) (vB) equation were obtained with Ford's (1933), Walford's (1946), Gulland's method (1964), Beverton and Holt (1959) and two types of regression: simple non linear vB equation and weighted non linear vB equation to find the best fit (Salgado–Ugarte et al., 2005). Weight–length relationship was calculated with the function W = a * Lb (Mendenhall, 1987; Zar, 1996). The weight for every age was obtained with the growth data in length and the weight–length function; and weight growth by substituting Lt and L∞ for W and W∞, in von Bertalanffy's (1938) equation.
Monthly values of the condition factor (CF), equivalent to the "a" parameter of weight–length equation, were obtained for total and eviscerated weight (Safran, 1992), and compared with the confidence interval, to explain changes in the gonad and liver throughout the year. Gastric repletion state was classified according to Gallardo–Cabello and Gual–Frau (1984) as: GRI = number of full stomach/total number of stomach. The gonadosomatic index (GSI) was determined with the equation described by Rodríguez–Gutiérrez (1992): GSI = Wg/W*100, where Wg is gonad weight and Wt is the total weight of the individual. The hepatosomatic index (HSI) was calculated according to Rodríguez–Gutiérrez (1992) as HSI = Wl/W *100, where Wl is the liver weight and Wt is the total weight of the individual. Two condition factors were used: Clark's (1928, with eviscerated weight) and Fulton's (1902, with total weight). Gonadic maturity index was determined In situ, according to Nikolsky (1963). Size of first maturity was obtained by the logistic method (Salgado–Ugarte et al., 2005), which fits the sexual mature individuals' proportion in relation to total length (Lt). Age limit or longevity (95% of L∞ was determined with Taylor's equation (1958, 1960): A0.95 = ln(1 – 0.95)/K + t0.
RESULTS
The relation between total length (Lt) versus standard length (Ls) for L. guttatus (Fig. 1) presents a potential relation of isometric type b = 1.00 (R2 = 0.96; t(1579, 0.05) = 0.007), which means that the magnitude of growth is proportional between the Lt and Ls. Fig. 2 shows an isometric relation between Lt and height (H) with slope value of b = 0.94 (R2 = 0.88; t(1579, 0.05) = 0.047), which indicates that the length and the height of the organism maintain their same proportion as it increases in age. The relation between total weight (W) and Lt showed a slope of b = 2.96 (R2 = 0.96; t(1579, 0.05) = 1.92) (Fig. 3) which indicates an isometric growth with the organism maintaining a proportional weight to its length as it increases its age.
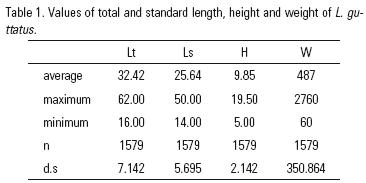
Table 1 shows maximum, minimum and average values of Lt, Ls, H and Wt, and in the Table 2 values of these measures related to length classes of L. guttatus.

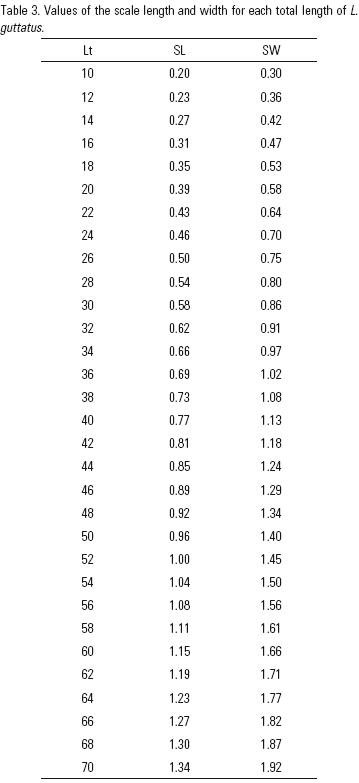
The relation between length (SL) and width (SW) of the scale (Fig. 4) presents an isometric growth b = 0.96 (R2 = 0.97, t(983, 0.05) = 0.728) reason why the scales do not change their morphology conserving their form throughout the time. Table 3 shows the relation between length and width of the scales for different values of total length of L. guttatus. Likewise, the relation between Lt versus SL is isometric (Fig. 5): b = 0.99 (R2 = 0.96, t(983, 0.05) = –8.489), which verifies this type of structure to determine age and use of the growth equation of von Bertalanffy.
During the sampled months a maximum period of growth ring formation was observed in the scales: April (0.65 mm ± 0.30 Std. Dev.), which indicates that a growth ring is formed per year and scales are valid to determine age of L. guttatus.
Analysis of growth rings in scales allowed the identification of 3 age groups. The percentage of scales that showed perfectly defined growth rings was 100 %.
Table 4 shows the values of von Bertalanffy's constants calculated by different methods, which are very similar among them. Nevertheless the nonlinear method provided the best correlation and the parameters are statistically better fitted to the observed data: L∞ = 96.60 cm, K = 0.22 years–1 and to = –0.10 years with an adjustment of p > 0,00 (Chi2 = 0.99, g.l. = 5 and α = 0.05).
Table 5 shows the average values of length and weight, as well as the instantaneous growth rate in length and weight for each age group. Fig. 6 shows observed and calculated values of the growth, with length and weight data of L. guttatus.
Table 6 summarizes values of the von Bertalanffys's constants for L. guttatus obtained by different authors in diverse areas from the Mexican Pacific, and Pauly's growth index ( ') (1979), which helps compare different growth curves. Values calculated in the present study are within the confidence intervals of this growth index (Table 7).
') (1979), which helps compare different growth curves. Values calculated in the present study are within the confidence intervals of this growth index (Table 7).

The months of highest increase in the condition factor for L. guttatus were February and June; for both Fulton's and Clark's indexes a lapse of two months are preceded by higher values of the gastric repletion index (Fig. 7).
The values obtained with KDE are shown in Table 8, for ages 1 to 3, and compared with the results obtained with scales readings.

The mean values for the indexes are: CF Fulton = 7.950 (± 0.235 s.d.), CF Clark = 6.040 (± 0.317 s.d.), GRI = 3.990 (± 0.198 s.d.), GSI = 4.760 (± 0.253 s.d.) and HIS = 5.430 (± 0.370 s.d.). The highest values the GSI appeared in April and August which correspond with the lower values of the HSI (Fig. 7).
The period of massive spawning of L. guttatus occurs during August. Table 9 shows the spawning periods of this species in different localities from the Mexican Pacific by different authors. The first sexual maturity length of L. guttatus was 30.63 cm Lt. This length is defined as that at which 50% of all the individuals are sexually mature and its determination is particularly useful to separate the immature organisms of those sexually mature.
The longevity of L. guttatus determined in this paper is 13.5 years.
DISCUSSION
The scales of L. guttatus were obtained in the area protected by the left pectoral fin because it is the area that has less regenerated scales, however FAO (1982) mentions that the best scales to determine age are those obtained in the scapula (between the head and the dorsal fin). Scales turned out to be suitable structures to identify growth rings of L. guttatus which agrees with Espino–Barr (1996), and Sarabia (2005).
The amplitude of the growth rings on scales showed a progressive diminution as the organism aged. In this study the infinite length of L. guttatus was 96.60 cm Lt, the maximum length observed was 62 cm and the maximum reported by fishers was 75 cm. Amezcua–Linares (2008) reported a male of 80 cm for this locality and Espino–Barr et al. (2004) an organism of 87.31 cm in Jalisco. There are always big differences in sizes, depending on the sample, the latitude, the season of the year, the method to obtain the organisms, etc.
Values of longevity found in other areas by different authors are lower to those found in this paper, except for the reported in Oaxaca by Ramos–Cruz (2001). Differences with results obtained by other authors in the same area could be due to the use of standard length instead of total (as the case of Madrid–Vera, 1990), to the use of an indirect method (length frequencies) or the fishing aspects as effort, location and sampling strategy. However, values of the growth index for this species demonstrate its validity. The greater growth in length of L. guttatus happens during its first year of life with the intention to reduce natural mortality (Gallardo–Cabello et al., 2007 and Espino–Barr et al., 2008).
Recruitment age of L. guttatus is at one year, for this paper it was possible to obtain individuals of the 0 age group (less than one year) through the stomach analysis content of an individual of L. guttatus of 32 cm Lt, and a flounder Cyclopsetta quema of 32 cm Lt (sample from February 2006), who ate individuals of L. guttatus from 1.7 and 1.65 cm Lt, the scales of these organisms showed the absence of growth rings, which means that they belonged to age group 0.
Mean lengths data obtained by scale readings are very similar to those obtained by KDE method, which validates both methods: direct and indirect, that is, that the growth rings formed on scales and the polymodal curve analyses give a good fit to determine the age in this species. Increase of the gastric repletion index values due to greater availability of food, are reflected in the index of the condition factor and in the hepatosomatic index after two months as a result of assimilation of nutrients through the processes of catabolism. On the other hand the gastric repletion index and condition factor diminish during spawning period, as a result of corporal wearing down of the fish (Santamaría et al., 2003a; Gallardo–Cabello et al., 2007; Espino–Barr et al., 2008).
In this study, six maturity stages were identified, similar to those described by Rojas (1997) in the Gulf of Nicoya, Costa Rica and those reported by Piñón et al. (2009) for Lutjanus argentiventris in the Gulf of California. This species spawning period happens during August, which agrees with Arellano et al. (2001) observations in the coasts of Guerrero, nevertheless, other authors as Cruz–Romero et al. (1991) and Cruz–Romero & Espino–Barr (2006) found other spawning period months for L. guttatus in the coasts of Jalisco and Colima. Organisms born during August appeared in the fishing grounds during April and June and those born in April recruited in the fishing area from November to January. They become recruited to the fishing gear over a year after. Recruitment length is 16 cm, and the period with greater recruitment of adult population happens during April and August.
L. guttatus reaches sexual maturity at a length of 30.63 cm Lt, value similar to that found by Cruz–Romero et al. (1991) of 29 cm Lt in Colima. On the other hand, Rojas (1997) reported a minimum maturity length of 31.5 cm Lt in the Gulf of Nicoya, Costa Rica. Similar values were reported by Rojo et al. (1999) and Santamaría et al. (2003a).
Of the 1,579 sampled organisms, 649 (41.10%) were sexually immature individuals. This is because the fishers prefer to capture organisms whose lengths approximately oscillate between 30 and 35 cm Lt and 400 g of weight, being these sizes the ones that pay higher price, because they fit in the plate and tourism prefers its consumption. Organisms of greater size have to be filleted and its commercial value descends. As a measure for protecting this species of overfishing, we suggest a closed season during August and September, to give a longer period of possibilities of spawning, and a first size of capture at 45 cm of length equivalent to 2.5 years of age, when most females have reproduced.
ACKNOWLEDGEMENTS
We thank the artisanal fishermen in Bufadero Bay, Michoacán, who always allowed us to take samples from their catch; and several institutions for financial assistance: UNAM, CONACyT and INAPESCA.
REFERENCES
Allen, G. R. & D. R. Robertson. 1994. Peces del Pacífico Oriental Tropical. CONABIO, Agrupación Sierra Madre y CEMEX. México. 327 p. [ Links ]
Amezcua, F., C. Soto–Ávila & Y. Green–Ruiz. 2006. Age, growth, and mortality of the spotted rose snapper Lutjanus guttatus from the southeastern Gulf of California. Fisheries Research 77: 293–300. [ Links ]
Amezcua–Linares, F. 2008. Peces demersales del Pacífico de México. Instituto de Ciencias del Mar y Limnología, Universidad Nacional Autónoma de México. México. 281 p. [ Links ]
Arellano, M. M., A. Rojas H., F. García D., FJ. P. Ceballos V. & M. Villarejo F. 2001. Ciclo reproductivo del pargo lunarejo Lutjanus guttatus (Steindachner, 1986) en las costas de Guerrero, México. Biología Marina y Oceanografía 36 (1): 1–8. [ Links ]
Bhattacharya, C. G. 1967. A simple method of resolution of a distribution into Gaussian components. Biometrics 23: 1 15–135. [ Links ]
Beverton, R. J. H. & S. J. Holt. 1 959. A review of the lifespan and mortality rates of fish in nature, and their relation to growth and other physiological characteristics. Ciba Found. Symposium on the Lifespan of Animals. London UK. 142 – 177. [ Links ]
Castro–Aguirre, J. L., H. S. Espinosa Pérez & J.J. Schmitter–Soto. 1 999. Ictiofauna estuarino–lagunar y vicaria de México. Serie Biotecnologías. Instituto Politécnico Nacional y Ed. Noriega–Limusa. México. 711 p. [ Links ]
Chiappa–Carrara, X., A. Rojas H. & M. Mascaro. 2004. Coexistencia de Lutjanus peru y Lutjanus guttatus (Pisces: Lutjanidae) en la costa de Guerrero, México: relación con la variación temporal en el reclutamiento. Revista de Biología Tropical 52 (1): 177–185. [ Links ]
Clark, F. 1928. The weight–length relationship of the Californian sardine (Sardina coerulea) at San Pedro. Fishery Bulletin U.S. 12: 22–44. [ Links ]
Cruz–Romero, M., E. Espino–Barr, P. del Monte–Luna, A. García–Boa, A. Ayala–Cortés, J.J. González–Ruiz & S. Sánchez–González. 2000. Huachinango del Pacífico. In: M.A. Cisneros–Mata & L. Beléndez M. (Eds.). Sustentabilidad y Pesca Responsable en México. Evaluación y Manejo 1999–2000. INP–SEMARNAP. México, D.F. 1047 p. [ Links ]
Cruz–Romero, M., E. Espino–Barr, J. Mimfela L., A. García–Boa, L. F. Obregón A. & E. Girón F. 1991. Biología reproductiva en tres especies del género Lutjanus en la costa de Colima, México. Informe Final. Clave CONACYT: P220CCoR892739, México. 118 p. [ Links ]
Cruz–Romero, M. & E. Espino–Barr. 2006. Desarrollo y resultados de la investigación de la pesca ribereña. In: P. Guzmán–Amaya & D. Fuentes–Castellanos (eds.). Pesca, Acuacultura e Investigación en México. Comisión de Pesca, Cámara de Diputados. CEDRSSA. México, D.F. 407 p. [ Links ]
Daniel, W. W. 1991. Bioestadística. Base para el análisis de las ciencias de la salud. Ed. Noriega–Limusa. México. 667 p. [ Links ]
Ehrhardt, N. 1981. Curso sobre métodos en dinámica de poblaciones. 1a.Parte. Estimación de parámetros poblacionales. Instituto Nacional de Pesca. México, D.F. 150 p. [ Links ]
Espino–Barr, E., M. Cruz–Romero & A. García–Boa. 1994. Métodos comparativos para determinar edad y crecimiento de pargos de la familia Lutjanidae. Memorias de resúmenes del IV Congreso Nacional de Ictiología, Morelia, Mich. [ Links ]
Espino–Barr, E. 1996. Edad y crecimiento del huachinango Lutjanus peru (Nichols y Murphy, 1922), en las costas de Colima, México. Tesis de Maestría, Facultad de Ciencias; Universidad Nacional Autónoma de México. México DF. 73 p. [ Links ]
Espino–Barr, E., E. G. Cafral–Solís, A. García–Boa & M. Puente–Gómez. 2004. Especies marinas con valor comercial de la costa de Jalisco, México. SAGARPA – INP. México. 145 p. [ Links ]
Espino–Barr, E., M. Gallardo–Cabello, E. G. Cafral Solís, A. García–Boa & M. Puente–Gómez. 2008. Growth of the Pacific jack Caranx caninus (Pisces: Carangidae) from the coast of Colima, México. Revista de Biología Tropical 56 (1): 171–179. [ Links ]
FAO. 1982. Métodos de recolección y análisis de datos de talla y edad para la evaluación de poblaciones de peces. Circular de Pesca No. 736. Roma, Italia. 1–101. [ Links ]
Fischer, W., F. Krupp, W. Schneides, C. Sommer, K. E. Carpenter & U. H. Niem (Eds.). 1 995. Guía Fao para la identificación de especies para los fines de la pesca. Pacífico Centro Oriental. (2 y 3): 644–1813. [ Links ]
Ford, E. 1933. An account of the herring investigations conducted at Plymouth during the years from 1924 to 1933. Journal of Marine Biology Association. U.K. 19: 305 – 384. [ Links ]
Fulton, T. 1 902. Rates of growth of sea–fishes. Annual Report of the Fishery Board of Scotland 1902 (3): 326–446. [ Links ]
Gallardo–Cabello, M. & A. Gual–Frau, 1984. Consideraciones bioecológicas durante el crecimiento de Phycis blennoides (Brünnich, 1768) en el Mediterráneo Occidental (Pisces: Gadidae). Anales del Instituto de Ciencias. del Mar y Limnología 1 1 (1): 225–238. [ Links ]
Gallardo–Cabello, M., E. Espino–Barr, A. García–Boa, E. G. Cafral–Solís & M. Puente–Gómez. 2007. Study of the growth of the green jack Caranx caballus Günther 1868, in the coast of Colima, México. Journal of Fisheries and Aquatic Science 2 (2): 131–139. [ Links ]
Grimes, C. A. 1987. Reproductive biology of the Lutjanidae. In: J.J. Polovina & S. Ralston (eds). Tropical snapper and grouper Biology and Fisheries management. Boulder: Westview Press Inc. USA. 239–294. [ Links ]
Gulland, J. A. 1964. Manual of methods of fish population analysis. FAO Fish. Tech. Paper 40: 1–60. [ Links ]
Holden, M.J. & D.F.S. Raitt. 1975. Manual de Ciencia Pesquera. Parte 2.– Métodos para investigar los recursos y su aplicación. ONU/FAO. Documento Técnico Sobre Pesca 115 (1): 1 –207. [ Links ]
Lai, H. L. & H. Liu. 1 979. Age and growth of Lutjanus sanguineus in the Arafura sea and north of west shelf. Acta Oceanographica Taiwanica 10: 164–175. [ Links ]
Madrid–Vera, J. 1 990. Ecología de algunas especies de peces de importancia comercial. Tesis de maestría. Universidad Nacional Autónoma de México, México. 179 p. [ Links ]
Mendenhall, W. 1987. Introduction to probability and statistics. PWS–Kent Publishing Co. USA. 884 p. [ Links ]
Nikolsky, G. V. 1 963. The Ecology of Fishes. Academic Press. London, UK. 351 p. [ Links ]
Pauly, D. 1 979. Theory and management of tropical multispecies stocks: a review with emphasis on the Southeast Asian demersal fisheries. ICLARM Study Review 1: 1–35. [ Links ]
Petersen, C. G. J. 1892. Fiskenes biologiske forhold I Holbaek Fjord, 189–091. Beret. Danm. Biol. St. 1890 (1): 121–183. [ Links ]
Piñón, G. A. 2003. Contribución al conocimiento de la biología de las especies Hoplopagrus guentherii, Lutjanus argentiventris, Lutjanus colorado y Lutjanus guttatus de la Bahía de Mazatlán y Santa María la Reforma. Tesis de Maestría Instituto de Ciencias del Mar y Limnología, Universidad Nacional Autónoma de México, México. 106 p. [ Links ]
Piñón A., F. Amezcua & N. Duncan. 2009. Reproductive cycle of female yellow snapper Lutjanus argentiventris (Pisces, Actinopterygii, Lutjanidae) in the SW Gulf of California: gonadic stages, spawning seasonality and length at sexual maturity. Journal of Applied Ichthyology 25: 18–25. [ Links ]
Ramos–Cruz, S. 2001. Evaluación de la pesquería de huachinango Lutjanus peru en la zona costera de Salina Cruz, Oaxaca, México, durante 1 995. Ciencia Pesquera 14: 151–157. [ Links ]
Rodríguez–Gutiérrez, M. 1 992. Técnicas de evolución de la madurez gonádica en peces. AGT Ed. México. 79 p. [ Links ]
Rojas–Herrera, A. A. 2001. Aspectos de dinámica de poblaciones del huachinango Lutjanus peru (Nichols y Mmurphy, 1922) y del flamenco Lutjanus guttatus (Steindachner, 1869) (Pisces: Lutjanidae) del litoral de Guerrero, México. Tesis de Doctorado, Facultad de Medicina, Veterinaria y Zootecnia, Universidad de Colima, 194 p. [ Links ]
Rojas, M. J. R. 1 997. Fecundidad y época de reproducción del pargo mancha Lutjanus guttatus (Pisces: Lutjanidae) en el Golfo de Nicoya, Costa Rica. Revista de Biología Tropical 45: 477–487. [ Links ]
Rojo V., J. A., F. Arreguín–Sánchez, E. Godínez D. & M. Ramírez R. 1 999. Selectividad de redes de enmalle para el pargo lunarejo (Lutjanus guttatus) y el pargo alazán (Lutjanus argentiventris) en Bahía de Navidad, Jalisco, México. Ciencias Marinas 25 (1): 145–152. [ Links ]
Ruiz–Durá, M. F., Y. Orijel–Arenas & G. Rodríguez–Hernández. 1 970. Líneas de crecimiento en escamas de algunos peces de México. Instituto Nacional de Investigaciones Biológico Pesqueras. Serie Investigación Pesquera 2: 1–97. [ Links ]
Ruiz–Luna, A., E. Girón F., J. Madrid V. & A. González F. 1985. Determinación de edad, crecimiento y algunas constantes biológicas del huachinango del Pacífico Lutjanus peru (Nichols y Murphy , 1922). Memorias del VII Congreso Nacional de Zoología, Morelia Mich., México. 188–201. [ Links ]
Safran, P. 1 992. Theoretical analysis of the weight–length relationship in fish juveniles. Marine Biology 1 12: 545–551. [ Links ]
Salgado–Ugarte, I. H. 1 992. El análisis exploratorio de datos biológicos. Fundamentos y aplicaciones. Marc Ed. UNAM. México. 243 p. [ Links ]
Salgado–Ugarte, I. H. 2002. Suavización no paramétrica para análisis de datos. FES Zaragoza, UNAM. México. 139 p. [ Links ]
Salgado–Ugarte, I. H., J. Gómez–Márquez & F. Peña–Mendoza. 2005. Métodos actualizados para el análisis de datos Biológicos–Pesqueros. ENEP Zaragoza UNAM. México. 240 p. [ Links ]
Santamaría M., A., J. Elorduy G., M. Villarejo F. & A. A. Rojas H. 2003a. Desarrollo gonadal y ciclo reproductivo de Lutjanus peru (Pisces: Lutjanidae) en Guerrero, México. Revista de Biología Tropical 51 (2): 17–25. [ Links ]
Santamaría M., A., J. Elorduy G. & A. A. Rojas H. 2003b. Hábitos alimentarios de Lutjanus peru (Pisces: Lutjanidae) en las costas de Guerrero, México. Revista de Biología Tropical 51 (2): 1–17. [ Links ]
Sarafia, M. M. 2005. Determinación de la edad y crecimiento del pargo flamenco Lutjanus guttatus (Steindachner, 1869) (Pisces: Lutjanidae), mediante el análisis de escamas en Bahía Buíadero, Michoacán, México. Tesis de Licenciatura, Facultad de Ciencias, Universidad Nacional Autónoma de México. México, D.F. 54 p. [ Links ]
Sheaves, M. 1 995. Large Lutjanid and Serranid fishes in tropical estuaries: Are they adults or juveniles? Marine Ecology Progress Series 129: 31–40. [ Links ]
Taylor, C.C. 1958. Cod growth and temperature. Journal du Conseil 23 (3): 366–370. [ Links ]
Taylor, C.C. 1 960. Temperature, growth and mortality – the Pacific cockle. Journal du Conseil 26 (1): 1 17–124. [ Links ]
von Bertalanffy, L. 1938. A quantitative theory of organic growth (inquiries on growth laws. II). Human Biology 10 (2): 181–213. [ Links ]
Walford, L.A., 1946. A new graphic method of describing the growth of animals. Biological Bulletin 90 (2): 141–147. [ Links ]
Zar, J.H., 1 996. Biostatisticalanalysis. 3rd ed. Prentice Hall. USA. 662 p. [ Links ]














