Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Hidrobiológica
versão impressa ISSN 0188-8897
Hidrobiológica vol.19 no.3 Ciudad de México Dez. 2009
Artículos
Vitamin A effects and requirements on the juvenile Kuruma Prawn Marsupenaeus japonicus
Efectos y requerimientos de vitamina A en los juveniles del camarón Kuruma Marsupenaeus japonicus
Luis Héctor Hernandez Hernandez1*, Shin–Ichi Teshima2, Manabu Ishikawa2, Shunsuke Koshio2, Francisco Javier Gallardo–Cigarroa3, Orhan Uyan4 and Md. Shah Alam5
1 Laboratorio de Producción Acuícola, Facultad de Estudios Superiores Iztacala, Avenida de los Barrios s/n, Los Reyes Iztacala, Tlalnepantla, Estado de México, C.P. 54090, México. *E–mail: luish3@yahoo.com
2 Laboratory of Aquatic Animal Nutrition, Faculty of Fisheries, Kagoshima University, Shimoarata 4–50–20, Kagoshima 890–0056, Japan.
3 Yamaha Nutreco Aquatech Co. LTD, Suite 702 Abundant'95, Hakataekihigashi 3–12–1, Fukuoka 812–0013, Japan.
4 Aquaculture Research Group, DSM Nutritional Products France, Animal Nutrition & Health Research, CRNA– BP170, 68305 SAINT–LOUIS Cedex, France.
5 Center for Marine Science, Aquaculture Program, University of North Carolina at Wilmington, 7205 Wrightsville Ave., Wilmington, NC 28403, USA.
Recibido: 9 de mayo de 2008.
Aceptado: 5 de junio de 2009.
ABSTRACT
A 40–day feeding experiment was conducted to assess the effect and requirements of dietary vitamin A (vitA) of juvenile Kuruma Prawn Marsupenaenus japonicus. Nine semi–purified diets were prepared with supplementations of 0, 3,000, 6,000, 9,000, 12,000, 15,000, 18,000, 21,000 and 24,000 international units of vitA per kg (IU vitA/kg) and fed once a day to triplicate groups of 15 juveniles per tank (initial weight of 0.14 ± 0.001 g, mean ± standard error). Weight gain (WG), specific growth rate (SGR) and feeding efficiency ratio (FER) were significantly lower in the juveniles fed with the diets contained 0 and 3,000 IU vitA/kg than in the other groups. Similar values of total retinol (ROL) in whole body were detected only in the juveniles fed on the diets supplemented with 21,000 and 24,000 IU vitA/kg: 10.22 ± 2.74 and 10.70 ± 0.14 μg ROL /g of wet weight, respectively (mean ± standard error). It was not possible to detect retinal in the whole body samples in any of the experimental groups. According to the WG analyzed by the broken line regression, the dietary requirements of vitA should be of 7,500 IU vitA/kg.
Key words: Marsupenaeus japonicus , vitamin A , retinol, retinal, Kuruma Prawn.
RESUMEN
Un experimento de alimentación de 40 días se realizó para determinar el efecto y requerimientos de vitamina A (vitA) en la dieta de juveniles de camarón Kuruma Marsupaeneus japonicus. Nueve dietas semipurificadas se prepararon con suplementaciones de 0, 3,000, 6,000, 9,000, 12,000, 15,000, 18,000, 21,000 y 24,000 unidades internacionales de vitA por kg (IU vitA/kg) y se utilizaron para alimentar una vez por día a grupos por triplicado de 15 juveniles por tanque (peso inicial de 0.14 ± 0.001 g, promedio ± error estándar). La ganancia en peso (WG), la tasa de crecimiento especifico (SGR) y la tasa de eficiencia del alimento (FER) fueron significantemente más bajos en los juveniles alimentados con las dietas que contenían 0 y 3,000 IU vitA/kg que en los otros grupos. Valores similares de retinol (ROL) total en el cuerpo fueron detectados exclusivamente en los juveniles alimentados con las dietas suplementadas con 21,000 y 24,000 IU vitA/kg: 10.22 ± 2.74 and 10.70 ± 0.14 µg ROL/g de peso húmedo, respectivamente (promedio ± error estándar). No fue posible detectar retinal en las muestras de cuerpo en ninguno de los grupos experimentales. De acuerdo con el WG analizado con una regresión de línea de rompimiento, los requerimientos de vitA en la dieta deben de ser 7,500 IU vitA/kg.
Palabras clave: Marsupenaeus japonicus, vitamina A, retinol, retinal, camarón Kuruma.
INTRODUCTION
Vitamin A (vitA) is the generic descriptor for compounds with the qualitative biological activity of all–trans–retinol, and can be found in the animals as retinol (ROL), retinal (RAL) and retinyl esters (RNE) (Bearer–Rogers et al., 2001). VitA has been related to multiple physiological processes in vertebrates, including vision, growth, cell differentiation and reproduction (Combs, 1998). In crustaceans, vitA has been related to the vision process (Dall, 1995; Conklin, 1997; Goldsmith & Cronin, 1993; Srivastava & Goldsmith, 1997), but also it has been reported as a necessary compound for normal growth in species such as Litopenaeus vannamei Bonne, 1931(He et al. 1992), Penaeus monodon Fabricius, 1798 (Reedy et al., 1999), Marsupenaeus japonicus (Kanazawa, 1985) and P. chinensis Osbeck, 1765 (Chen & Li, 1994). As well, vitA has been related to the normal ovarian development of M. japonicus (Alava et al., 1993), L. vannamei (Liñan–Cabello et al., 2002; Liñan–Cabello et al., 2003), Cherax quadrucarinatus Martens, 1868 (Liñan–Cabello et al. , 2004) and P. chinensis (Mengqing et al., 2004).
For crustaceans, only limited information on the vitA quantitative requirement is available: Akiyama et al. (1992) reported that commercial species of penaeids might require a supplementation of 10,000 International Units of vitA per kg diet (IU vitA/kg, 1 IU = 0.3 μg of all–trans–ROL), while Conklin (1997) reported an inclusion of 5,000 IU vitA/kg for general diets of penaeid species. Recently, Shiau & Chen (2000) reported that juvenile P. monodon requires a minimum inclusion of 8,200 IU vitA/kg for optimal growth.
As for the Kuruma Prawn M. japonicus, also little information on dietary vitA requirement has been reported. Kanazawa (1985) reported that the shortage of vitA in the diets of the larval stage caused the cessation or retardation of metamorphosis, growth and high mortality. As well, Alava et al. (1993) reported that a supplementation of 15,000 IU vitA/kg, enhanced the ovarian development in the brookstock of Kuruma Prawn. The quantitative requirements of dietary vitA have been not established for this species and for thus, the aim of the present study was to assess the dietary requirement of vitA for growth in the juvenile Kuruma Prawn, one of the most important species of aquaculture in Japan.
MATERIALS AND METHODS
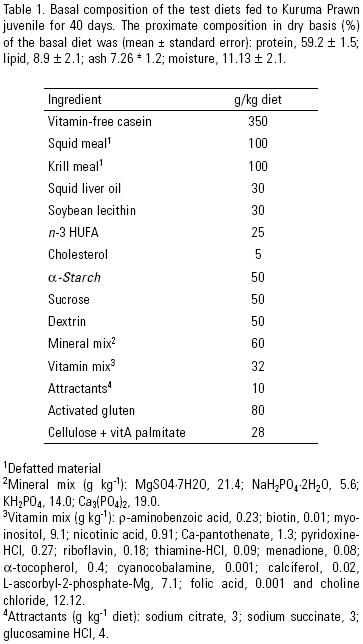
Test diets. The table 1 was formulated according to the recent of nutrient requirement information for Kuruma Prawn (Teshima et al., 2001). Defatted krill meal and squid meal (Nippon Suisan Kaisha Ltd., Tokyo, Japan), and vitamin–free casein (Wako Pure Chemical Ind., Osaka, Japan) were used as protein sources. Squid liver oil (Riken Vitamin, Tokyo Japan), soybean lecithin (Wako Pure Chemical Ind., Osaka, Japan), cholesterol (Nacalai Tesque Inc., Kyoto, Japan) and n–3 highly unsaturated fatty acids (n–3 HUFA, DHAce, Oriental Co., Chiba, Japan) were used as lipid sources; while α–starch, dextrin (Wako Pure Chemical Ind., Tokyo, Japan) and sucrose (Wako Pure Chemical Ind., Tokyo, Japan) were used as carbohydrate sources. VitA palmitate (1,000 IU vitA/mg, Nacalai Tesque Inc. Kyoto, Japan) was added to the test diets to provide concentrations of 0, 3,000, 6,000, 9,000, 12,000, 15,000, 18,000, 21,000 and 24,000 IU vitA/kg of diet (table 2). Diets were prepared by similar methods as described by Teshima et al. (2001). Briefly, all dry ingredients were mixed with the lipid mix (including vitA palmitate and the other fat–soluble vitamins), then, water (40 %) was added and mixed until a dough–like was obtained. The dough was passed through a pelletizer to obtain 1.6–mm pellets, which were dried at 60 °C in a constant temperature oven for 60 min. To improve the stability in water, dried pellets were steamed for 1 min at 100 °C. All diets were stored until used at –24 °C.

Feeding experiment. Ovigerous Kuruma Prawns were obtained from Matsumoto Suisan, Miyazaki, Japan and transported to the Kamoike Marine Production Laboratory (Faculty of Fisheries, Kagoshima University, Japan) and were placed in a 500 L tank for spawning. Hatched larvae were reared by feeding live food (Chaetoceros sp. and Artemia nauplii) and a micro–diet (Shrimp Seed Production Micro–diet number 0–1, Himashigaru Co. Ltd., Kagoshima, Japan).
Ten days before starting the feeding trial, the juveniles were fed on the diet without vitA supplementation in order to get them adjusted to the test diets and deplete their vitA reserves, as reported by Shiau & Chen (2000). At the beginning of the feeding trial, juveniles were sorted to obtain equal size. Twenty–seven plastic tanks of 30 l capacity (filled with 20 l sea water) were used and 15 juveniles (initial weight of 0.14 ± 0.001 g, mean ± standard error) were randomly stocked per tank and each test diet was fed to triplicate groups of juveniles. The juveniles were fed by hand the respective diets at 10 % of the mean body weight per day. The daily ration was offered once a day, during the evening. The juveniles were weighted every ten days and the ration size was adjusted accordingly. Fecal matter and uneaten diet were removed by siphoning from the tank. Uneaten diets were quantified for measurement of feed intake.
Each tank was equipped with an under–gravel filter covered with a sand bed and water was circulated through the action of an air–lift device. The water flow rate on each tank was 0.8 l min–1. Through the feeding experiment water quality parameters (mean ± SD) were: dissolved oxygen 5.8 ± 0.1 mg/l, pH 8.02 ± 0.1, salinity 35 ± 0.3 g/l, and water temperature ranged from 28 to 30 °C All tanks were maintained under a natural photoperiod of 12 h light, 12 hrs dark. The feeding experiment using the test diets was conducted for a period of 40 days.
At the end of the feeding experiment, the juveniles were starved for 24 h, then weighed and examined externally for symptoms of deficiency or excess of vitA. Five prawns for each dietary replicate tank were chosen randomly, pooled, dried in a freeze dryer and keep at –24 °C until proximate composition analysis. The rest of the prawns of each treatment were pooled and store at –80 °C until vitA analysis.
Proximate analysis and vitA analysis. The diets and the whole body of Kuruma Prawn were analyzed according to the Association of Official Analytical Chemists methods (1990) for moisture, crude protein and ash contents. Total lipids were determined by the method reported by Bligh & Dyer (1959).
VitA analysis in the diets and the whole body was performed by high performance liquid chromatography (HPLC). Total ROL was extracted and measured by the techniques reported by Estevez & Kanazawa (1995), while RAL was extracted and measured according with Hernandez et al. (2004). The HPLC system consisted of a pump LC–3A (Shimadzu Corp. Tokyo, Japan), a UV detector SPD–6A (Shimadzu Corp. Tokyo, Japan) set at 325 nm, a data processor Chromatopac C–R7Ae–plus (Shimadzu Corp. Tokyo Japan), and a silica column Shim–pack CLC–SIL (4.6 x 150 mm, Shimadzu Corp. Tokyo, Japan). Hexane–isopropanol (95:5) and hexane–dichlo–rometane (2:1) were used as mobile phase for total ROL and RAL, respectively. A flow rate of 1 ml/min was used in both determinations.
Statistical analysis. Data on final body weight (BW), weight gain (WG), specific growth rate (SGR), feed efficiency ratio (FER), daily feed intake (DFI), survival rate, proximate analysis of whole body of each treatment were tested using one–way ANOVA (package super–ANOVA, Abacus Concepts, California, USA). Significant differences among the treatments were evaluated with Duncan's new multiple range test (Steel &Torrie, 1980). Statistical significance of differences was determined by setting the error at 5% (p < 0.05) for each set of comparisons. Dietary vitA requirement was estimated by the broken–line method (Robbins et al., 1979). Regression analysis was performed using the software package Stat–ViewTM (Abacus Concepts, California, USA).
RESULTS
The means of BW, WG, SGR, FER, DFI and survival rate of the juvenile Kuruma Prawn are presented in table 3. Significant lower values of BW, WG, SGR and FER were observed in the juveniles fed with the diets containing 0 and 3,000 IU vitA/kg than the other treatments. The growth of the Kuruma Prawn in both groups started to decrease around the 30th day of the feeding trial. The highest values of BW, WG and SGR were observed in the juveniles fed with 18,000 IU vitA/kg, but no significant differences were observed with those fed the diets with 6,000, 9,000, 12,000, 15,000, 21,000 and 24,000 IU vita/kg.
Significant lower survival rate was observed in the juveniles fed with the diets with 0, 12,000 and 24,000 IU vitA/kg than those fed with 18,000 IU vitA/kg. However, the mortality observed in those groups during the feeding trial was not related to the treatments with vitA. About 20 % of the juveniles fed the diet with 0 IU vitA/kg showed a lighter coloration than that observed in the other groups.
No significant differences were observed on the contents of moisture, ash and protein of the whole body among the groups (table 4). There was a tendency of low lipid content in the groups fed with no or low supplementation, but only lipid content was significantly lower in the groups fed with 3,000 and 6,000 IU vitA/ kg than the group fed with 21,000 IU vitA/kg.

Total ROL was detected only in the juveniles fed on the diets supplemented with 21,000 and 24,000 IU vitA/kg. The contents of total ROL (mean of three samples ± standard error) in these groups were: 10.22 ± 2.74 and 10.70 ± 0.14 μg ROL/g of wet weight, respectively. No significant differences were observed among the means of both groups. As well, it was not possible to detect RAL in the whole body samples in any of the experimental groups.
Fig. 1 shows the dietary requirement of vitA for growth in terms of WG determined by the broken–line analysis. Based on mean WG, the regression equations were y = 0.02x + 179 (r = 0.9) and y = 0.001x + 337 (r = 0.6) and requirement was estimated to be 7,500 IU vitA/kg.
DISCUSSION
As vitA is mainly concentrated in the eyes of crustaceans, it is believed that its role is related to vision, and thus the requirements must to be lower than those reported for other species (Conklin, 1997). Dall (1995) reported that diets for penaeids might not need a vitA supplementation when fish oils are added to diets, while Conklin (1997) reported a maximum inclusion of 5,000 IU vitA/kg in the diets for commercial penaeids. The data of the present study indicated that dietary vitA was necessary for optimal growth of the juveniles of Kuruma Prawn M. japonicus, as also reported for L. vannamei (He et al., 1992), P. monodon (Reedy et al., 1999) and P. chinensis (Chen & Li, 1994). Based on the data of WG (table 3 and Fig. 1), the minimum dietary supplementation of vitA must be of 7,500 IU vitA/kg. This value is comparable to the value found for the juveniles of P. monodon (about 8,400 IU vitA/kg) (Shiau & Chen, 2000), the only species with quantitative requirements reported. A higher supplementation (15,000 IU vitA/kg) was recommended for the development of the ovaries of broodstock of Kuruma Pranw (Alava et al., 1993), but as in the case of other nutrients, vitA seems to be required in higher levels during the reproduction cycle (Conklin, 1997).
The most typical signs of nutrient deficiency reported for crustaceans are either the growth depression or mortality (Conklin, 1997; Chen & Li, 1994). The reduce growth observed in the juveniles of Kuruma Prawn fed with the diets of 0 and 3,000 IU IU vitA kg–1 was considered as a vitA deficiency symptom. As well, the light coloration observed in the juveniles fed on those diets might be a sign of vitA deficiency, and similar results were reported for juveniles of P. monodon when fed a vitA deficient diet (Shiau & Chen, 2000).
Shiau & Chen (2000) reported that juveniles of P. monodon showed increased content of total lipids in the hepatopancreas with increasing levels of dietary vitA. There was a similar tendency in the contents of total lipid in the whole body of juvenile Kuruma Prawn. These findings contrasts with those reported for other aquatic species, as high contents of dietary vitA decreased the total lipid content in whole body of fish, such as guppy Poecilia reticulata Peters, 1859 (Shim & Tan, 1990), greasy grouper Epinephelus tauvina Foskskål, 1775 (Mohamed et al., 2003) and Japanese flounder Paralichthys olivaceus (Hernandez et al., 2005). However, the mechanisms of how might vitA affects the lipid deposition in crustaceans have been not studied yet and thus, require further research.
The role of vitA in crustaceans was believed to be related to the vision process, and indeed RAL (the vitA metabolite that supports the synthesis of photopigments in the eyes; Combs, 1998) has been reported to be up to 85% of total vitA in the eye (Srivastava & Goldsmith, 1997) and an important compound for broodstock (Liñan–Cabello et al., 2002, 2003) of some species of crustaceans. In contrast to those findings, it was not possible to detect RAL in the samples of whole body of Kuruma Prawn and total ROL was detected only in the samples of the juveniles fed on supplementations higher than 21,000 IU/kg. This might suggests that kuruma prawn storages vitA as ROL (either as free ROL or RNE) rather than RAL when fed high levels of vitA. Srivastava et al. (1996) reported that the crayfish Procambarus and the lobster Homarus sp. are able to storage ROL and RNE. During this study, all vitA analysis were performed on whole body basis and it is difficult to establish where vitA is storage in the body of the Kuruma Prawn, but a similar trend of increased levels of total ROL were found in juveniles of P. monodon, when fed increasing levels of vitA (Shiau & Chen, 2000).
In conclusion, vitA was a necessary compound for the optimal growth of juveniles of Kuruma Prawn and the minimum supplementation for growth of vitA as ROL palmitate in this species must be of 7,500 IU vitA/kg. As well, it was possible to observed that juveniles of Kuruma Prawn are able to storage ROL when fed diets with inclusions more than 21,000 IU vitA/kg.
ACKNOWLEDGMENTS
The authors kindly acknowledge the financial support for this research from the Ministry of Education, Culture, Sports, Science and Technology (Monbukagakusho) of Japan.
REFERENCES
Akiyama D. M., W. G. Dominy & A. L. Lawrence. 1992. Penaeid shrimp nutrition. In: Fast A.W. & L.J. Lester (Eds.). Marine shrimp culture: principles and practices. Elsevier, The Netherlands, pp. 535–568. [ Links ]
Alava V. R., A. Kanazawa, S. Teshima & S. Koshio. 1993. Effects of dietary vitamins A, E and C on the ovarian development of Penaeus japonicus. Nippon Suisan Gakkaishi 59: 1235–1241. [ Links ]
Association of Official Analytical Chemists. 1990. Official methods of analysis of the Association of Analytical Chemist, 15th ed. AOAC, Arlington, USA. 868 p. [ Links ]
Bligh E. G. & W. J. Dyer. 1959. A rapid method of total lipid extraction and purification. Canadian Journal of Biochemistry and Physiology. 37: 911–917. [ Links ]
Bearer–Rogers J., A. Diefferbacher & J. V. Holm. 2001. Lexicon of lipid nutrition (IUPAC technical report). Pure Applied Chemistry. 73: 685–744. [ Links ]
Chen S. Q. & A. J. Li. 1994. Investigation on nutrition of vitamin A for shrimp Penaeus chinesis. I. Effects of vitamin A on shrimp's growth and visual organ. Acta Zoologica Sinica 40: 266–273. [ Links ]
Combs, G. F. 1998. The vitamins, fundamental aspects in nutrition and health. 2nd ed. Academic Press, San Diego, USA. 618 pp. [ Links ]
Conklin, D. E. 1997. Vitamins. In: D'Abramo L. R., D.E. Conklin & D. M. Akiyama (Eds.) Crustacean nutrition. World Aquaculture Nutrition. Luisiana, USA., pp. 123–149. [ Links ]
Dall W. 1995. Carotenoids versus retinoids (vitamin A) as essential growth factors in penaeid prawns (Penaeus semisulcatus). Marine. Biology 124: 209–213. [ Links ]
Estevez A. & A. Kanazawa. 1995. Effect of (n–3) PUFA and vitamin A Artemia enrichment on pigmentation success of turbot Scophthalmus maximus (L). Aquaculture Nutrition 1: 159–168. [ Links ]
Goldsmith T. H. & T. W.Cronin. 1993. The retinoids of seven species of mantis shrimp. Visual Neuroscience 10: 915–929. [ Links ]
He H., A. L. Lawrence & R. Liu. 1992. Evaluation of dietary essentiality of fat–soluble vitamins A, D, E and K for penaeid shrimp (Penaeus vannamei). Aquaculture 103: 177–185. [ Links ]
Hernandez H. L. H., S. Teshima, M. Ishikawa, S. Cosió & Y. Tanaka. 2004. Effects of dietary vitamin A on juvenile red sea bream Chrysophrys major. Journal of the World Aquaculture Society 35: 436–444. [ Links ]
Hernandez H. L. H., Teshima S., Ishikawa M., Alam S., Koshio S. & Y. Tanaka. 2005. Dietary vitamin A requirements of juvenile Japanese flounder Paralichthys olivaceus. Aquaculture Nutrition 11: 3–9. [ Links ]
Kanazawa A. 1985. Nutrition of penaeid prawn and shrimp. In: Taki Y., L.H. Primavera, & J.A. Lobrera (Eds.). Proceedings of the first international conference on culture of penaeid prawns/shrimp. Southeast Asian Fisheries Development Center. Iloilo, The Philipines. pp. 123–130. [ Links ]
Liñan–Cabello M. A., J. Paniagua–Michel & P. VJohnston. 2002. Bioactive roles of carotenoids and retinoids in crustaceans. Aquaculture Nutrition. 8: 299–239. [ Links ]
Liñan–Cabello M. A., J. Paniagua–Michel & T. Zenteno–Savín. 2003. Carotenoids and retinal levels in captive and wild shrimp Litopenaeus vannamei. Aquaculture Nutrition 9: 383–389. [ Links ]
Liñan–Cabello M. A., R. Medina–Zandejas, M. Sánchez–Barajas & H.A. Mena. 2004. Effects of carotenoids and retinol in oocyte maturation of crayfish Cherax quadrucarinatus. Aquaculture Research 35: 905–911. [ Links ]
Mengqing L., J. Wenjuan, C. Qing & W. Jialin. 2004. The effect of vitamin A supplementation in broodstock feed reproductive performance and larval quality in Penaeus chinensis. Aquaculture Nutrition 10: 295–300. [ Links ]
Mohamed J. S., V. Sivaram, T. S. C. Roy, S. P. Mariam, S. Murudagass & M. R. Hussain . 2003. Dietary vitamin A requirements of juvenile greasy grouper (Epinephelus tauvina). Aquaculture 219: 693–701. [ Links ]
Reedy H. R. V., M. G. Naik, & T. S. Annappaswamy. 1999. Evaluation of the dietary essentiality of vitamins for Penaeus monodon. Aquaculture Nutrition 5: 267–275. [ Links ]
Robbins k., H. W. Norton & D. H. Baker. 1979. Estimation of nutrient requirements from growth data. Journal of Nutrition 109: 1710–1714. [ Links ]
Shiau S. Y. & Y. Chen. 2000. Estimation of dietary vitamin A requirement of juveniles grass shrimp Penaeus monodon. Journal of Nutrition 130: 90–94. [ Links ]
Shim K. F. & C. H. Tan. 1990. The dietary requirements of vitamin A in guppy (Poecilia reticulata Peters). In: Takeda M. & T. Watanabe (Eds.). The current status of fish nutrition in aquaculture, Proceedings of Third International Symposium on Feeding and Nutrition of Fish, Toba, Japan, August 28 – September 1. Japan Translation Center, Japan. pp. 133–140. [ Links ]
Steel R. D. G. & J. H. Torrie. 1980. Principles and procedures of statistics, a biometrical approach. McGraw–Hill, New York, USA. 633 pp. [ Links ]
Srivastava R., & T. H.Goldsmith. 1997. On the mechanism of isomeriza–tion of ocular retinoids by the crayfish Procambarus clarkii. Journal of experimental Biology 200: 625–631. [ Links ]
Srivastava R., D. Lau & T. H. Goldsmith. 1996. Formation and storage of 11–cis retinol in the eyes of lobster (Homarus) and crayfish (Procambarus). Visual Neuroscience 13: 215–222. [ Links ]
Teshima S., S. Koshio, M. Ishikaea & A. Kanazawa. 2001. Protein requirement of the prawn Marsupenaeus japonicus estimated by a factorial method. Hydrobiologia 449: 293–300. [ Links ]














