Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Hidrobiológica
versión impresa ISSN 0188-8897
Hidrobiológica vol.19 no.2 Ciudad de México may./ago. 2009
Artículos
Two new species of the genus Notropis Rafinesque, 1817 (Actinopterygii, Cyprinidae) from the Lerma River Basin in Central Mexico
Dos nuevas especies del género Notropis Rafinesque, 1817 (Actinopterygii, Cyprinidae), de la cuenca del río Lerma, México central
Omar Domínguez–Domínguez1, Rodolfo Pérez–Rodríguez2, Luis Humberto Escalera–Vázque2 and Ignacio Doadrio3
1 Laboratorio de Biología Acuática, Facultad de Biología, Universidad Michoacana de San Nicolás de Hidalgo, Morelia, Michoacán, México. *E–mail: goodeido@yahoo.com.mx
2 Posgrado en Ciencias Biológicas, Universidad Nacional Autónoma de México, D. F., México
3 Departamento de Biodiversidad y Biología Evolutiva, Museo Nacional de Ciencias Naturales, CSIC, Madrid, España.
Recibido: 26 de agosto de 2008.
Aceptado: 30 de junio de 2009.
RESUMEN
Diversos estudios sugieren la existencia de especies no descritas de ciprínidos en el centro de México. Las especies del género Notropis se distribuyen a lo largo del centro de México y regiones adyacentes, algunas especies llegan a cuencas del sur del país. Se han reconocido dos grupos: un clado sureño y uno en la parte central del país. En este último, Notropis calientis fue descrito como un complejo de especies que habita las partes altas de las cuencas donde se distribuyen. Con base en características morfométricas, merísticas y genéticas, se describen dos nuevas especies que emanan del complejo Notropis calientis. Notropis marhabatiensis sp. nov. se diagnostica por presentar: 7, rara vez 8 radios ramificados en la aleta pélvica (vs. 8 rara vez 7 o 9 en las otras especies dentro del complejo N. calientis) y 9 rara vez 8 escamas transversales (vs. 10 rara vez 11 o 9 en N. grandis y N. calientis). Una línea lateral oscura y delgada corre a partir del origen de la aleta pectoral hasta el pedúnculo caudal. Veintiséis posiciones nucleotídicas fijadas en el gen citocromo b. Notropis granáis sp. nov. presenta 6, rara vez 7 radios ramificados en la aleta anal (vs. 7, rara vez 8 o 6 en N. marhabatiensis, N. calientis y N. aulidion y 8, rara vez 7 o 9 en N. calbazas y N. amecae); 42, rara vez 40–41 y 43–45 escamas en una línea lateral (vs. 40 rara vez 37–39 en N. calabazas; 35–36, rara vez 37–39 y 33–34 en N. amecae; 35, rara vez 31–34 y 36 en N. marhabatiensis y N. calientis y 34 rara vez 30–33 y 35 in N. aulidion) y 11, rara vez 10 o 12 branquiespinas en el primer arco branquial. La línea lateral se extiende a partir del origen de la aleta pectoral hasta el origen de la aleta dorsal, con un segmento ligeramente convexo. Los adultos de esta especies son de mayor tamaño (longitud estándar=  = 42.6, SD= 4.69) (vs. N. calientis (n=55,
= 42.6, SD= 4.69) (vs. N. calientis (n=55,  = 33.3, SD=3.28) y N. marhabatiensis sp. nov. (n=30,
= 33.3, SD=3.28) y N. marhabatiensis sp. nov. (n=30,  =30.5, SD=7.57) (F = 16.87; p < 0.001)). Presentan treinta y una posiciones nucleotídicas fijadas en la secuencia del gen mitocondrial citocromo b y cuatro cambios aminoacídicos. Las distancias genéticas entre N. marhabatiensis y otras especies del complejo variaron entre
=30.5, SD=7.57) (F = 16.87; p < 0.001)). Presentan treinta y una posiciones nucleotídicas fijadas en la secuencia del gen mitocondrial citocromo b y cuatro cambios aminoacídicos. Las distancias genéticas entre N. marhabatiensis y otras especies del complejo variaron entre 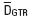 = 6.1% a 7.4%. Para N. granáis fueron de
= 6.1% a 7.4%. Para N. granáis fueron de 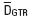 = 6.3% a 8.1%.
= 6.3% a 8.1%.
Palabras clave: Nueva especie, Notropis, México central, Cyprinidae, especie en peligro crítico.
ABSTRACT
Prior findings suggest the existence of undescribed species among the cyprinids of central Mexico. Within the genus Notropis distributed across central Mexico and adjacent areas sometimes reaching southern basins, two groups have been recognized: a Southern Mexican clade and a central Mexican clade. Within this last clade, Notropis calientis has been defined as a species complex of four small minnows inhabiting upland areas. Here we describe two new species of this complex based on morphometric, meristic and genetic characters. Notropis marhabatiensis sp. nov. was diagnosed according to the following set of characters: 7, rarely 8, branched pelvic fin rays (vs. 8, rarely 7 or 9, in other species of the N. calientis complex) and 9, rarely 8, scales in a transverse series (vs. 10, rarely 9 or 11 in N.grandis & N. calientis); a dark, narrow lateral stripe running from around the pectoral fin to the caudal peduncle origin; and twenty–six fixed nucleotide positions in the cytochrome b gene. The diagnosis of Notropis grandis sp. nov. was based on: 6, rarely 7, branched fin rays (vs. 7, rarely 6 or 8, in N. marhabatiensis, N. calientis and N. aulidion and 8, rarely 7 or 9, in N. calbazas and N. amecae), 42, rarely 40–41, or 43–45 scales in a lateral series (vs. 40, rarely 37–39, in N. calabazas; 35–36, rarely 37–39, or 33–34 in N. amecae; 35, rarely 31–34, or 36 in N. marhabatiensis and N. calientis and 34, rarely 30–33, or35 in N. aulidion) and 11 rarely 10 or 12 gill rakers in the first arch; a dark lateral stripe widening from approximately the pectoral fin origin to the dorsal fin origin, forming a slightly convex segment; adult animals larger (n=30,  =42.6, SD=4.69) relative to N. calientis (n=55,
=42.6, SD=4.69) relative to N. calientis (n=55,  = 33.3, SD= 3.28) and N. marhabatiensis sp. nov. (n=30,
= 33.3, SD= 3.28) and N. marhabatiensis sp. nov. (n=30,  = 30.5, SD=7.57) (F = 16.87; p < 0.001); and finally 31 fixed nucleotide positions in the mitochondrial gene cytochrome b sequence along with four amino acid changes. Calculated genetic distances between the new species and other species of the complex ranged between
= 30.5, SD=7.57) (F = 16.87; p < 0.001); and finally 31 fixed nucleotide positions in the mitochondrial gene cytochrome b sequence along with four amino acid changes. Calculated genetic distances between the new species and other species of the complex ranged between 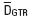 = 6.1% to 7.4% for N. marhabatiensis and
= 6.1% to 7.4% for N. marhabatiensis and 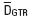 = 6.3% to 8.1% for N. grandis.
= 6.3% to 8.1% for N. grandis.
Key words: New species, Notropis, Central Mexico, Cyprinidae, critically endangered species.
INTRODUCTION
The central region of Mexico boasts a high diversity and endemicity of its fresh water fishes, attributed to a complex geological and zoogeographic history (Barbour, 1973; Echelle & Echelle, 1984; Domínguez–Domínguez et al., 2006). The Central Mexico is the northern limit for some neotropical families of freshwater fishes, whereas it constitutes the southern limit for others of nearctic origin. Thus, the region is a large zone of contact between nearctic and neotropical fish families. Cyprinid species are of nearctic origin, and the southern margin of their distribution range is Central Mexico and adjacent areas, such as the Balsas, Atoyac and Papaloapan river basins. Although cyprinids are among the most diverse group of fishes in the world, this southern limit of North American cyprinids shows less diversity, despite co–distributed fish groups (e.g. Goodeidae and Atherinopsidae) containing a high number of endemic species. The fish fauna of Central Mexico includes three genera of cyprinids, Notropis Rafinesque, 1818, Algansea Girard, 1856 and Yuriria Jordan & Evermann, 1896, totaling 15 species.
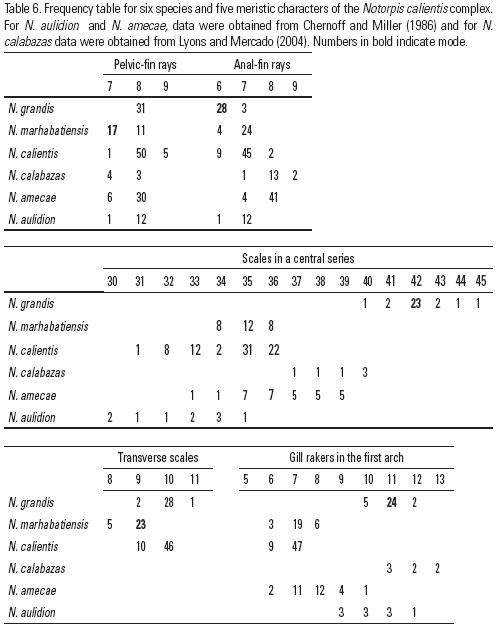
Several genetic approaches have revealed the presence of cryptic species among the cyprinids of Central Mexico (Schönhuth & Doadrio, 2003; Schönhuth et al., 2008). Some of these have been recently described (Domínguez–Domínguez et al., 2007). Within the genus Notropis inhabiting the Central Mexico and southern areas two groups have been identified (Schönhuth & Doadrio, 2003) based on complete sequences of the mitochon–drial cytochrome b gene: a Southern Mexican clade, comprising the species Notropis boucardi (Günther, 1868), N. moralesi (de Buen, 1955), N. imeldae Cortes, 1968, N. cumingii (Günther, 1868) and an undescribed species from Oaxaca; and a Central Mexican clade, including the species N. sallaei (Günther, 1868), N. calientis (Günther, 1868) and Yuriria alta (Jordan, 1880). In a recent phylo–genetic study by Schönhuth et al. (2008) based on four genes, the genus Yuriria was assigned a basal position with respect to the two Notropis clades (Southern and Central clades). The species N. amecae (Chernoff & Miller, 1986), N. aulidion (Chernoff & Miller, 1986) and N. calabazas (Lyons & Mercado–Silva, 2004) were not included in either study (Schönhuth et al., 2008).
Notropis calientis has been defined as a species complex by Chernoff and Miller (1986), who described two of its species and portrayed the complex as a group of three small minnows inhabiting upland areas of Central Mexico: N. aulidion, N. amecae and N. calientis. All these species are characterized by their brilliant yellow to golden breeding coloration, small eyes, rela–tively few anal–fin rays and an incomplete pored–lateral line of scales (absent in N. aulidion). More recently, another species has been described, N. calabazas Lyons & Mercado–Silva (2004), from the upper Rio Verde in the Pánuco River Basin. This species was assigned by the authors to the N. calientis complex because of its reduced number of head and lateral sensory canals, which are also incomplete.
The species of the N. calientis complex mainly inhabit small mountain streams, springs and small spring–fed lakes and their outlets. The four species included so far are highly sensitive to environmental disturbances and their populations are in decline. The species N. aulidion is believed to be extinct, N. amecae (Chernoff & Miller, 1986) is considered very rare and N. calabazas is uncommon. Notropis calientis, on the other hand, is widespread throughout most of the Lerma and Verde de Santiago drainage, the Rio Santa María in Pánuco drainage and the Cuitzeo Lake and its tributaries, although the decline of populations in some drainages has also been reported (Lyons & Mercado–Silva, 2004).
In a recent phylogenetic study of the cyprinids of central Mexico, the populations of Notropis from Zacapu Lake and San Miguel spring showed high genetic divergence with respect to populations of N. calientis and have been proposed as new undescribed taxa (Schönhuth & Doadrio, 2003; Schönhuth et al., 2008). The purpose of this study was to describe two new species within the Notropis calientis complex based on morphologic and genetic characters. These two new species inhabit the Zacapu Lake and San Miguel spring in Central Mexico.
MATERIALS AND METHODS
The designations of the new species were based on morphological analyses of the type material listed in the species descriptions and the following additional material (see Table 1 and Fig. 1): Notropis calientis, 30 specimens, CPUM–1621 (La Paz Dam near the city of Aguascalientes, Verde River drainage, Santiago River Basin, state of Aguascalientes); and N. calientis, 25 specimens, CPUM–1622 (La Mintzita Spring near the city of Morelia, Cuitzeo lake drainage, Michoacan).

The holotype and paratype series of the two new species were deposited in the Colección de Peces, Universidad Michoacana de San Nicolás de Hidalgo, Morelia, Mexico (CPUM), Colección Nacional de Peces, Universidad Nacional Autónoma de México (CNP–IBUNAM) and Museo Nacional de Ciencias Naturales de Madrid, España (MNCN).
Morphological analysis. Measurements were performed following Chernoff and Miller (1986), Doadrio et al. (2002) and Domínguez–Domínguez et al. (2007). Nineteen morphometric characters were determined using the computer program ImageTool 3.00, and seven meristic variables were counted with the help of a stereoscopic microscope. The following abbreviations were used for morphometric and meristic characters: SL, standard length; HL, head length; PrOL, preorbital length; ED, eye diameter; PoOL, postorbital length; Hd, head depth; SbP snout to origin of the pectoral fin; SbD, snout to origin of the dorsal fin; SbV, snout to origin of the ventral fin; SbA, snout to origin of the anal fin; DFL, dorsal fin length; PFL, pectoral fin length; VFL, ventral fin length; AFL, anal fin length; ACD, origin of the anal fin to origin of the caudal fin; DCD, origin of the dorsal fin to origin of the caudal fin; BD, body depth; BLD, body least depth; ADD, origin of the anal fin to origin of the dorsal fin (Fig. 2). Abbreviations for meristic characters are: C, caudal fin branched rays; D, dorsal fin branched rays; V, ventral fin branched rays; A, anal fin branched rays; SCS, scales in a central series; SLL, scales in a lateral line series; ST, scales in a transverse series according to Kottelat and Phreihof (2007); PS, pored scales; GR, gill rakers.

To identify the variables contributing most to the differences between the populations sampled, principal component analyses (PCA) were performed on the meristic and morphometric data collected from all the specimens, using a covariance matrix for the morphometric characters and correlation matrix for meristic characters. The PCA results for the morphometric data (not shown) indicated that all weighted characters on the first principal component (PC I) showed the same sign and were of similar magnitude, suggesting that this axis represents general size–related variation (Jolicoeur & Mosimann, 1960; Humphries et al., 1981; Bookstein et al., 1985). To test the possibility that one of the populations included in the dataset showed a larger size of specimens, as was empirically observed, the dataset was subjected to one way ANOVA and Tukey analysis. Burnaby's method was used to correct for the size effect (Burnaby, 1966; Rohlf & Bookstein, 1987; Doadrio et al., 2002) and all subsequent analyses were conducted on the corrected matrix. A second PCA was undertaken of the morphometric characters and subjected to canonical analysis of variance (CVA) to summarize differences among populations. The statistics package PAST v 1.75b was used for all the tests (Hammer et al., 2001). Morphometric and meristic characters were analyzed independently.
Genetic analysis. Seven sequences of Notropis (AF469137–AF469143) were obtained from GenBank (see Table 1). Six specimens, including those of the type locality of N. calientis and N. amecae and the outgroup N. sallaei, were collected and sequenced (Table 1 and Fig. 1). Sequences were obtained as follows. Total cellular DNA was isolated from tissues by standard protei–nase K and phenol/chloroform extraction procedures (Sambrook et al., 1989). Two overlapping fragments of the cytochrome b gene (1140 bp) were amplified via polymerase chain reaction (PCR) for each individual DNA sample. The primers used for cytochrome b in all species were those described in Zardoya and Doadrio (1998). The amplification process involved an initial denaturation step at 94°C for 2 min, and 35 cycles performed as follows: dena–turation at 94°C (1 min), aligning at 48°C (1 min), and extension at 72°C (1.45 min), with a final extension of 7 min at 72°C. PCR mixtures were prepared in 25 µl reaction volumes with final concentrations of 0.4 µM of each primer, 0.2 mM of each dNTP, 1.5 mM MgCl2, and 1U of Taq DNA polymerase (Biotools). PCR products were checked on 1.5% agarose gels, and cloned using the pGEM–T vector (Promega) into Escherichia coli JM109. Positive clones were sequenced using the FS–Taq Dye Deoxy Terminator cycle–sequencing kit (Applied Biosystems). DNA sequences of both strands were obtained using M13 universal (forward and reverse) sequencing primers. All samples were sequenced using an Applied Biosystems 3700 DNA sequencer following the manufacturer's instructions. Chromatograms and alignments were visually checked and verified. To obtain the model that best fitted our data we used a Bayesian information criterion implemented in the program Modeltest 3.7 (Posadas & Crandall, 1998). The aligned data were analyzed using the Bayesian inference method in the program Mr. Bayes 3.1.1 (Huelsenbeck & Ronquist, 2001), simulating four Markov chains for 1,000,000 cycles. Based on the GTR model provided by Modeltest, genetic distances (DGTR) between populations of Notropis and the new species were obtained using the program Sequencer 6.1.0 (written by B. Kessing and available at http://nmg.si.edu/). Accession numbers of the submitted sequences are GQ249850–GQ249856.
RESULTS
Notropis marhabatiensis sp. nov. (Figure 3A, Table 2)
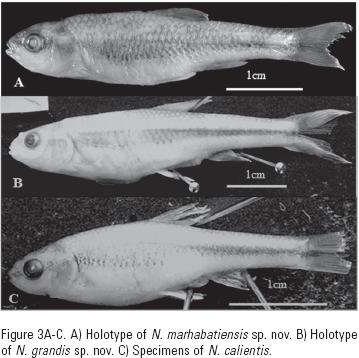
Holotype: MNCN–205640, 36.64 mm SL. Ojo de Agua de San Miguel in Maravatio town, Lerma Basin, Michoacan, Mexico. Geographic coordinates: latitude 19.86667 N, longitude 100.4333 W. Coll. I. Doadrio, E. González–Luna, A. de Sostoa and O. Domínguez–Domínguez, 6 June 2000.
Paratypes: 14 specimens MNCN–205629–205641; 13 specimens CPUM–2299; 3 specimens CNP–IBUNAM 14622. Same data as holotype.
Diagnosis: Notropis marhabatiensis sp. nov. was diagnosed according to the following combination of characters: 7, rarely 8, branched pelvic fin rays (vs. 8 rarely 7 or 9 for the other species of the N. calientis complex; Table 3) and 9, rarely 8, scales in a transverse series (vs. 10, rarely 9 or 11, in N. grandis and N. calientis). A dark, narrow lateral stripe runs from around the pectoral fin to the origin of the caudal peduncle. Twenty–six fixed nucleotide positions, or autopomorphies, and one amino acid change were observed in the cytochrome b sequence with respect to Notropis sp., N. calientis and N. amecae (Table 4). Genetic distances for N. marhabatiensis were: 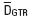 = 6.1% with respect to N. calientis;
= 6.1% with respect to N. calientis; 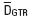 = 7.4% with respect to N. amecae; and
= 7.4% with respect to N. amecae; and 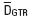 = 7.4% with respect to N. grandis sp. nov. (Table 5).
= 7.4% with respect to N. grandis sp. nov. (Table 5).



Description: D= II 7; A= I–II 6 (7); V= I (7)8; GR= 6(7)8; SCS = 34(35)36; ST= (8) 9. As in the other species of the Notropis calientis complex, the lateral line is incomplete and curved, SLL = 0 to 7 pored scales. All morphometric and meristic measurements are provided in Table 2. Scales thin, moderately imbricate and deciduous. The body is moderately deep, laterally compressed and elongated. Deepest body section approximately at the origin of the dorsal fin, but its depth is similar in the anterodorsal part of the body, tapering to the posterodorsal portion. Maximum body depth is 3.7–4.9 ( = 4.34 ±0.32) times the standard length and minimum body depth 8.5–12.6 (
= 4.34 ±0.32) times the standard length and minimum body depth 8.5–12.6 ( = 9.6 ±0.72) times the standard length. Caudal peduncle relatively long, with least depth just before the hypural plate. Distance origin anal fin to origin caudal fin is 2.9–3.6 (
= 9.6 ±0.72) times the standard length. Caudal peduncle relatively long, with least depth just before the hypural plate. Distance origin anal fin to origin caudal fin is 2.9–3.6 ( = 3.2 ±0.17) times the standard length and origin dorsal fin to origin caudal fin 1.9–2.4 (
= 3.2 ±0.17) times the standard length and origin dorsal fin to origin caudal fin 1.9–2.4 ( = 2.0 ±0.08) times the standard length. Head slightly longer than deep, snout rounded to slightly pointed not projecting beyond the upper lip. Head length is 3.5–4.5 (
= 2.0 ±0.08) times the standard length. Head slightly longer than deep, snout rounded to slightly pointed not projecting beyond the upper lip. Head length is 3.5–4.5 ( = 3.9 ±0.26) times the standard length and head depth 4.6–5.4 (
= 3.9 ±0.26) times the standard length and head depth 4.6–5.4 ( = 4.9 ±0.21) times the standard length. Eye centered at halfway head length. Eye diameter is 12.8–16.4
= 4.9 ±0.21) times the standard length. Eye centered at halfway head length. Eye diameter is 12.8–16.4  = 14.7 ±1.16) times the standard length. Dorsal fin about midway between snout and caudal peduncle at same level or slightly anterior to the ventral fin origin along the same axis. Distance from snout to the dorsal fin origin is 1.7–2 (
= 14.7 ±1.16) times the standard length. Dorsal fin about midway between snout and caudal peduncle at same level or slightly anterior to the ventral fin origin along the same axis. Distance from snout to the dorsal fin origin is 1.7–2 ( = 1.88 ±0.05) times the standard length and distance snout to ventral fin origin 1.7–1.9 (
= 1.88 ±0.05) times the standard length and distance snout to ventral fin origin 1.7–1.9 ( = 1.79 ±0.06) times the standard length. Pectoral fin length is 4.6–7.6 (
= 1.79 ±0.06) times the standard length. Pectoral fin length is 4.6–7.6 ( = 6.5 ±0.94) times the standard length. Dorsal fin length is 4.2–6.6 (
= 6.5 ±0.94) times the standard length. Dorsal fin length is 4.2–6.6 ( = 4.9 ±0.53) times the standard length. Ventral fin length is 5.3–9.1 (
= 4.9 ±0.53) times the standard length. Ventral fin length is 5.3–9.1 ( = 6.9 ±0.94) times the standard length. Anal fin length is 4.7–8.1 (
= 6.9 ±0.94) times the standard length. Anal fin length is 4.7–8.1 ( = 6.2 ±0.77) times the standard length. Snout slightly rounded. Mouth terminal to slightly subterminal and oblique. Barbels absent.
= 6.2 ±0.77) times the standard length. Snout slightly rounded. Mouth terminal to slightly subterminal and oblique. Barbels absent.
Pigmentation pattern: Preserved specimens show a yellowish to light brown coloration of the body. A dark, narrow lateral stripe runs from approximately the origin of the pectoral fin to the caudal peduncle. In the predorsal region, a diffuse elongated spot appears in the middle body portion; in some specimens this spot is made up of a number of smaller spots. These spots occur across most of the body and are more conspicuous at the opercle. Some specimens show a small dark patch at the caudal peduncle and anterior part of the caudal fin. Fine pigmented predorsal stripes in the dorsal region. Upper head pigmented with small dark spots. Interorbital region darkly pigmented. All fins transparent and in some individuals the anal, caudal and dorsal fin show a dark coloration.
Etymology: The name "marhabatiensis" is derived from its type locality, the town of Marhabatio (P'urepecha word of Maravatío). The P'urepecha word Marhabatio means beautiful thing or place.
Common name: Maravatío shiner.
Distribution: The type locality of N. marhabatiensis is the San Miguel Spring in the southeast region of Maravatio town in the upper Lerma River drainage, state of Michoacan. The species is only known in the San Miguel Spring (Fig. 1).
Remarks: The spring inhabited by N. marhabatiensis is highly degraded and used for recreation purposes. The main pool is arround 2 m deep and 5 m wide. A small outlet stream runs along 200 m and then discharges into the town's (Maravatío) waste–water channel. The species is absent from the stream. Fish collected with N. marhabatiensis from the type locality were the native goodeids Girardinichthys multiradiatus (Meek, 1904) and Goodea atripinnis Jordan, 1880, and the introduced fish were the cichlid Oreochromis sp. and the poeciliids Poecilia reticulata Peters, 1859 and Heterandria bimaculata (Heckel, 1848). No vegetation was found around the pool. Within the spring grow aquatic plants of the genus Ceratophyllum. The substrate is composed mainly of sand and gravel with many boulders at the bottom of the pool and mud in the stream.
Conservation status: The species is only known in the type locality, San Miguel Spring, and only a small population was found in surveys conducted in 2000 and 2002. In recent visits to the area (2004 to 2007), the species was not found. This small spring is surrounded by the houses of Maravatio and suffers intense human pressure as a recreation and washing place. The recent introduction of the exotic species P. reticulata, H. bimacu–lata and Oreochromis sp. could explain the possible absence of this species from the spring. Because of its restricted distribution range, recent unsuccessful sampling efforts and threats to the habitat and species in the San Miguel Spring, we consider this species as Critically Endangered according to UICN criteria (CR B–1 b i, iii, iv). Further, according to the Risk Evaluation Method (Método de Evalucaión de Riesgo–MER) (Sánchez et al., 2007), the species should be considered in danger of extinction (14 A,I; B,I; C,I; D,I).
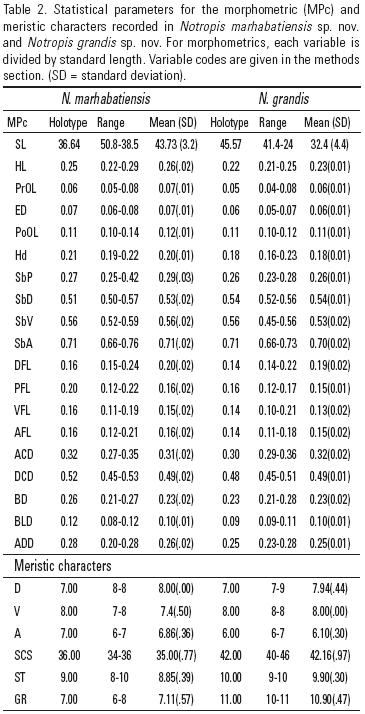
Notropis grandis sp. nov. (Figure 3B, Table 2)
Holotype: CPUM– 2300, 45.57 mm SL. La Angostura, Laguna de Zacapu, Zacapu, Michoacan, Mexico. Geographic coordinates: latitude 19.81667 N, longitude 101.78333 W. Coll. R. Pérez–Rodríguez and R. Rosas–Valdez, 9 June 2005.
Paratypes: 26 specimens CPUM–1623; 5 specimens CNP–IBUNAM 14624; 4 specimens MNCN–262768–262771. Same data as holotype.
Diagnosis: Notropis grandis sp. nov. was diagnosed according to the following combination of characters: 6, rarely 7, branched anal fin rays (vs. 7, rarely 6 or 8, in N. marhabatiensis, N. calientis and N. aulidio and 8, rarely 7 or 9, in N. calbazas and N. amecae), 42, rarely 40–41, or 43–45 scales in a lateral series (vs. 40, rarely 37–39, in N. calabazas; 35–36, rarely 37–39, or 33–34 in N. amecae; 35, rarely 31–34, or 36 in N. marhabatiensis and N. calientis (Table 3) and 34, rarely 30–33, or 35 in N. aulidion) and (10) 11 (12) gill rakers in the first arch. A dark lateral stripe widens from approximately the pectoral fin origin to the dorsal fin origin, forming a slightly convex segment. Adult animals large (n =30 ,  =42.6, SD =4.69) relative to N. calientis (n =55,
=42.6, SD =4.69) relative to N. calientis (n =55,  =33.3, SD =3.28) and N. marhabatiensis sp. nov. (n =30,
=33.3, SD =3.28) and N. marhabatiensis sp. nov. (n =30,  =30.5, SD =7.57) (F = 16.87; p < 0.001). Twenty–three fixed nucleotide positions, or autopomorphies, and two amino acid changes were observed in the cytochrome b sequence with respect to N. calientis, N. marhabatiensis and N. amecae (Table 4). Genetic distances for N. grandis were:
=30.5, SD =7.57) (F = 16.87; p < 0.001). Twenty–three fixed nucleotide positions, or autopomorphies, and two amino acid changes were observed in the cytochrome b sequence with respect to N. calientis, N. marhabatiensis and N. amecae (Table 4). Genetic distances for N. grandis were: 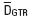 = 6.3% with respect to N. calientis;
= 6.3% with respect to N. calientis; 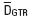 = 8.1% with respect to N. amecae; and
= 8.1% with respect to N. amecae; and 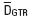 = 7.4% with respect to N. marhabatensis sp. nov. (Table 5).
= 7.4% with respect to N. marhabatensis sp. nov. (Table 5).
Description: D =II 6(7)8; A = I–II (6)7; V = I 8; GR = 10(11)12; SCS =40–41(42)43–45; ST =9(10). All morphometric and meristic measurements are provided in Table 2. As in other species of the Notropis calientis complex, the lateral line is incomplete and curved, SLL = 1 to 17 pored scales. Scales thin, moderately imbricate and deciduous. The body is moderately deep, laterally compressed and elongated. Dorsal and ventral profiles moderately arched. Deepest body section approximately midway between the dorsal fin and the occiput. Maximum depth is 3.6–4.8 ( = 4.5 ±0.28) times the standard length and minimum body depth 9.2–11.7 (
= 4.5 ±0.28) times the standard length and minimum body depth 9.2–11.7 ( = 10.4 ±0.48) times the standard length. Caudal peduncle relatively long and thick, and narrowest portion of the body occurs just at the end of the caud al peduncle. Distance origin anal fin to origin caudal fin is 2.8–3.4 (
= 10.4 ±0.48) times the standard length. Caudal peduncle relatively long and thick, and narrowest portion of the body occurs just at the end of the caud al peduncle. Distance origin anal fin to origin caudal fin is 2.8–3.4 ( = 3.1 ±0.16) times the standard length and origin dorsal fin to origin caudal fin 2–2.2 (
= 3.1 ±0.16) times the standard length and origin dorsal fin to origin caudal fin 2–2.2 ( = 2.1 ±0.06) times the standard length. Head moderately longer than deep, snout rounded to slightly pointed not projecting beyond the upper lip, head length 4.0–4.7 (
= 2.1 ±0.06) times the standard length. Head moderately longer than deep, snout rounded to slightly pointed not projecting beyond the upper lip, head length 4.0–4.7 ( = 4.3 ±0.16) times the standard length and head depth 4.4–6.12 (
= 4.3 ±0.16) times the standard length and head depth 4.4–6.12 ( = 5.6 ±0.36) times the standard length. Eye is relatively small and centered at halfway head distance. Eye diameter is 14.4–18.6 (
= 5.6 ±0.36) times the standard length. Eye is relatively small and centered at halfway head distance. Eye diameter is 14.4–18.6 ( = 16.8 ±1.13) times the standard length. Dorsal fin appears about midway between snout and caudal peduncle at same level or slightly behind the ventral fin origin along the same axis. Distance snout to dorsal fin origin is 1.8–1.9 (
= 16.8 ±1.13) times the standard length. Dorsal fin appears about midway between snout and caudal peduncle at same level or slightly behind the ventral fin origin along the same axis. Distance snout to dorsal fin origin is 1.8–1.9 ( = 1.86 ±0 .04) times the standard length and snout to ventral fin origin 1.8–2.2 (
= 1.86 ±0 .04) times the standard length and snout to ventral fin origin 1.8–2.2 ( = 1.9 ±0.08) times the standard length. Pectoral fin length is 3.5–4.4 (
= 1.9 ±0.08) times the standard length. Pectoral fin length is 3.5–4.4 ( = 3.9 ±0.19) times the standard length. Dorsal fin is 4. 7–7.3 (
= 3.9 ±0.19) times the standard length. Dorsal fin is 4. 7–7.3 ( = 5.5 ±0.76) times the standard length. Pectoral fin is 5.9–8.1 (
= 5.5 ±0.76) times the standard length. Pectoral fin is 5.9–8.1 ( = 6.84 ±0.55) times the standard length. Ventral length is 4.8–9.83 (
= 6.84 ±0.55) times the standard length. Ventral length is 4.8–9.83 ( = 7.6 ±0.89) times the standard length. Anal fin is 5.7–8.5 (
= 7.6 ±0.89) times the standard length. Anal fin is 5.7–8.5 ( = 6.9 ±0.73) times the standard length. Snout slightly rounded. Mouth is terminal to slightly subterminal and oblique. Barbels absent.
= 6.9 ±0.73) times the standard length. Snout slightly rounded. Mouth is terminal to slightly subterminal and oblique. Barbels absent.
Pigmentation pattern: Preserved specimens show a yellowish to light brown coloration of the body. A dark lateral stripe widens from approximately the origin of the pectoral fin to the dorsal fin origin forming a slightly convex segment; the stripe becomes narrow and more conspicuous in the postdorsal region of the body. The lateral stripe ends in a small dark patch in the caudal peduncle and caudal fin origin. Fine pigment stripes around the base of the dorsal fin. Upper part of the head pigmen–ted. All fins clear and unpigmented.
Etymology: The name "grandis" refers to the larger size of this species relative to the other members of the N. calientis complex.
Common name: Zacapu shiner.
Distribution: The type locality of N. grandis sp. nov. is Lake Zacapu in the town of Zacapu, Lerma River drainage, state of Michoacán. It is only known in the Zacapu Lake and its outlet (Fig. 1).
Remarks: The water body is fed by at least 12 large springs that maintain the level of the lake. The water in the spring area is clean and sustains dense vegetation, mainly Potamogeton illinoensis Morong , P. pectinatus Linnaeus, Myriophyllum sp., Sagittaria sp., and Ceratophyllum demersum Linnaeus. The rest of the lake contains turbid water with less vegetation than the spring area. In the zone around the lake, grow Taxodium, Salix, Berula erecta Hudson, Scirpus sp., and Typha latifolia Linnaeus. The introduced Eichhornia crassipes Martius is also found. The fish species found are the goodeids Hubbsina turneri De Buen, 1940, Goodea atripinnis, Xenotoca variata (Bean, 1887), Allotoca zacapuensis Meyer, Radda & Domínguez–Domínguez, 2002, Alloophorus robustus (Bean, 1892), Skiffia lermae Meek, 1902, Zoogoneticus quitzeoensis (Bean, 1898), the poeciliid Poeciliopsis infans (Woolman, 1894), the atheri–nopsid Chirostoma humboltianum (Valenciennes, 1835), the cyprinid Algansea tincella (Valenciennes, 1844), and the introduced cypri–nids Cyprinus carpio Güldenstädt, 1773 and Ctenopharyngodon idella (Valenciennes, 1844).
Conservation status: The species is only known in Lake Zacapu. Although since 2003 the lake forms part of a protected area, the introduction of the exotic carp species Cyprinus carpio and Ctenopharyngodon idella and their parasite Bothriocephalus acheilognathi Yamaguti, 1934 seems to have had a negative impact on N. grandis sp. nov. Indeed, the repercussions of introducing carp species and their parasites on native fish species and their habitats in Mexico have been well documented (Lyons et al., 1998; Soto–Galera et al., 1998; Tapia & Zambrano, 2003; Salgado–Maldonado & Pineda–López, 2003; Mejía–Madrid et al., 2005; Domínguez–Domínguez et al., 2006). The lake's water quality has been affected by a drop in the water table reducing water inflow to the springs that feed the lake. Moreover, at the beginning of the XIX century, drying of the original marshy area (around 15,000 ha) for agricultural purposes reduced the lake to a mere 32 ha (Guzmán, 1985). This decrease in the water mass had detrimental effects on the Zacapu Lake population of Zoogoneticus quitzeoensis (Bean, 1898), severely compromising genetic diversity and increasing the extinction risk of its fish populations (Domínguez–Domínguez et al., 2007). According to these events and pending further population and genetic studies, we propose that the new species should be considered Critically Endangered according with the UICN criteria (CR B–1 a i, iii, iv). Under Mexican environmental Law and following the criteria of MER (Sánchez et al., 2007), the new species should be considered in danger of extinction (14 A,II;B,I; C,II, D,I).
DISCUSSION
Morphometrics. As mentioned above, the specimens of Notropis from Zacapu and Ojo de Agua de San Miguel showed diagnostic meristic and genetic characters differentiating them from their close relatives in the Ameca River (N. amecae), Mezquital River (N. aulidion), Pánuco River (N. calabazas) and Lerma and Santiago river drainages (N. calientis). Although only two juvenile specimens of N. amecae were included in our genetic analyses and no specimens of N. aulidion and N. calabazas were collected, we obtained some comparative meristic characters from the literature (Table 6). Another factor supporting our findings is the biogeographic scenario, since the rivers inhabited by N. aulidion, N calabazas and N. amecae (Mezquital, Pánuco and Ameca, respectively) constitute a different drainage system and a different biogeographic region from the current habitat of N. calientis, N. grandis sp. nov. and N. marhabatiensis sp. nov. in the Lerma River and Verde River of the Santiago River drainage (Domínguez–Domínguez et al., 2006).
In an exploratory PCA of morphometric measurements, PCI was able to explain 92.46 % of the variance and eigenvectors showed close values with the same sign, suggesting the influence of standard length on the results (Bookstein et al., 1985; Doadrio et al., 2002). Accordingly, the original data matrix was size–corrected following Burnaby's method (Burnaby 1966; Rohlf & Bookstein 1987). Despite the differences found in the PCA and the use of the corrected matrix in subsequent analyses, one way ANOVA and Tukey analysis revealed the species N. grandis to be larger than the other two species examined (N. marhabatiensis and N. calientis). Moreover, according to the mean sizes given by Chernoff and Miller (1989) and Lyons & Mercado–Silva (2005), N. grandis also appears to be larger than N. aulidion, N. amecae and N. calabazas.
In a second PCA of morphological characters, PCI accounted for 41.26% of the variance and PCII for a cumulative variance of 53.02%. The characters that contributed most to this variance were PrOL and SbP for PCI and PFL and PVL for PCII (Table 6). Variance was more influenced by PCI and no well–defined groups emerged. Neither was there any evidence of diagnostic characters among the three species examined (N. calientis, N. grandis and N. marhabatiensis) (Fig. 5A). Although morphology has been largely used to describe fish species, several studies have shown that the morphologic characters of cyprinids may not significantly differ between closely related species, and this seems to be a particular feature of the genus Notropis (Chernoff & Miller, 1986, Lyons & Mercado–Silva, 2005; Domínguez–Domínguez et al., 2007). Moreover, it is widely recognized that phenotypic plasticity depends on habitat conditions and that allometry may affect morphometric characters (Hood & Heins, 2000; Trapani et al., 2005). Thus, other sources of information are needed to identify all the species of the N. calientis complex.
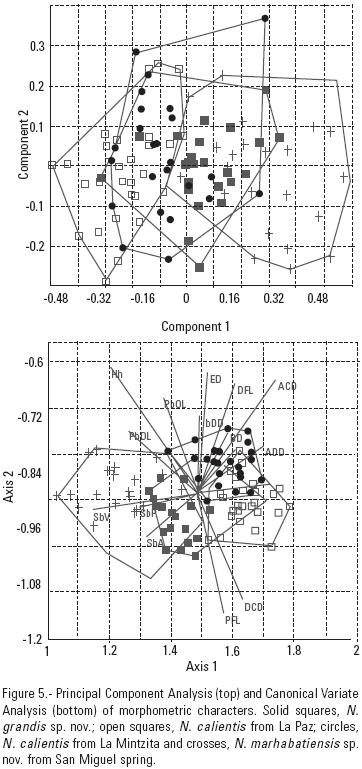
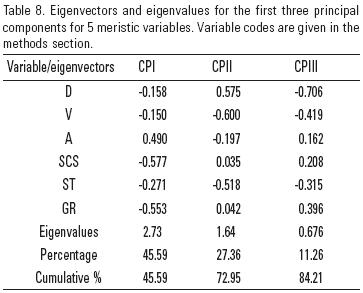
The additional sources of information used here for species recognition were genetic and meristic characters. In our PCA analysis of meristic characters comparing N. calientis (two populations), N. grandis and N. marhabatiensis, PCI explained 45.59% of the variance, and PCII accounted for a cumulative variance of 72.95%. The characters that most contributed to the variance in PCI were transverse scales, gill rakers and branched anal fin rays, this component clearly separated the species N. grandis from N. calientis and N. marhabatiensis. The variables that contributed most to the variance in PCII were ventral fin rays (Table 8) and scales in a transverse series, with this component separating, although not too clearly, the species N. calientis and N. marhabatiensis (Fig. 5). With respect to all other species within the N. calientis complex, the frequency tables (Table 6) indicate clear differences in N. grandis in terms of its lateral scales and gill rakers in the first arch, whereas N. marhabatiensis differed from other species in terms of its pelvic fin rays.
Genetics. Of the characters obtained for the Notropis cytochrome b sequences examined, 163 were variable, and 133 were parsimony informative. Third codon positions were the most informative (106 informative characters) followed by the first codon position (24 characters). Saturation of transition and transversion changes was checked by plotting the absolute number of changes of each codon position against patristic distances. There was no ingroup evidence of saturation at any of the three positions (not shown). The GTR+G model was selected as the model that best fitted the dataset. Rate matrix parameters were: –lnL= 2622.7678; K= 9; AIC= 5263.5356. Base frequencies were: freqA= 0.2634; freqC= 0.2805; freqG= 0.1574; freqT= 0.2987. The proportion of variable sites=0 and gamma distribution shape parameter = 0.1655.
The phylogenetic hypothesis obtained through Bayesian analysis after burning 500 chains (Fig. 6) indicated four well–differentiated groups, each representing one of the species analyzed (N. calientis, N. amecae, N. grandis sp. nov. and N. marhabatiensis sp. nov.), although relationships among them were not resolved. This could be the outcome of the long genetic distances found among the species (Table 5) with an overall average 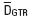 = 5.5%. Since most drainage and several populations within the Lerma–Santiago river system were surveyed, these high genetic differences could be the consequence of ancient isolation of the species in their respective water bodies, and a greater dispersal capacity of N. calientis inhabiting the Cuitzeo Lake and Lerma and Santiago river drainages.
= 5.5%. Since most drainage and several populations within the Lerma–Santiago river system were surveyed, these high genetic differences could be the consequence of ancient isolation of the species in their respective water bodies, and a greater dispersal capacity of N. calientis inhabiting the Cuitzeo Lake and Lerma and Santiago river drainages.
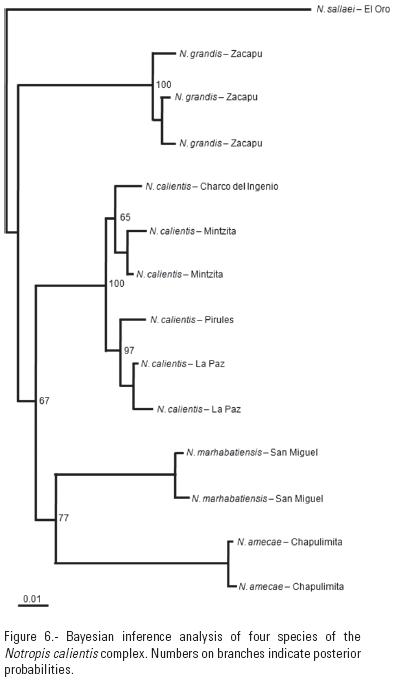

The Zacapu Lake seems to have served as a diversification area for other species groups of aquatic fauna, as has been reported for the endemic goodeid Allotoca zacapuensis, the amphibian Ambystoma andersoni (Krebs & Brandon. 1984) and the bivalve Anodonta grandis Say, 1829. However, divergences estimated for N. grandis were significantly greater (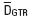 = 5.8 to 8.1%) compared to the other three Notropis species, than the divergences observed for A. zacapuensis (DP = 2.5 to 2.6%) when compared to their closely related species (Aguilar–Medrano et al., unpublished data), and even greater when compared to those shown by other goodeid population pairs living in Zacapu and contiguous regions;
= 5.8 to 8.1%) compared to the other three Notropis species, than the divergences observed for A. zacapuensis (DP = 2.5 to 2.6%) when compared to their closely related species (Aguilar–Medrano et al., unpublished data), and even greater when compared to those shown by other goodeid population pairs living in Zacapu and contiguous regions; 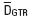 = < 1% for most species (Domínguez–Domínguez, in press). If we consider the molecular clock generally assumed for other cyprinids of 1.05% divergence per million years (Dowling et al., 2002), the cladogenetic event for N. grandis would be dated around 6 Mya, during the upper Miocene (Messinian). This dating significantly exceeds that for the formation of Zacapu Lake, estimated to have occurred in the Upper Pliocene (Demant, 1992).
= < 1% for most species (Domínguez–Domínguez, in press). If we consider the molecular clock generally assumed for other cyprinids of 1.05% divergence per million years (Dowling et al., 2002), the cladogenetic event for N. grandis would be dated around 6 Mya, during the upper Miocene (Messinian). This dating significantly exceeds that for the formation of Zacapu Lake, estimated to have occurred in the Upper Pliocene (Demant, 1992).
Notropis marhabatensis sp. nov. is confined to a small spring in the upper Lerma River close to Maravatio city. The biogeographic differences between the upper Lerma and its lower reaches have been widely discussed (see Barbour, 1973; Domínguez–Domínguez et al., 2006) and are supported by the species endemic to this region Algansea barbata Alvarez & Cortés, 1964, Girardinichthys multiradiatus and N. sallaei, the sister species of the N. calientis complex (Schönhuth and Doadrio, 2003). This region of the Upper Lerma was distinguished by Barbour (1973) as an ancient Maravatio basin, indicating the formation of an ancient lake in the region. Genetic distances between N. marhabatiensis and the other species of the N. calientis complex were 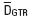 = 6.1 to 7.4%, and its divergence with respect to N. sallaei was in the range DP = 8.5 to 10% (Schönhuth and Doadrio, 2003). This information suggests that different events of isolation between and within the upper Lerma and the rest of the Lerma River occurred over 5 Mya. The former would have led to the formation of the N. sallaei species, possible isolated in the ancient Aztlán Paleolake ca. 10 Mya, and the latter related to the isolation and formation of N. marhabatiensis in the ancient Maravatio basin (Barbour, 1973) ca. 7 Mya.
= 6.1 to 7.4%, and its divergence with respect to N. sallaei was in the range DP = 8.5 to 10% (Schönhuth and Doadrio, 2003). This information suggests that different events of isolation between and within the upper Lerma and the rest of the Lerma River occurred over 5 Mya. The former would have led to the formation of the N. sallaei species, possible isolated in the ancient Aztlán Paleolake ca. 10 Mya, and the latter related to the isolation and formation of N. marhabatiensis in the ancient Maravatio basin (Barbour, 1973) ca. 7 Mya.
Interestingly, the other two native species found in San Miguel Spring (Goodea atripinnis and Girardinichthys multiradiatus) displayed a smaller genetic distance, 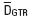 < 1.5%, with respect to other populations inhabiting contiguous basins (Domínguez–Domínguez et al., in press). This information, and the fact that N. marhabatiensis has only been identified at its type locality, would appear to indicate that the dispersal capacity or capacity to invade new areas of this species is lower than the capacity shown by its sympatric species.
< 1.5%, with respect to other populations inhabiting contiguous basins (Domínguez–Domínguez et al., in press). This information, and the fact that N. marhabatiensis has only been identified at its type locality, would appear to indicate that the dispersal capacity or capacity to invade new areas of this species is lower than the capacity shown by its sympatric species.
Based on their endemicity and on the threatened nature of the reduced water bodies inhabited by N. grandis and N. marhabatiensis, we recommend the two species are included as endangered in the Norma Oficial Mexicana (Mexican red list).
ACKNOWLEDGEMENTS
The authors thank Adolfo de Sostoa, Hugo Mejía and Rogelio Rosas for their help during field trips, Luis Boto for his help and comments and Lourdes Alcaraz and Carlos Pedraza for laboratory work. Part of this research was financed by a grant awarded to OD by Chester Zoo Garden, England and by the project CGL2006–12325/BOS. OD, RP and LE thank the Consejo Nacional de Ciencia y Tecnología for fellowship awards.
REFERENCES
Barbour, C. D. 1973. A biogeographical history of Chirostoma (Pisces: Atherinidae): A species flock from the Mexican Plateau. Copeia: 533–556. [ Links ]
Bookstein, F. B., B. Chernoff, R. L. Elder, J. M. Humphries, G. Smith & G. strauss. 1985. Morphometrics in Evolutionary Biology. Academy of Natural Sciences of Philadelphia. Special Publication 15: 1–277. [ Links ]
Briolay, J., N. Galtier, R. M. Brito & I. Bouvet. 1998. Molecular phylogeny of Cyprinidae inferred from cytochrome b DNA sequences. Molecular Phylogenetics and Evolution 9: 100–108. [ Links ]
Burnaby, T. P. 1966. Growth–invariant discriminant functions and generalized distances. Biometrics 22: 96–110. [ Links ]
Buth, D. G. 1979. Biochemical systematic of the cyprinid genus Notropis–I. The subgenus Luxilus. Biochemical Systematics and Ecology 7: 69–79. [ Links ]
Cavender T. M. & M. M. Coburn. 1992. Phylogenetic relationships of North American Cyprinidae. In: Mayden, R. L. (Ed). Systematics, historical ecology and North American Freshwater fishes. Stanford University Press, pp. 328–373. [ Links ]
Coburn M. M. & T. M. Cavender. 1992. Interrelationships of North American cyprinid fishes. In: Mayden R. L, (Eds.). Systematics, historical ecology, and North American freshwater fishes. Stanford University Press, pp. 328–373. [ Links ]
Chernoff, B. & R. R. Miller. 1986. Fishes of the Notropis calientis complex with a key to the southern shiners of México. Copeia (1): 170–183. [ Links ]
Dimmick, W. W. 1987. Phylogenetic relationships of Notropis hubbsi, N. welaka and N. emiliae (Cypriniformes: Cyprinidae). Copeia 2: 316–325. [ Links ]
Doadrio, I., J. A. Carmona & C. Fernández–Delgado. 2002. Morphometric study of the Iberian Aphanius (Actinopterygii, Cyprinodontiformes), with description of a new species. Folia Zoologica 51: 67–79. [ Links ]
Domínguez–Domínguez, O., I. Doadrio & G. Pérez–Ponce De León. 2006. Historical biogeography of some river basins in Central México evidenced by their goodeine freshwater fishes: a preliminary hypothesis using secondary Brooks parsimony analysis (BPA). Journal of Biogeography 33: 1437–1447. [ Links ]
Domínguez–Domínguez, O., A. Pompa–Domínguez & I. Doadrio. 2007b. A new species of the genus Yuriria Jordan & Evermann, 1896 (Actinopterygii, Cyprinidae) from the Ameca basin of the Central Mexican Plateau. Graellsia 2 (63): 259–271. [ Links ]
Echelle, A. A. & A. F. Echelle. 1984. Evolutionary generics of a "species flock": Atherinid fishes on the Mesa Central of México. In: Echelle, A.A. & I. Kornfield (Eds.). Evolution of Fish Species Flocks. University of Main at Orono Press, pp. 93–110. [ Links ]
Gilbert, C. R. & R. M. Bailey. 1972. Systematics and zoogeography of the American cyprinid fish Notropis (Opsopoeodus) emiliae. Occasional Papers of the University of Michigan Museum of Zoology 664: 1–33. [ Links ]
Guzmán, A. J. N. 1985. La desecación de la Ciénega de Zacapu: orígenes y consecuencias. Tzintzun 2:26–37. [ Links ]
Hammer, Ø., D. A. T. Harper, & P. D. Ryan. 2001. PAST: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontologia Electronica 4(1):1– 9. http://palaeo–electronica.org/2001_1/past/issue1_01.htm [ Links ]
Hood, C. S. & D. C. Heins. 2000. Ontogeny and Allometry of Body Shape in the Blacktail Shiner, Cyprinella venusta. Copeia 2000: 270–275. [ Links ]
Howes, G. L. 1991. Systematic and biogeography: an overview. In: Winfield, I. J. & J. S Nelson (Eds). Cyprinids fishes: Systematics, biology and exploitation. Chapman and Hall, London, pp: 1–33 [ Links ]
Hubbs, C. L. & R. R. Miller. 1977. Six distinctive cyprinid fish species referred to Dionda inhabiting segments of the Tampico Embayment drainage of México. San Diego Natural History Transactions 18: 267–336. [ Links ]
Humphries, J. M., F. L. Bookstein, B. Chernoff, G. R. Smith, R. L. Elder & S. G. Poss. 1981. Multivariate discrimination by shape in relation to size. Systematic Zoology 30: 291–308. [ Links ]
Huelsenbeck, J. P. & F. R. Ronquist. 2001. MrBayes: Bayesian inference of phylogeny. Bioinformatics 17: 754–755. [ Links ]
Jolicoeur, P. & J. E. Mosimann. 1960. Size and shape variation in the painted turtle. A principal component analysis. Growth 24: 339–354 [ Links ]
Lyons, J., G. González–Hernández, E. Soto–Galera & M. Guzmán–Arroyo. 1998. Decline of fishes and fisheries in selected drainages of west–central Mexico. Fisheries 23: 10–18. [ Links ]
Lyons J., & N. Mercado–Silva. 2004. Notropis calabazas (Teleostei; Cyprinidae): New Species from the Río Pánuco Basin of Central México. Copeia 4: 868–875. [ Links ]
Mayden, R. L. 1989. Phylogenetic studies of North American minnows, with emphasis on the genus Cyprinella (Teleostei: Cypriniformes). In: Mengel R.M. & R. F.Johnston (Eds.). Miscellaneous publications of the Museum of Natural History, University of Kansas 80: 1–189. [ Links ]
Mayden, R. L. 1991. Cyprinids of the new world. In: Winfield I.J & J. S Nelson (Eds.). Cyprinid fishes: systematics, biology and exploitation. London: Chapman & Hall, pp. 240–263. [ Links ]
Mejía–Madrid, H. H., O. Domínguez–Domínguez & G. Pérez–Ponce De León. 2005. Adult Endohelminth Parasites of Goodeinae (Cyprinodontiformes: Goodeidae) from México with Biogeographical Considerations. Comparative Parasitology 72: 200–211. [ Links ]
Meyer, M. K., A. C. Radda & O. Domínguez–Domínguez. 2001. Notes on the genera Neoophorus Hubbs & Turner, 1937 and Allotoca Hubbs & Turner, 1937, witha description of a new species of Allotoca from Laguna de Zacapu, Michoacan, Mexico (Teleostei, Cyprinodontiformes, Goodeidae). Annalen aus dem Naturhistorisches Museum Wien 103: 453–460. [ Links ]
Pérez–Ponce De León, G. 2003. Biodiversity and biogeographic patterns in the Mesa Central of México: insights from host–parasite systems. Journal of Parasitology 89: 126–133. [ Links ]
Rohlf, F. J. & F. L. Bookstein. 1987. A comment on shearing as a method for size correction. Systematic Zoology 36: 356–367. [ Links ]
Salgado–Maldonado, G. & R. F. Pineda–López. 2003. The Asian fish tapeworm Bothriocephalus acheilognathi: a potential threat to native freshwater fish species in México. Biological Invasions 5: 261–268. [ Links ]
Sambrook, J., E .F. Fritsch & T. Maniatis. 1989. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory. New York. 545 p. [ Links ]
Schönhuth, S. & I. Doadrio. 2003. Phylogenetic relationships of Mexican minnows of the genus Notropis (Actinopterygii, Cyprinidae). Biological Journal of the Linnean Society 80: 323–327. [ Links ]
Schönhuth, S., I. Doadrio, O. Domínguez–Domínguez, D. M. Hillis & R. L. Mayden Molecular evolution of southern North American Cyprinidae (Actinopterygii), with the description of the new genus Tampichthys from central Mexico. Molecular Phylogenetics and Evolution 47: 729–756 [ Links ]
Trapani, J., Y. Yamamoto & D. W. Stock. 2005. Ontogenetic transition from unicuspid to multicuspid oral dentition in teleost fish: Astyanax mexicanus, the Mexican tetra (Ostariophysi: Characidae). Zoological Journal of the Linnean Society 145: 523–538. [ Links ]
Zardoya, R. & I. Doadrio. 1998. Phylogenetic relationships of Iberian cyprinids: systematic and biogeographical implications. Proceedings of the Royal Society of London 265: 1365–1372. [ Links ]
Zardoya, R. & I. Doadrio. 1999. Molecular evidence on the evolutionary and biogeographical patterns of European cyprinids. Journal of Molecular Evolution 49: 227–237. [ Links ]














