Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Hidrobiológica
versión impresa ISSN 0188-8897
Hidrobiológica vol.20 no.1 Ciudad de México abr. 2010
Artículos
Lipid peroxidation and metallothionein induction by chromium and cadmium in Oyster Crassostrea virginica (Gmelin) from Mandinga Lagoon, Veracruz
Lipoperoxidación e inducción de metalotioneínas por cromo y cadmio en ostión Crassostrea virginica (Gmelin) de la Laguna de Mandinga, Veracruz
Guadalupe Barrera–Escorcia1,* and Irma Wong–Chang 2
1 Laboratorio de Ecotoxicología, Departamento de Hidrobiología, Div. CBS, Universidad Autónoma Metropolitana–Iztapalapa, México, D.F. A.P. 55 –535. *E–mail: gube@xanum.uam.mx
2 Laboratorio de Contaminación Marina, Instituto de Ciencias del Mar y Limnología, Universidad Nacional Autónoma de México, México, D.F., A.P. 70–305.
Recibido: 26 de julio de 2009.
Aceptado: 06 de enero de 2010.
ABSTRACT
Metallothionein and lipid peroxidation are associated to metals toxicity, it is possible that accumulation in tissue might as well be related to their increase. Both biomarkers under Cr and Cd exposure were evaluated in the American oyster Crassostrea virginica (Gmelin) by Semi–static bioassays, 96 h long. Metallothionein content was determined by silver saturation, lipid peroxidation by reactive components to thiobarbituric acid, and metals concentration by atomic absorption spectrophotometry. Metallothioneins increased in digestive gland and abductor muscle under Cr exposure, after 6 h. Under Cd exposure, metallothioneins showed variations, with high values after 48 h in digestive gland, abductor muscle and gill, and important dispersion of data. Lipid peroxidation under Cr exposure reached after 48 h. In Cd, values higher than controls were observed after 6 h exposure. Metallothioneins in gill and digestive gland were positively correlated with the Cr concentration in water and in oysters. In contrast, these proteins correlation turned out to be negative to the Cr Bioconcentration Factor in digestive gland and muscle. The higher peroxidation in oysters which accumulated less, suggests deterioration and a metabolic effort to control the Cr internal concentration. Under Cd exposure, metallothioneins showed positive correlations between water and oysters metal content, and with the Bioconcentration Factor. The correlations, indicates incapability to control the Cd stored levels. The results indicate that the protective role of metallothioneins was limited in the case of this non esencial metal, in oyster originated in native populations from Mandinga Lagoon.
Key words: Metallothionein, lipid peroxidation, oyster, chromium, cadmium.
RESUMEN
Las metalotioneínas y la lipoperoxidación están asociadas a la toxicidad de los metales, su incremento posiblemente se relaciona también con su acumulación en tejido. Se evaluaron ambos biomarcadores en el ostión americano Crassostrea virginica (Gmelin), bajo exposición a Cr y Cd con bioensayos semiestáticos de 96 h. Las metalotioneínas se determinaron por saturación de plata, la lipoperoxidación por los componentes reactivos al ácido tiobarbitúrico, y la concentración de metales por espectrofotomería de absorción atómica. Las metalotioneínas se incrementaron en la glándula digestiva y músculo abductor, después de 6 h de exposición a Cr. En el caso de exposición a Cd las metalotioneínas mostraron variaciones, con valores elevados e importante dispersión de datos en glándula digestiva, músculo y branquia, posteriores a 48 h. La lipoperoxidación se incrementó después de 48 h bajo exposición a Cr. Valores superiores al control fueron observados después de 6 h de exposición a Cd. Las metalotioneínas en branquia y glándula digestiva se correlacionaron positivamente con la concentración de Cr en agua y ostión. En contraste, esta correlación fue negativa respecto al Factor de Bioconcentración de Cr en glándula digestiva y músculo. La mayor peroxidación en ostiones que acumularon menos Cr, sugiere deterioro y un esfuerzo metabólico para controlar la concentración interna. Las correlaciones positivas de las metalotioneínas con el Cd en agua y ostión, así como con el Factor de Bioconcentración, indica la incapacidad para controlar el Cd almacenado. Los resultados indican que el papel protector de las metalotioneínas fue limitado para este metal no esencial, en ostiones provenientes de poblaciones nativas de la Laguna de Mandinga.
Palabras clave: Metalotioneína, lipoperoxidación, ostión, cromo, cadmio.
INTRODUCTION
Metals, such as Cr and Cd, tend to increase in diverse coastal environments of the Mexican Gulf, hoarding in the sediment (Paez, 2005) and in filtering organisms, like oysters, where critical levels for consumption can be exceeded (Guzman et al., 2005). The oyster Crassostrea virginica (Gmelin, 1791) is found in the Atlantic coast from North America and literature about its physiology is profuse (Roesijadi, 1996); it is considered an adequate indicator to assess the contaminants contributions, since the metals concentrations incorporated in tissue fluctuates reflecting the environmental concentration. In Mexico, they are also distributed in the Gulf coastal lagoons and there are some studies about Cr and Cd levels accumulated in organisms (Contreras & Castañeda, 1995; Botello, 1994; Villanueva & Botello, 1998). This specie represents a regionally important exploitable resource and it is extracted directly from the lagoons. It has been considered that metals concentrations collected in tissue, constitute potential biological impact indicator of environmental concentration, since the metals bioavailability is automatically considered (Borgmann, 2000). Wood et al. (1997) state that this kind of approach can be useful to predict the metals toxicity induced from water to benthonic organisms. In filtering organisms, such as C. virginica, tissue hoarding is particularly important since, even though there are several access routes for both metals into the animals, the main route is the water (Neff, 2002; Rebougas et al., 2005).
Besides the accumulated metal, it is feasible to assess different biomarkers as specific reflections of its presence, under chronic or sublethal exposure conditions. Biomarkers are identifiable signs that provide early damage warnings (Koeman, 1991), and generate quantifiable responses. For instance, the metallothionein synthesis associated with the essential metals requirements (Simkiss et al., 1982; Klaasen & Eaton, 1991). In C. virginica play an important role in the regulation of Zn, a metal which enters in large amounts and is promptly cleared out from the organism, which is related with the Ca fixation in valves and, therefore, with their resistance and, consequently, with the organism development, moreover, metallothionein regulate Cu levels (Savva, 1999). In presence of excessive Zn amounts, or other essential or non–essential metals, such as a Cd, the metallothionein synthesis increase (Roesijadi, 1996). Due to its regulatory activity with essential–metals, its production has also been correlated with detoxifying mechanisms linked with toxic metals, principally nonessential ones, in several species, including C. virginica (Köhler & Riisgard 1982; Stegeman et al., 1992; Roesijadi et al., 1996).
Another important biomarker is the oxidative stress damage, a common phenomenon under physiological stress conditions. The production of unbound radicals manifests, amongst other effects, increase in the membrane lipids oxidation, event also known as lipid peroxidation. The peroxidative damage can also result from the action of electrophilic intermediaries which interact with nucleophilic sites in the cell, including glutathione and the thiol–group proteins, producing oxidative stress in the entire cell. Moreover, due to the presence of nucleophilic sites in the nucleic acids, the production of unbound radicals is associated with mutagenesis and carcinogenesis (Goyer, 1991). The effect of Cd has been demonstrated in lipid peroxidation, however, the mechanisms are not entirely acknowledged (Souza et al., 1996), on the other hand, albeit scant information about chromium exists, it is necessary to consider that Cr6+ is an oxidant and corrosive agent therefore, it can be associated with oxidative stress.
The purpose of this work was to determine the effects over metallothionein and lipid peroxidation in Crassostrea virginica native from a mexican coastal tropical lagoon under sublethal Cr and Cd exposure.
MATERIALS AND METHODS
Adult commercial–size oysters (longer than 5 cm), extracted from Mandinga lagoon, located in Veracruz state, Mexico (19° 03' N and 96° 05' W) were selected. Measures in situ of: dissolved oxygen (± 0.005 mg/L), temperature (± 0.05 °C), pH (± 0.005), salinity (± 0.05‰) and turbidity (0.5 UT), were taken with a Horiba U–10 multianalyzer. Simultaneously, three water samples were taken for further Cd and Cr concentration analysis in the laboratory. These samples were placed in one–litter plastic containers and titrated to pH < 2 with nitric acid 0.5 mL as indicates the American Public Health Association (APHA, 1995), placed in ice chests for transportation and frozen at – 20 °C, until metal–content analysis performance.
The oysters were transported at 4°C temperature in plastic bags to the laboratory. After arrival, the organisms were washed according to the APHA method (1995) using plastic brushes, with abundant water, eliminating epibionts and mud that could interfere with results. Oysters were washed with alcohol 70% and ten minutes later with water again. Homogenous organisms (69 ± 8 mm length, mean standard deviation) were selected. Twelve specimens were used for metals determination.
A total of 144 oysters were introduced in a 600 L capacity maintenance system with closed water circulation, continuous air flow (> 5 mg/L of dissolved oxygen), a carbon and anthracite particle filter and a wet/dry trickle biological filter. The salinity was similar to that registered in the field and it was adjusted by 0.5% per day, up to the bioassays selected value (22‰). Artificial salted water Instant Ocean (Shumway & Kbehn, 1982), prepared and filtered a month earlier, was used. The temperature was maintained at 25 °C and the air flow was continuous (> 5 mg/L of dissolved oxygen). During the 23 days the organisms remained in the system, they were fed with Tetraselmis suecica Kylin (15 to 20 X 106 cells per organism per day, according to Castrejon et al., 1994) and fasted 24 h prior the experimental phase.
Semi–static bioassays, 96 h long, were carried out in these filtering organisms to provide an adequate water renewal in a relatively little space as recommends Buikema et al. (1982). System fixed conditions were 22‰ salinity and 25 °C temperature. The physicochemical parameters were monitored daily and 25% of the water volume was replaced, with reposition of metal in each concentration.
The metal concentrations chosen for the bioassays were lower than the LC1 obtained for C. virginica of the Mandinga Lagoon (Barrera, 2006), lower than the Mexican legislation accepted limits for residual water (DOF, 1997), similar to those concentrations used in other investigations in sublethal bioas–says with this species (Conners & Ringwood, 1997) but higher than those detected in the lagoon. Five conditions were selected: control (without metals), 88 µgCr/L, 144 µgCr/L, 110 µgCd/L and 210 µgCd/L. A total of 140 specimens were used (120 for the selected biomarkers and 20 for metal accumulated), 28 per concentration, placed in 40 L glass water–tanks, at the rate of 8 oysters per tank. After 6, 48 and 96 h of exposure, 8 organisms were extracted from each concentration, including controls. The remaining four animals were used to determine metals concentrations in gill, abductor muscle and digestive gland.
Metallothionein production (µg/g of tissue) was determined by the silver saturation method as described by Scheuhammer & Cherian (1991) in gill, abductor muscle and digestive gland. Oyster tissue samples were homogenized individually (360 samples) with four 0.25 M sucrose volumes and froze to – 85 °C for further processing. Metallothionein coupled to metal was replaced by silver in glycine buffer; a blood haemolyzed of lamb was used in order to drag unbound metal. Subsequently, unwanted molecules were cleared out through water bath (2 min) and 4,000 rpm centrifugation, recovering the over floating remanent, which was centrifuged at 13,000 rpm for 5 min. The metallothionein concentration (µg/g of wet tissue) was determined through the remnant silver concentration.
Lipid peroxidation mediated damage was analysed only in the digestive gland (120 samples). This procedure was carried out straight after extracting the organisms from the water tanks. The thiobarbituric acid reactive components were expressed as malondialdehyde concentration equivalents (MDA nmol/mg protein), which represent more than 80% of the components associated to lipid peroxidation (Bucio et al., 1995). A part of the sample was separated for protein determination. Tissue was immediately frozen at – 20 °C, in order to perform further analysis through the Lowry's technique (Cooper, 1997), based on colorimetric readings, indicated by the Folin's reactive, and on a bovine–albumin curve–pattern.
Metal levels were analysed in water and the organisms arriving at the end of the bioassay. The dissected tissues were dehydrated and subsequently grinded. Metals concentrations in the water were also determined at the beginning, the middle and the end of the assay. Tissue and water samples were titrated to pH < 2 with nitric acid for further analysis. Samples digestion was carried out in a CEM–MDS–81D microwave oven for 10 minutes at 80% oven's power. The method proposed by Huan (1994) was used for tissue processing, using 0.25 g of dry weight (DW). Metals were determined with an atomic absorption spectropho–tometer Varian AA20, equipped with acetylene–air flame (three readings per sample). Reference samples from the Metrology National Centre (Centro Nacional de Metrología) were used, with 4.0 ± 0.12 mgCr/L (where our reading was 4.02 mgCr/L) and with 1.45 mgCd/L ± 0.059 (where our reeding was 1.40 mgCd/L). The bioconcentration factor (BCF) of the analysed samples was determined (Buikema et al., 1982).
For statistical analysis, the software used was: Windows environment Excel 95 and XP2000 version and Statistica software by Statsoft Ser. (1997). The Kruskal–Wallis test and the Spearman's rank correlation coefficient (Marques de Cantú, 1991) were applied. The tests significance was p < 0.05.
RESULTS
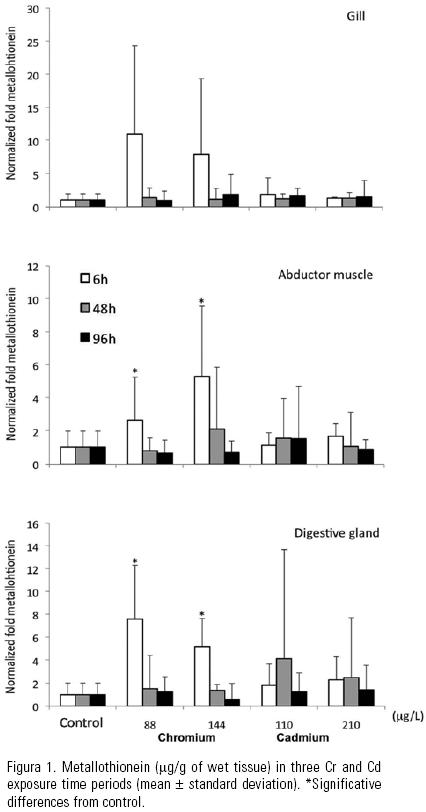
Metallothionein. Metallothionein levels were higher in digestive gland, than gill and abductor muscle. Significant statistical differences were confirmed, after 6 h the test began (Fig. 1), in the control oysters digestive gland (33.0 ± 37.2 µg/g, mean ± standard deviation) compared to Cr exposed animals (211.5 ± 132.8 µg/g). Similarly, the control oysters abductor muscle (34.5 ± 21.3 µg/g) had lower values than the exposed animals (137.3 ± 73.1 µg/g). The gill did not show differences among control and organisms under both metals exposure. Metallothionein levels demonstrated variations amongst the analysed days in Cd exposed oysters, with high values after 48 h, nevertheless, no differences between groups were found.
Lipid peroxidation. During the assay, the mean value in the digestive gland of control organisms was 155.8 ± 42.2 MDA nmol/ mg of protein. The oysters under 88 µgCr/L exposure reached significantly higher concentrations after 48 h (271.7 ± 98.3 MDA nmol/mg of protein). In 144 µgCr/L exposure, values lower than controls were observed after 96 h (97.0 ± 14.5 MDA nmol/mg of protein). In Cd exposure oysters presented higher values than controls after 6 h exposure, 213.0 ± 111.6 and 262.6 ± 109.1 MDA nmol/mg of protein (Fig. 2).

Metal content. Oysters from Mandinga lagoon presented 1.20 ± 0.63 µgCr/g DW and 2.33 ± 1.11 µgCd/g DW. In the water the concentrations were 80 ± 44 µgCr/L and 77 ± 8 µgCd/L. During the bioassay control oysters showed similar concentrations in the analysed tissues, with mean values of 6.3 ± 4.92 µgCr/g DW and 1.7 ± 2.36 µgCd/g DW (Fig. 3). These basal values implied high Cr BCF in control oysters.
In Cr exposed animals, the abductor muscle had similar Cr concentration to control specimens (10.76 ± 3.42 µgCr/g DW). The rest of the tissues reported higher values: in gills of organisms exposed to 88 µgCr/L and 144 ugCr/L the values were 27.47 ± 18.65 µgCr/g DW and 19.41 ± 2.87 µgCr/g DW, respectively. The levels reached in the digestive gland in oysters exposed to 144 µgCr/L were 28.19 ± 12.85 µgCr/g DW. These values 2–folded the accumulation value under exposure to 88 µgCr/L of 13.89 ± 5.69 µgCr/g DW.
The gill and the digestive gland hoarded Cd in similar proportions. The registered values in gill were 34.54 ± 10.42 µgCd/g DW and 47.51 ± 52.11 µgCd/g DW, and the digestive gland were 39.84 ± 5.66 µgCd/g DW and 48.69 ± 49.38 µgCd/g DW. These concentrations contrasted with the ones registered in muscle (10.80 ± 2.93 µgCd/g DW), once more, the lowest concentrations amongst the studied tissues.
The Cr BCF in gill and abductor muscle was higher under 88 ugCr/L than in 144 ug/L exposure. The Cd BCF was higher than Cr BCF and it was associated to the 110 µgCd/L concentration. In the abductor muscle it implied lower accumulation compared to the rest of the tissues (Fig. 4).
Biomarkers behaviour regarding the accumulated metal. Significantly negative correlations between Cr water concentrations and BCF in the three tissues were found (table 1). In gill and digestive gland, the metallothionein production was directly correlated with the Cr concentration in water and in oysters. In contrast, the correlation turned out to be negative to the BCF in digestive gland and muscle. In other words, there was a higher metallothionein concentration in organisms with lower BCF. High levels of lipid peroxidation revealed a negative correlation (r = – 0.75) with the oysters Cr concentration. This implies larger peroxidative damage in oysters with lower metal concentration.
Metallothionein production in organisms exposed to Cd showed positive metal content correlations between water and oysters, however, in contrast with Cr exposure, the BCF showed also a positive metallothionein production correlation in the digestive gland and in the gill. There were no relevant correlations observed in the abductor muscle. There was a positive correlation between the lipid peroxidation and the Cd concentration in water (r = 0.99), that is, there was larger peroxidative damage associated with the Cd exposure concentration.
DISCUSSION
A larger induction of metallothionein in the digestive gland, compared to other tissues, has been demonstrated in Mytilus edulis Linnaeus, 1758, M. galloprovincialis Lamark, 1819, C. gigas Thunberg, 1793 and other molluscs (Geret et al., 1997; Geret & Cosson, 2000; Mourgaud et al., 2002). Our results were consistent with those reported by Köhler & Riisgard (1982), where higher concentrations in the digestive gland, then in the gill and finally in the abductor muscle of Mytilus edulis exposed to Cd, were found. The hoarding in digestive gland, as consequence of Cd exposure, has been attributed to the fact that metal–bound proteins are not functional and, consequently, they are stored in the tissue. The cells involved in metals transportation, such as digestive, hepatic and renal cells, are more affected (Goyer, 1991), therefore, deterioration in these tissues is expected. A relationship has been found between high levels of body Cd and atrophy of digestive gland in C. virginica (Gold–Bouchot et al., 1995).
Metallothionein induction in digestive gland and abductor muscle, as consequence of Cr exposure, was confirmed after 6 h with values 7.4– and 5.4–fold higher than controls, respectively. Cd exposure showed a different behavior, even though high concentrations were observed in Cd exposed specimens (810.1 µg/g, in the digestive gland) after 48 h, the difference against the controls was not confirmed. Data dispersion was important and time variation was present in Cd exposed oysters. Apparently, in natural environments, and even in the contaminated ones, previous exposure to low cadmium concentrations are the underlying basis of raise in metallothionein, more than induction itself, due to Cd stabilization and accretion, while basal levels are synthesized (Roesijadi, 1999). The increase in metallothionein concentrations during the bioassay can be interpreted as a consequence of the presence of metals in the exposed organisms. Experiments with Crassostrea gigas have shown increases in the expression of metallothioneins mRNA in time, up eleven days (Choi et al., 2008). But increase in control organisms can only be associated to a stress related to laboratory conditions. The metallothionein expression is regulated by a complex behaviour associated to essential metals, as Zn and Ca. Since the organisms were not fed during the experiment, it is possible that lack of food could have generated a metabolic unbalance (Roesijadi, 1999). This behaviour could be expected in all the organisms and prevents the possibility of demonstrating differences among controls and experimental animals after 48 and 96 h.
There are several works reporting metallothionein induction for Cd exposure in molluscs; as described in the Asiatic clamp Corbicula fluminea, (O. F. Muller, 1774), were concentrations 2.5 times higher than in controls were found (Baudrimont et al.,1997). Langston & Zhou (1987), reported in Macoma baltica (Lannaeus, 1758) the increase from 35 ug/g in controls, to 450 ug/g in organisms under 100 µgCd/L exposure. Viarengo et al. (1997), and Mourgaud et al. (2002) determined increase of metallothioneins associated to metals exposure including Cd in Mytilus galloprovincialis. In Mytilus edulis, exposed to levels from 200 µgCd/L to 500 µgCd/L the metallothioneins increased approximately up to 500 µg/g (Bebiano & Langston, 1991; Köhler & Riisgard, 1982). Moreover, an increase has been observed in C.gigas larvae exposed to 200 µgCd/L, approximately 2.5 times higher than in controls (Gautier et al., 2006). Other studies have reported increases in metallothioneins in gill, mantle and abductor muscle of C. virginica after Cd exposure (Carpene, 1993; Roesijadi, 1994, 1996; Roesijadi & Klerks, 1989; Roesijadi et al., 1996). Although in these studies the metallothionein raise derived from Cd exposure is similar to the obtained values, the data can only be compared, strictly speaking, when it has been obtained through the same technique (Amiard et al., 2006).
Metallothionein induction in Cr (an essential metal) exposed specimens was observed after 6 h. However, the values were low in further time periods. This finding might be the result of a toxic effect previous to a detoxifying process, as stated by Amiard et al. (2006).
Several results presented high data dispersion, heterocedasticity and the presence of extreme cases. The absence of normality and homocedasticity can influence the interpretation with parametrical techniques, therefore Kruskal–Wallis test, a robust technique, was used (Tukey, 1977). The analysis must consider the biological specific features of this specie too, for instance, the valves closure, in C. virginica can last for up to 7 h under normal conditions (Shumway, 1982), and the valves closure duration proportionally increases according to the environment toxics concentration. This closure might produce the responses delay and, therefore, the broad dispersion of data. Furthermore, if closure is proportional to the toxic concentration, it is possible that the greater effect observed occasionally in lower concentrations in metallothionein, as well as in lipid peroxidation, could be related to this behavior. A narcotic effect has also been attributed to metals (Smith, 1985; Lin et al., 1992), which might affect the organism activity, the slow valves closure could be an effect of metals exposure.
The dispersion of data could be a consequence of the origin of organisms too. The oysters came from the Mandinga lagoon, they were selected with commercial size and morphologically homogenous, but there is natural variability in organisms from native populations. The variability in results of bioassays using organisms from natural environments could be expected, but the evaluation of their responses is desirable because they can be related to a real situation. On the other hand, the oyster culture in Mexico does not handle the entire life cycle and it is not possible to obtain organisms from controlled laboratories or cultures.
Besides, the previous exposure must to be considered because, in natural environments as Mandinga, several pollutants could be present. It has been demonstrated that Perna viridis (Linnaeus 1758), is more sensitive and reacts faster to Cd, after it has been previously exposed to this metal. These findings are relevant due to the high levels detected in the Mandinga lagoon (77 µgCd/L), which are far beyond those established by the Mexican regulations intended for the environment protection (0.2 µgCd/L) as indicates Comisión Nacional del Agua (2003).
Due to the essential metals regulation activity in bivalve organisms, the metallothionein has been associated with cellular detoxification and protection mechanisms; furthermore, in some studies it is considered to play the same roles in presence of metals without known biological function, such as Cd (Köhler & Riisgard, 1982; Stegeman et al., 1992; Roesijadi et al., 1996; Viarengo et al., 1997; Romeo et al., 1997). However, other studies specify that metallothionein–Cd complexes are metabolized slower than complexes with essential metals (Simkiss & Masson, 1983). Other protective roles of these proteins have been described, such as general antioxidative defence; e.g. it is considered that previously exposed organisms to Cd, might resist more effectively the oxidative stress catching the hydroxyl and superoxide radicals (Amiard et al., 2006). However, metallothioneins are not able to intercept all of the molecules, therefore, ionic–form metals are also able to bind to other sensitive cellular sites, namely enzymes and membranes, stimulating the production of free radicals, resulting in membrane destabilization (Roesijadi, 1996). Consequently, the protective role of these proteins seems to be limited.
Metallothionein induction, as result of Cr exposure in C. virginica, is a narrowly explored area. This metal is associated to a greater extent with oxidative stress, since it is involved in several enzymatic activations, for it bounds to the enzymes active sites in redox reactions with Fe and Cu; Cr3+ promotes insulin action and is an essential nutrient for the metabolism of sugars and lipids, performing several functions in vital processes. The organisms exposed to Cr6+ must transform it into Cr3+ within the cell. Cr6+ entry occurs through the sulphate transport system, and once inside, it is transformed through two routes: an enzymatic route, involving the cytochrome P450 and glutathione reductase, where Cr3+ is the final product; and another non–enzymatic route, which transforms Cr6+ in Cr5+ and Cr4+ by means of the ascorbic acid. These Cr–forms produce reactive–oxygen species (Moreno, 2003). The different Cr transformation routes could explain the reason why it accumulates less than Cd, and why the lipid peroxidation is larger with lower BCF values.
Nevertheless, the increase in lipid peroxidation could also be expected under Cd exposure, since this metal promotes equally the reactive–oxygen species production. Other studies state that Cd exposure induces metallothionein production and peroxidative damage in the freshwater Asiatic clam Corbicula fluminea (Legeay et al., 2005). Considering the time of expression of the cellular–protection processes in response to exposure and the role played by lysosomes in metals detoxification (Amiard et al., 2006), it is expected that metallothionein induction occurs first and the lipid peroxidation takes place later. The results obtained in C. virginica specimens exposed to Cr, sustain this theory, since the lipid peroxidation in the digestive gland occurred after 48 h exposure and the metallothionein induction ensued after 6 h. In contrast, the lipid peroxidation became evident after 6 h to Cd exposure; however, there was no evident association with the metallothionein increase. In other studies, significative correlations between Cd and Cu sedimentary levels and metallothionein, in juvenile C. virginica specimens, were found (Ringwood et al., 1999); however, there was no correlation between these meals levels and peroxidative damage. The difficulty to incorporate the Cd into different metabolic routes could explain why the larger the lipid peroxidation is, the higher the Cd BCF becomes.
The metallothionein, as metals exposure biomarker, is considered highly useful for environmental protection (Goyer, 1991; Viarengo et al., 1999); nonetheless, in order to corroborate if lipid peroxidation is associated to metallothionein induction, as result of Cd exposure, it would be suitable to consider the possible influence of previous exposure.
Water is the main route of exposure (Neff, 2002; Rebougas et al., 2005), but not only water content is important, levels in sediment must be considered, due the possibility of metal transference to the water column under different environmental conditions associated to changes in pH, temperature and salinity. Mandinga sediment concentrations determined by Botello (1994) indicated values of 45.74 μgCr/sDW and 1.22 μgCd/gDW. Those values represent an increase to previous analysis (Rosas, et al., 1983) and represent a risk to native organisms.
In that manner, the selected trial concentrations, slightly higher than those detected in water of the Mandinga lagoon, where the organisms were obtained, revealed answers in the analysed biomarkers. There was induction of metallothionein due to Cr exposure after 6 h in the digestive gland (where the highest concentrations were found) and in the abductor muscle, and lipid peroxidation in the digestive gland after 48 h exposure. The negative correlation amongst the metallothionein levels and the digestive gland BCF, suggests a metabolic effort to control the internal Cr concentration, however, higher peroxidation in oysters which accumulated less, suggests deterioration.
The higher Cd toxicity, a non essential metal, became evident due to lipid peroxidation after 6 h of exposure. The direct correlation amongst metallothioneins and the Cd BCF in the three analysed tissues, points out the organisms incapability to control the stored levels, therefore, the protective role of these proteins from this metal is very limited, which can be supported by the lipid peroxidation, which was proportional to the metal water concentration.
Mandinga native oysters had Cd pre–exposure, this fact could involve a lower resistance to the posterior Cd exposure, and could explain the limited protective role of metallothioneins in oyster from Mandinga Lagoon.
ACKNOWLEDGEMENTS
To Ricardo Rosas Cedillo for his consultancy in the assemblage of techniques for the analysis of metals in water and tissues, and the access to the atomic absorption equipment. We thank to Cecilia Vanegas Pérez and Concepción Gutiérrez Ruíz for their valuable comments and advices.
REFERENCES
American Public Health Association (APHA). 1995. Standard methods for the examination of water and wastewater. Eaton, A.D., L.S. Clesceri & A.E. Greenberg (Eds.). American Public Health Association, American Waterworks Association, Water Environment Federation. Washington, D.C.: 8–1 – 8–47. [ Links ]
Amiard, J. C., C. Amiard–Triquet, S. Barka, J. Pellerin & P. S. Rainbow. 2006. Metallothioneins in aquatic invertebrates: Their role in metal detoxification and their use as biomarkers. Aquatic Toxicology 76: 160–202. [ Links ]
Barrera, E.G. 2006. Toxicidad del cromo y cadmio en ostión Crassostrea virginica de la Laguna de Mandinga, Veracruz. Tesis Doctorado en Ciencias Biológicas, UAM, México, 229 p. [ Links ]
Baudrimont, M, J. Metivaud, R. F. Maury–Brachet & A. Boudou. 1997. Bioaccumulation and metallothionein response in the asiatic clam (Corbicula fluminea) after experimental exposure to cadmium and inorganic mercury. Environmental Toxicology and Chemistry 16: 2096–2105. [ Links ]
Bebianno; M. J. & W. J. Langston. 1991. Metallothionein induction in Mytilus edulis exposed to cadmium. Marine Biology 108: 91–96. [ Links ]
Borgmann, U. 2000. Methods for assessing the toxicological significance of metals in aquatic ecosystems: Bioaccumulation–toxicity relationships water concentrations and sediment spiking approaches. Aquatic Ecosystems Health Management 3: 277–289. [ Links ]
Botello, A. V. 1994. Estudio geoquímico y diagnóstico ambiental de las lagunas de los alrededores de la Central Nucleoelectrica de Laguna Verde, Veracruz. Informe Final 1992–1993. Comisión Federal de Electricidad/ICMyL/UNAM, México. 128 p. [ Links ]
Bucio, L., V. Souza, A. Albores, E. Chávez & M. C. Gutiérrez. 1995. Cadmium and mercury toxicity in the WRL–68 cells. Toxicology 99: 153–167. [ Links ]
Buikema, Jr. A.L., B. R. Niederlehner & J. Cairns. 1982. Biological monitoring. Part IV Toxicity testing. Water Research 16: 239–262. [ Links ]
Carpené, E. 1993. Metallothionein in marine molluscs. In: Dallinger, R. & P. Dainbow (Eds.). Ecotoxicology of metals in invertebrates proceedings. Lewis Publishers, Boca Raton. 461 p. [ Links ]
Castrejón, 0. L., D .D. Porras, & S. Ch. Band. 1994. Cultivo de alimento vivo para acuicultura. Instituto Nacional Indigenista. Universidad del Mar, Oaxaca. 118 p. [ Links ]
Comisión Nacional del Agua. 2003. Ley federal de derechos normas aplicables en materia de Aguas Nacionales y sus bienes públicos inherentes2003. Diario Oficial de la Federación, México, D.F. diciembre 31. 259 p. [ Links ]
Conners, D. E. & A. H. Ringwood. 1997. The effecs of glutathione depletion on copper cytotoxicity on oysters (Crassostrea virginica). 18th Annual Meeting Society of Environmental Toxicology and Chemistry, San Francisco, Ca.: p. 327. [ Links ]
Contreras, E. F. & C. 0. Castañeda. 1995. Los ecosistemas costeros del Estado de Veracruz. Gobierno Edo. De Veracruz/SEDAFP, México: 81–88. [ Links ]
Cooper, T. G. 1977. Biochemistry. John Wiley and Sons, New York: 53–55. [ Links ]
Choi, Y. K., P.G. Jo & Ch.Y. Choi. 2008. Cadmium affects the expression of heat shock protein 90 and metallothionein mRNA in the pacific oyster, Crassostrea gigas. Comparative Biochemistry and Physiology, Part C 147: 286–292. [ Links ]
Diario Oficial de la Federación (DOF). 1997. Norma Oficial Mexicana NOM–001–ECOL–1996 que establece los límites máximos permisibles de contaminantes en las descargas de aguas residuales en aguas y bienes nacionales. Secretaría de Medio Ambiente Recursos naturales y Pesca, México, enero 6: 68–86. [ Links ]
Gautier, D., C. Mouneyrac, F. Quiniou, E. His, M. Gnassia–Barelli & M. Roméo. 2006. Metal bioaccumulation and metallothionein concentrations in larvae of Crassostrea gigas. Environmental Pollution 140: 492–499. [ Links ]
Geret, F. & R.P. Cosson. 2000. Utilisation des métallothionéines comme biomarqueur de la contamination metallique: variabilité entre sites et organes chez l'huitre Crassostrea gigas. Oceanology Acta 23: 261–271. [ Links ]
Geret, F., F. Rainglet & R. P. Cosson . 1997. Comparison between two protocols for the asolation of metallothioneins within mussel Mytilus edulis. Journal de la Recherce Oceanographique 22: 151–156. [ Links ]
Gold–Bouchot, G., R. Simá–Álvarez, 0. Zapata–Pérez, J. G. Gémez–Ricalde. 1995. Histopatological effects of petroleum hydrocarbons and heavy metal on the American Oyster (Crassostrea virginica) from Tabasco, México. Marine Pollution Bulletin 31: 439–445. [ Links ]
Goyer, R. A. 1991. Toxic effects of metals. In: Amdur, M.O., J. Doull & C.D. Klaassen (Eds.). Casarett and Doull's Toxicology. Pergamon Press, New Cork, pp. 623–680. [ Links ]
Guzmán, A. P., F. S. Villanueva & A. V. Botello . 2005. Metales en tres lagunas costeras del estado de Veracruz. In: Botello, A.V., J. Rendón von Osten, G. Gold–Bouchot & C. Agraz–Hernández (Eds.). Golfo de México, contaminación e impacto ambiental: diagnóstico y tendencias. Universidad Autónoma de Campeche, Universidad Nacional Autónoma de México, INE, Campeche, pp. 361–372. [ Links ]
Huan, S.Ch.T. 1994. General guidelines for the acid digestion offish and shellfish. Reference 83. Method number NIEA C303.01T. Executive Yuan Environmental Protection Agency Republic of China Official Gazette 7: 1–6. [ Links ]
Klaassen, C.D. & D.L. Eaton. 1991. Principles of toxicology. In: Amdur, M.O., J. Doull & C.D. Klaassen (Eds.). Casarett and Doull's Toxicology. Pergamon Press, Toronto, pp. 12–49. [ Links ]
Koeman, J.H. 1991. From comparative physiology to toxicological risk assessment. Comparative Biochemical Physiology 100C: 7–10. [ Links ]
Kohler, K. & H. U. Riisgard. 1982. Formation of metallothioneins in relation to accumulation of cadmium in the common mussel Mytilus edulis. Marine Biology 66: 53–58. [ Links ]
Langston, W. J. & M. J. Zhou. 1987. Cadmium accumulation distribution and elimination in the bivalve Macoma balthica: neither metallothionein nor metallothionein–like proteins are involved. Marine Environmental Research 21: 225–237. [ Links ]
Legeay, A., M. Achard–Joris, M. Baudrimont, J.Ch. Massabuau & J.P. Bourdineaud. 2005. Impact of cadmium contamination and oxygenation levels on biochemical responses in the Asiatic clam Corbicula fluminea. Aquatic Toxicology 74: 242–253. [ Links ]
Lin, W., A. R. Mitchel & P.K. Chien. 1992. The effects of copper, cadmium and zinc on particle filtration and uptake of glycine in the Pacific oyster Crassostrea gigas. Comparative Biochemical Physiology 103C: 181–187. [ Links ]
Marques de Cantú, M.J. 1991. Probabilidad y estadística para ciencias químico–biológicas. McGraw–Hill, México. 657 p. [ Links ]
Moreno, G.M. D. 2003. Toxicología ambiental. McGraw Hill, México. 370 p. [ Links ]
Mourgaud, Y., E. Martinez, A. Geffard, B. Andral, J. Y. Stanisiere & J.C. Amiard. 2002. Metallothionein concentration in the mussel Mytilus galloprovincialis as a biomarker of response to metal contamination: validation in the field. Biomarkers 7: 479–490. [ Links ]
Neff, J. M. 2002. Bioaccumulation in marine organisms. Elsevier, New York. 452 p. [ Links ]
Páez–Osuna, F. 2005. Efectos de los metales. In: Botello, A.V., J. Rendón von Osten, G. Gold–Bouchot, C. Agraz–Hernández (Eds.). Golfo de México, contaminación e impacto ambiental: diagnóstico y tendencias. Univ. Autón. de Campeche, Univ. Nal. Auton. de México, INE, Campeche, p.p. 343–360. [ Links ]
Rebouças do Amaral, M. C., M. Freitas–Rebelo, J. P. Machado–Torres & W.Ch. Pfeiffer. 2005. Bioaccumulation and depuration of Zn and Cd in mangrove oysters (Crassostrea rhizophorae, Guilding, 1828) Transplanted to and from a contaminated tropical coastal lagoon. Marine Environmental Research 59: 277–285. [ Links ]
Ringwood, A. H., D. E. Conners, Ch. J. Keppler & A. A. Dinovo. 1999. Biomarker studies with juvenile oysters (Crassostrea virginica) deployed in–situ. Biomarkers 4: 400–414. [ Links ]
Roesijadi, G. 1994. Behavior of metallothionein–bound metals in a natural population of an estuarine mollusc. Marine Environmental Research 38: 147–168. [ Links ]
Roesijadi, G. 1996. Environmental Factors: Response to Metals. In: Kennedy, V. S., R. I. E. Newell & A.F. Eble (Eds.). The eastern oyster Crassostrea virginica. A Maryland Sea Grant Book College Park, Maryland, pp. 515–537. [ Links ]
Roesijadi, G. 1999. The basis for increased metallothionein in a natural population of Crassostrea virginica. Biomarkers 4: 467–472. [ Links ]
Roesijadi, G. & P.L. Klerks. 1989. Kinetic analysis of Cd binding to metallothionein and other intracellular ligands in oyster gills. Journal of Experimental Zoology 251: 1–12. [ Links ]
Roesijadi, G., K. M. Hansen & E. Unger. 1996. Cadmium–induced metallo–thionein expression during embrionic and early larval development of the mollusc Crassostrea virginica. Toxicological Applied Pharmacology 140: 356–363. [ Links ]
Romeo, M. R., P. Cosson, M. Gnassia–Barelli, Ch. Risso, X Stein & M. Jafaurie. 1997. Metallothionein determination in the liver of the sea bass Dicentrarchus labrax treated with copper and B(a)P. Marine Environmental Research 44: 275–284. [ Links ]
Rosas, I., A. Baez, R. Belmont. 1983. Oyster (Crassostrea virginica) as an indicator of heavy metal pollution in some lagoons of the Gulf of Mexico. Water Air and Soil Pollution 20: 127–135. [ Links ]
Savva, D. 1999. Cloning and analysis of the metallothionein gene of the shore crab Carcinus maenas. Consiglio Nazionale della Recherche Universitá degli Studi di Siena, Italia, Siena, p. p. 92–94. [ Links ]
Scheuhammer, A. M. & M. G. Cherian. 1991. Quantification of metallothionein by silver saturation. Methods in Enzimology 205: 78–82. [ Links ]
Shumway, S.E. 1982. Oxygen Consumption in Oysters: An Overwiew. Marine Biology Letters 3: 1–23. [ Links ]
Shumway, S. E. & R. K. Koehn. 1982. Oxygen consumption in the American oyster Crassostrea virginica. Marine Ecology Progress Series 9: 59–68. [ Links ]
Simkiss, K. & Masson. 1983. Metal ions: Metabolic and toxic effects. In: Hochachka, P. W. J K. M. Wilbur (Eds.) 1. The Mollusca. 2. Environmental Biochemistry and Physiology. Academic Press, San Diego, pp. 101–164. [ Links ]
Simkiss, K., M. Taylor & A.Z. Mason . 1982. Metal detoxification and bioac–cumulation in molluscs. Marine Biology Letters 3: 187–201. [ Links ]
Smith, J.R. 1985. Copper exposure and ciliary function in gill tissue of Mytilus californianus. Bulletin of Environmental Contamination and Toxicology 35: 556–563. [ Links ]
Souza, V., L. Bucio, M. C. Gutiérrez & E.Ch. Cossio. 1996. Acumulación de cadmio y su efecto en el transporte de calcio en las células WRL–68. In: Albert, A. L. & L. A. Osorio (Eds.). La toxicología en México. Estado actual y perspectivas. Sociedad Mexicana de Toxicología, México, pp. 63–66. [ Links ]
Stegeman, J. J., M. Browner, R. T. Giulio, L. Forlin, B. A. Fowler, B. M. Sanders & P. A. Van Veld . 1992. Molecular responses to environmental contamination: Enzime and protein systems as indicators of chemical Exposure effect. In: Hugget, R. J., R .A. Kimerle, P. M. Mehrle & H. L. Bergman (Eds.). Biomarkers, biochemical, physiological and histological markers of antropogenic stress. Lewis Publishers, Chelsea, pp. 235–336. [ Links ]
Tuckey, J. W. 1977. Exploratory data analysis. Addison–Wesley Co. Massachusetts, 688 p. [ Links ]
Viarengo, A., B. Burlando, F. Dondero, A. Marro & R. Fabbri. 1999. Metallothionein as a tool in biomonitoring programmes. Biomarkers 4: 455–466. [ Links ]
Viarengo, A., E. Ponzano, F. Dondero & R. Fabbri . 1997. A simple spectrophotometic method for metallothionein evaluation in marine organisms: An application to mediterranean and antartic molluscs. Marine Environmental Research 44: 69–84. [ Links ]
Villanueva, F.S. & A. V. Botello. 1998. Metal pollution in coastal areas of Mexico. Bulletin of Environmental Contamination and Toxicology 157: 53–94. [ Links ]
Wood, Ch. M., W. J. Adams, D.R. DiBona, S.N. Luoma, R.C. Playle, W.A. Stubblefield, H. L. Bergman, R. J. Erickson, J. S. Mattice & Ch. E. Schlekat. 1997. Environmental toxicology of metals. In: Bergman, H. L. & E. J. Dorward–King. (Eds.). Reassessment of metals criteria for aquatic life protection. SETAC Press, Pensacola, pp. 31–56. [ Links ]














