Servicios Personalizados
Revista
Articulo
Indicadores
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Hidrobiológica
versión impresa ISSN 0188-8897
Hidrobiológica vol.19 no.1 Ciudad de México abr. 2009
Artículos
Physicochemical properties of cowpea (Vigna unguiculata L. Walp.) meals and their apparent digestibility in white shrimp (Litopenaeus vannamei Boone)
Propiedades fisicoquímicas de harinas de frijol yorimón (Vigna unguiculata L. Walp.) y su digestibilidad aparente en camarón Litopenaeus vannamei Boone
Martha Elisa Rivas–Vega1,3, Ofelia Rouzaud–Sandez2, María Guadalupe Salazar–García2, Josafat Marina Ezquerra–Brauer2, Ernesto Goytortúa–Bores1 and Roberto Civera–Cerecedo1*
1 Laboratorio de Nutrición Acuícola. Centro de Investigaciones Biológicas del Noroeste (CIBNOR), Mar Bermejo No. 195, Col. Playa Palo de Santa Rita, La Paz, B. C. S., 23090, México.
2 Universidad de Sonora. Rosales y Transversal s/n, Hermosillo, Sonora, 83000, México.
3 Centro de Estudios Superiores del Estado de Sonora. Carretera a Huatabampo y Periférico Sur, Navojoa, Sonora, 85800, México. *Autor para correspondencia: rcivera04@cibnor.mx
Recibido: 2 abril de 2008.
Aceptado: 1 de marzo de 2009.
ABSTRACT
The effect of different feed processing methods on the physicochemical properties, and apparent digestibility of cowpea (Vigna unguiculata) meals as ingredients in diets for white shrimp (Litopenaeus vannamei) was investigated. Five experimental cowpea meals were prepared: whole raw (WRC), dehulled (DC), cooked (CC), germinated (GC) and extruded (EXC). The physicochemical properties of the meals were evaluated using differential scanning calorimetry. The meals were included at 15% in diets for L. vannamei (15.4 g) to determine firmness of pellets and in vivo digestibility of nutrients by using chromic oxide as inert marker. Six diets were evaluated: a control diet, and five diets containing the different cowpea meals. Transition enthalpy significantly decreased after thermal treatment, from 6.1 J/g in WRC to 1.4 J/g in CC, and disappeared in EXC. Firmness of pellets varied from 1.1 N in the EXC diet to 2.8 N in the WRC diet. A significant negative correlation between transition enthalpy and carbohydrate digestibility was found. Dry matter, protein, carbohydrate and lipid digestibility of cowpea meals significantly increased after germinating, cooking or extruding. It is concluded that germinated, cooked and extruded cowpea meals are highly digestible for shrimp and that enthalpy of transition is negatively correlated with the digestibility of carbohydrates.
Key words: Cowpea meals, digestibility, feedstuff, shrimp feeds.
RESUMEN
Se evaluó el efecto de diferentes procesos sobre las propiedades fisicoquímicas y digestibilidad aparente de la harina de frijol yorimón (Vigna unguiculata) como ingrediente en alimentos para camarón blanco (Litopenaeus vannamei). Se elaboraron cinco harinas de frijol yorimón: entero crudo (WRC), decorticado (DC), cocido (CC), germinado (GC) y extruido (EXC). Las características térmicas de las harinas fueron evaluadas usando calorimetría diferencial de barrido. Se elaboraron seis alimentos experimentales: un alimento control y cinco alimentos conteniendo 15% de las diferentes harinas de frijol yorimón. A estos alimentos se le determinó firmeza y digestibilidad in vivo de nutrientes para L. vannamei (15.4 g) usando óxido de cromo como marcador inerte. La entalpía de transición decreció después del tratamiento térmico, de 6.1 J/g en la WRC a 1.4 J/g en la CC, y desapareció en la EXC. La firmeza de los alimentos varió de 1.1 N en el alimento con EXC a 2.8 N en el alimento con WRC. Se encontró una correlación significativa negativa entre la entalpía de transición y la digestibilidad de carbohidratos de la harina del frijol yorimón. La digestibilidad de materia seca, proteína, carbohidratos y lípidos de las harinas de frijol yorimón aumentó significativamente con el germinado, la cocción y la extrusión. En el presente estudio se concluye que las harinas de frijol yorimón germinado, cocido y extruido son altamente digeribles para camarón L. vannamei, y la entalpía de transición se correlaciona significativamente con la digestibilidad de los carbohidratos.
Palabras clave: Alimento camarón, digestibilidad, harinas frijol yorimón, ingredientes.
INTRODUCTION
The sustainable development of aquaculture favors ingredients that allow the elaboration of low–cost environmentally friendly balanced feeds. Diverse vegetable ingredients for elaboration of balanced feeds for shrimp have been evaluated (Kumaraguru et al., 2006; Amaya et al., 2007, Venero et al., 2008). In aquaculture, cowpea has been identified as a high quality ingredient in diets for species such as the shrimp Penaeus monodon and tilapia (Oreochromis niloticus Linnaeus, 1758) (Eusebio, 1991; Keembiyehetty & De Silva, 1993; Olvera–Novoa et al., 1997; Kumaraguru et al., 2006). Cowpea has been used as protein source; it contains considerable proportion of carbohydrates, which can be used as energy by shrimp. It is known that carbohydrates are the most economical source of dietary energy. Carbohydrate digestibility in shrimp varies according to source and degree of gelatinization of starch (Davis & Arnold, 1993). Starch is the major component in carbohydrate–rich legumes seeds (Yáñez–Farías et al., 1997). Starch gelatinization is an important process that occurs during food processing operations such as cooking and extrusion (Biliaderis et al., 1980). Some studies have reported that starch gelatinization increases carbohydrate digestibility in L. vannamei (Davis & Arnold, 1993; Cousin et al., 1996). Rivas–Vega et al. (2006) reported that thermal processing, such as cooking and extruding, improved the nutritional quality of cowpea meals in diets for L. vannamei, and suggested this could be related to higher starch gelatinization of these meals. A rapid method to evaluate starch gelatinization is the Differential Scanning Calorimetry. This method could be used to evaluate the quality of ingredients for aquaculture feeds.
The objective of this research was to determine the effect of different feed processing methods on the physicochemical properties of cowpea meals and the apparent digestibility of nutrients in L. vannamei, and the relationship among these parameters.
MATERIALS AND METHODS
Experimental cowpea meals. Cowpea beans (V. unguiculata) were obtained from Sierra de Alamos, Sonora, Mexico. Whole raw cowpea (WRC) was subjected to different processes as described by Rivas–Vega et al. (2006):
1) Dehulled (DC) in a Strong & Scott 17810MR, Chicago, IL, USA, dehulling machine;
2) Cooked (CC), by soaking beans in distilled water (1:10 cowpea: water (w/v)) during 105 minutes at room temperature, boiled for 20 minutes and dried in a convection oven at 40°C for 24 hours;
3) Germinated (GC) on humid filter paper in a germination chamber (Biotronette Mark III, Lab–LineMR) at 33°C and 50% relative humidity for 3 days in complete darkness, then dried in a convection oven at 40°C for 24 hours; and,
4) Extruded (EXC) in a single screw extruder (Brabender GmbH & Co., Duisburg, Germany) with temperature of 80°C at entrance and of 180°C at exit, using 1000–1200 kPa of pressure. Material was fed into the conditioner at a rate of 25 kg/h.
The different cowpea products obtained were milled in a pulverizer (PULVEXMR 200, México, D.F.), sifted through 250 μm mesh sieve, and stored at 4 °C until used.
Formulation and elaboration of diets. A control diet containing 34% protein, 8% lipids, and 1% Cr2O3 (used as indirect marker for in vivo digestibility determinations) was formulated. Five experimental diets containing 84% of the control diet, 15% of cowpea test meals, and 1% Cr2O3 were also formulated (Table 1). Prior to preparing the experimental diets, all ingredients were pulverized and sieved through a 250 μm mesh sieve. The dry ingredients of each diet were mixed thoroughly in a food mixer before a mixture of fish oil and soybean lecithin was added. Water was added at approximately 40% of the total "as is" ingredient weight, and mixed. The resulting mixture was pressure pelleted using a meat grinder and a 2 mm die. The pellets were dried in a convection oven at 45 °C for 12 hours.
Differential Scanning Calorimetry (DSC). The phase transition temperatures and enthalpies of cowpea meals were measured using a differential scanning calorimeter 1020 Series DSC7 (Perkin–Elmer, Norwalk, Connecticut). The instrument was calibrated using Indium and Zinc as standards. The cowpea meals were weighed (5–15 mg, wet weight) and distilled water was added (200% w/w) in DSC hermetic pans (PE No. 0319–0218); three replicates by treatment were used. Determinations of transition temperatures were run at a heating rate of 10°C/min, from 26°C to 145°C. An empty pan was used as a reference. Enthalpy change (ΔH, J/g) was determined, measuring the area under the curve of the thermogram using the 1022 Series Thermal Analysis software from Perkin Elmer. The maximum transition temperature of the peaks was recorded.
Apparent digestibility trial. Juvenile white shrimp L. van–namei with a mean weight of 15.4 g ± 0.9 g were stocked in 60 L rectangular tanks (58 × 48 × 25 cm) at a density of 5 shrimp/tank. Three replicate tanks were randomly assigned for each diet. Shrimps were maintained in filtered seawater at 27.1 ± 0.01°C temperature, 39.7 ± 0.00 mg/L salinity, and 4.4 ± 0.01 mg/L dissolved oxygen. Shrimps were fed ad libitum three times daily during 7 days with the experimental diets before beginning faeces collection. Uneated feed and faeces were removed from tanks before the shrimp were given an initial feeding. Faeces were collected three time at day, one hour after each feeding, faecal strands were collected by siphoning (including faeces from the first feeding), gently rinsed with distilled water, and frozen at –80°C. At the end of the trial, collected faeces from each tank were pooled and freeze–dried. Diets and fecal samples were analyzed for crude protein (AOAC, 1990), carbohydrates (Dreywood, 1946), lipids (Folch–Less & Sloane–Stanley, 1957) and chromic oxide (Olvera–Novoa, 1994). Fifty mg samples were digested in 5 mL of nitric acid, and later in perchloric acid at 300°C until a red ring in the surface of the solution appeared. After digestion, 25 mL of distilled water were added, then absorbance was read at 350 nm. Apparent Digestibility Coefficients (ADC) for dry matter and nutrients in the diets were determined according to Cho et al. (1982) by using the following equations:
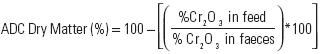
Apparent Digestibility Coefficients of Ingredients (ADCI) were calculated based on the percentage substitution of the test ingredient (Forster, 1999) by using the following equation:
Where:
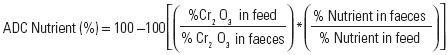
a= Nutrient contribution of reference diet to nutrient content of test diet= (level of nutrient in reference diet)*(100–i).
b= Nutrient contribution of test ingredient to nutrient content of test diet= (level of nutrient in test ingredient)*i.
i= Level of test ingredient in test diet.

Pellet firmness. The method 66–50 of the American Association of Cereal Chemists was used to evaluate pellet firmness. Analysis was conducted after 30 minutes of soaking pellets in distilled water. The maximum cutting force was measured using a Texturometer Instron 4465 (Instron Corporation, Canton, MA, USA). Force was applied using a 1 mm thick knife and a crosshead speed of 1 mm/min, with 50% deformation of the pellet diameter. Pellets 2.75 mm diameter and 5.91 mm long were used.
Statistical analysis. Apparent Digestibility Coefficients of ingredients were analyzed using non–parametric Kruskal Wallis test to determine significant differences among treatments, and a Newman–Keuls multiple range test was used to identify differences among means. Calorimetric data were analyzed using one–way ANOVA to determine significant differences among treatments, and a Tukey's multiple range test was used to identify differences among means. A regression analysis of Apparent Digestibility Coefficients of carbohydrates and transition enthalpy of cowpea meals was conducted. All statistical analyses were performed at 0.05 significance level using STATISTICATM 7.0 (StatSoft, Inc., Tulsa, OK, USA).
RESULTS AND DISCUSSION
Calorimetric methods have been applied to study the structure and phase transitions of starch in pure and complex food systems. The presence of ordered chain domains and the interactions between starch and food constituents can be probed by DSC, trough changes in the heat flow, while the sample is heated over a range of temperatures (Biliaderis, 1992). This is a thermoanalytical technique in which the difference in the amount of heat required to increase the temperature of a sample and reference are measured as a function of temperature (Biliaderis et al., 1980).Thermograms of cowpea meals obtained by differential scanning calorimetry (Figure 1) showed a first peak or transition, commonly known as starch gelatinization. Whole raw (WRC), dehulled (DC) and germinated (GC) cowpea meals showed a maximum temperature of the first transition between 81–82°C. Transition temperature of cooked (CC) cowpea meal was 61.8°C. Transition temperature in extruded cowpea (EC) meal was not detected (Table 2). The results coincide with Henshaw et al., (2003); they found a maximal temperature of the first transition between 78.1–82.2°C for 12 cowpea varieties. However, Phaseolus vulgaris and Lens esculenta show transition temperature of 74.9°C and 63.8°C, respectively, without heat treatment (Yáñez–Farías et al., 1997). It has been suggested that differences in transition temperature of starches are due to differences in shape and size of starch granules, amylose content and internal molecular arrangement of starch fractions within the granule (Yáñez–Farías et al., 1997).
Cowpea meal is a heterogeneous system, where the major macromolecules (starch and protein) contribute to the heat changes. Enthalpy may be better designated as overall transition enthalpy encompassing all heat changes associated with components in the system capable of thermal transition (Henshaw et al., 2003). Enthalpic changes of the first transition significantly diminished after food processing from 6.1 J/g for raw cowpea to 4.3 J/g for dehulled cowpea, and 2.5 J/g for germinated cowpea. Considering that starch is the major component of cowpea meal, this is explained as the energy needed (fusion enthalpy) to break the intermolecular bonds in starch granules to achieve gelatinization is lower, indicating that the native starch content has been reduced after dehulling and germination, since there is a smaller number of intermolecular connections to be broken in the starch chains. During dehulling, the speed of the abrasive disks increases the system temperature, causing changes in the cowpea components. During the germination process conducted under conditions of high humidity, amylases act on the starch components (Mayer & Poljakoff–Mayber, 1982). This process reduces the number of intermolecular bonds in starch, causing a reduction in the energy required to transition.
The first transition temperature detected in cooked cowpea beans was approximately 20°C lower than that of raw cowpea beans. This can be interpreted as gel fusion in the crystallized starch (Biliaderis, 1992). Once the gelatinized starch cools down, structural changes in the gel causes crystallization. The change of enthalpy of this process was 1.4 J/g. Extruded cowpea beans transition temperature was not detected since the starch was completely gelatinized under the conditions of temperature and humidity used during the extrusion.
The endothermic transition temperatures of second peak in the samples of dehulled and germinated cowpea were 93.7 and 95°C, respectively. The amylose molecule coils in a helix form, and it can form occlusion complexes between lipids and carbohydrates. Applying a temperature gradient modifies the starch molecular structure, thus allowing the formation of occlusion complexes. Osman–Ismail (1972) found that formation of occlusion complexes occur at a range of temperature between 23 to 85°C, and temperature at which these occlusion complexes occur depends on the type of starch and of volatile compounds. In this study, the formation of the occlusion complex detected in the thermal analysis could have occurred after increase of system temperature during the dehulling process (Russell & Juliano, 1983).
No significant differences in temperature, salinity and dissolved oxygen were found among treatments in the digestibility trial. Temperature was maintained within the optimum range of 25 to 28°C (Lee & Wickins, 1992; Clifford, 1994). Dissolved oxygen was maintained above the lower limit (3 mg/L) recommended for shrimp culture (Boyd, 1989; Fast & Lester, 1992).
The dry matter digestibility of the raw whole cowpea meal was 76.5%, and significantly increased after cooking, germination and extrusion processes (104.7, 103.1 and 97.1%, respectively) (Table 3). Protein and lipid digestibility of the cowpea meals also increased by cooking, germination and extrusion. The digestibility of carbohydrates increased after germinating, dehulling, cooking and extruding. Assuming that the trypsin inhibitor in cow pea decreases digestibility then the increase in apparent digestibility due to cooking and extrusion may be due to decreased trypsin inhibitor activity, and also to the loss of protein and starch original configuration, which facilitates the enzymatic hydrolysis occurring during shrimp digestive processes. Ghavidel & Prakash (2007) found a significant negative correlation between in vitro starch digestibility and antinutritional factors of germinated and dehulled cowpea meals. Rivas–Vega et al. (2006) found that trypsin inhibitor activity reduced after cooking and extruding cowpea beans. On the other hand, dry matter, protein and carbohydrate digestibility of diets containing these meals was improved (Rivas–Vega et al., 2006).
Some studies have reported that starch gelatinization improves digestibility of carbohydrate by L. vannamei (Davis & Arnold, 1993; Cousin et al., 1996). In the present study, a significant correlation (R2 = 0.93) between the enthalpy change of the first transition and in vivo carbohydrate digestibility of cowpea meals was observed (Figure 2). These results provide important information to consider Differential Scanning Calorimetry as a rapid and effective method to predict carbohydrate digestibility of ingredients used in diets for shrimps. Another advantage of this method is that the thermal characteristics of the samples can be evaluated in situ. It is important to highlight that our results were obtained from the same legume species using different technological processes, but it is important to evaluate the Differential Scanning Calorimetry on different sources of carbohydrates, and to test the sensibility of this method to predict in vivo digestibility.
The apparent digestibility coefficients of dehulled, cooked, germinated and extruded cowpea meals, in some cases, were greater than 100%. Physiologically this cannot be explained, but similar results have been reported in different studies on the digestibility of plant ingredients by shrimp (Brunson et al., 1997, Divakaran et al., 2000; Cruz–Suárez et al., 2001). Some authors attribute it to an interaction between the ingredients of the feed. Divakaran et al. (2000) suggested that dietary inclusion levels of soybean meal (35 and 46.3%) can affect the passage of chromium oxide through the digestive tract of L. vannamei, since they found a significant interaction (p<0.05) between these two inclusion levels. Brunson et al. (1997) obtained values of 101, 110 and 107% for dry matter, protein and energy digestibility of wheat gluten for P. setiferus, and attributed it to possible interactions between the nutrients of the ingredients.
Firmness of pellets, determined after 30 minutes of soaking, varied from 1.1 N for the EXC diet to 2.8 N for the WRC diet (Figure 3). Although pellet texture is an important factor for feed consumption by shrimp (Cruz–Suárez, 1998), very little information exists about this property. Cerecer–Cota et al. (2004) reported that feed firmness is negatively correlated to feed consumption in L. vannamei. Feed consumption was not measured in our study, but no significant correlation between pellet firmness and in vivo digestibility of cowpea meals in L. vannamei was found.
CONCLUSIONS
Cowpea meals were highly digestible for L. vannamei. Carbohydrate digestibility increased after germinating, dehulling, cooking and extruding. Temperature and enthalpy of the first transition of cowpea meals decreased after food processing, especially after thermal processing. The carbohydrate digestibility could reasonably be predicted based on the first transition enthalpy.
ACKNOWLEDGEMENTS
This work was possible thanks to financial support of CONACYT (Grant No. 129253 to Martha Rivas), CIBNOR (project AC 1.22) and SAGARPA (project 2003–CO2–149). We thank Rosalina Ramírez (Universidad de Sonora) for comments and suggestions to the manuscript, Juan Manuel Vargas López (Universidad de Sonora) for extruding cowpea, and Ana María Ibarra and José Luis Ramírez (Shrimp Genetic Improvement Program–CIBNOR) for providing the experimental organisms.
REFERENCES
Amaya, E., D. A. Davis & D. B. Rouse. 2007. Alternative diets for the Pacific white shrimp Litopenaeus vannamei. Aquaculture 262: 419–425. [ Links ]
AOAC INTERNATIONAL. 1990. Official methods of analysis. 15th Ed. Association of Official Analytical Chemistry, Washington, D.C. 1094 p. [ Links ]
Biliaderis, C. G. 1992. Structures and phase transitions of starch in food systems. Food Technology 46: 98–109. [ Links ]
Biliaderis, C. G., T. J. Maurice & R. Vose. 1980. Starch gelatinization phenomena studied by differential scanning calorimetry. Journal of Food Science 45: 1669–1674. [ Links ]
Boyd, C. 1989. Water quality management and aeration in shrimp farming. Fisheries and Allied Aquaculture, Departmental Series # 2. Auburn University, Alabama. [ Links ]
Brunson, J. F., R. P. Romaire & R. C. Reigh. 1997. Apparent digestibility of selected ingredients in diets for white shrimp Penaeus setiferus L. Aquaculture Nutrition 3: 9–16. [ Links ]
Cerecer–Cota, E. E., D. Ricque–Marie, B. Ramírez–Wong, M. G. Salazar–García, M. Velasco–Escudero & L. E. Cruz–Suárez. 2004. Correlación del consumo del alimento peletizado con la dureza del pellet en alimentos con diferente aglutinante. In: Cruz Suárez, L.E., Ricque Marie, D., Nieto López, M.G., Villarreal, D., Scholz, U. & González, M. (Eds.) Avances en Nutrición Acuícola VII. Memorias del VII Simposium Internacional de Nutrición Acuícola. November 16–19 2004, Hermosillo, Sonora, México. pp. 84. [ Links ]
Cho, C. y., S. J. Slinger & H. S. Bayley. 1982. Bioenergetics of salmonid fishes: energy intake, expenditure and productivity. Comparative Biochemistry and Physiology 73B: 24–41. [ Links ]
Clifford, H. C. 1994. Semi–intensive sensation: a case of study in marine shrimp management. World Aquaculture, 25, 6–12, 98–104. [ Links ]
Cousin, M., G. Cuzon, J. Guillaume & Aquacop. 1996. Digestibility of starch in Penaeus vannamei: in vivo and in vitro study on eight samples of various origins. Aquaculture 40: 361–372. [ Links ]
Cruz–Suárez, L. E. 1998. Digestión en camarón y su relación con formulación y fabricación de alimentos balanceados.. In: Cruz–Suárez, L.E., Ricque–Marie, D. & Mendoza, R. (Eds.) Avances en Nutrición Acuícola III. Memorias del Tercer Simposium Internacional de Nutrición Acuícola, 11–13 de Noviembre de 1996. Monterrey, Nuevo León, México. pp. 207–232. [ Links ]
Cruz–Suárez, L. E., D. Ricque–Marie, M. Tapia–Salazar, I. M. McCallum, & D. Hickling. 2001. Assessment of differently processed feed pea (Pisum sativum) meals and canola meal (Brassica sp.) in diets for blue shrimp (Litopenaeus stylirostris). Aquaculture 196: 87–104. [ Links ]
Davis, D. A. & C. R. Arnold. 1993. Evaluation of five carbohydrate sources for Penaeus vannamei. Aquaculture 114: 285–292. [ Links ]
Divakaran, S., M. Velasco, E. Beyer, I. Forster & A. Tacon. 2000. Soybean meal apparent digestibility for Litopenaeus vannamei, including a critique of methodology. In : Cruz–Suárez, L.E., D. Ricque–Marie, M. Tapia–Salazar, M.A. Olvera–Novoa y R.Civera–Cerecedo. (Eds). Avances en Nutrición Acuícola V. Memorias del V Simposium Internacional de Nutrición Acuícola. 19–22 noviembre, 2000. Mérida, Yucatán, México. [ Links ]
Dreywood, R. 1946. Qualitative test for carbohydrate material. Industrial & Engineering of Analytical Chemistry, Edmonton 18: 499–505. [ Links ]
Eusebio, P. S. 1991. Effect of dehulling on the nutritive value of some leguminous seeds as protein sources for tiger prawn, Penaeus monodon, juveniles. Aquaculture 99: 297–308. [ Links ]
Fast, W. A. & L. J. Lester. 1992. Marine shrimp culture: principles and practices. Developments in aquaculture and fisheries science, volume 23. Elsevier Science Publishers B.V. New York, USA. [ Links ]
Folch–Lees, J. & G. H. Sloane–Stanley. 1957. A simple method for the isolation and purification of total lipid from animal tissues. Journal of Biological Chemistry 26: 497–509. [ Links ]
Ghavidel, R. A. & J. Prakash. 2007. The impact of germination and dehulling on nutrient, antinutrients, in vitro iron and calcium bioavailability and in vitro starch and protein digestibility of some legume seeds. LWT – Food Science and Technology 40:1292–1299. [ Links ]
Henshaw, F. O., K. H. McWatters, J. O. Akingbala & M. S. Chinnan. 2003. Termal properties of cowpea flour: A study by differential scanning calorimetry. Nahrung/Food 47: 161–165. [ Links ]
Keembiyrhrtty, C. N. & S. S. De Silva. 1993. Performance of juvenile Oreochromis niloticus (L.) reared on diets containing cowpea, Vigna catiang, and black gram, Phaseolus mungo, seeds. Aquaculture 112: 207–215. [ Links ]
Kumaraguru, K. P. , T. Balasubramanian & R. Venkatesan. 2006. Apparent digestibility of differently processed grain legumes, cow pea and mung bean in black tiger shrimp, Penaeus monodon Fabricius and associated histological anomalies in hepatopancreas and midgut. Animal Feed Science and Technology 132: 250–266. [ Links ]
Lee, D. O. & J. F. Wickins. 1992. Crustacean Farming. Halsted Press, Wiley, New York, 392 p. [ Links ]
Mayer, A. M. & A. Poljakoff–Mayber. 1982. The germination of seeds. Pergamon Press. Ltd. U.K. 85–138 p. [ Links ]
Olvera–Novoa, M. A. 1994. Cuantificación de óxido de cromo en heces y alimentos. Nutrition of fish and crustaceans. 1–33 p. In: FAO (Ed.). A Laboratory Manual Project. Field Document 19. Rome: FAO. [ Links ]
Olvera–Novoa. M. A., F. Pereira–Pacheco, L. Olivera–Castillo, V. Perez–Flores, L. Navarro & J. Sámano. 1997. Cowpea (Vigna unguiculata) protein concentrate as replacement for fish meal in diets for tilapia (Oreochromis niloticus) fry. Aquaculture 158: 107–116. [ Links ]
Osman–Ismail, F. 1972. In: Arora S. K. 1983. Chemistry and biochemistry of legumes. Edward Arnold Publishers Ltd. U.K. 64–66 p. [ Links ]
Rivas–Vega, M. E., E. Goytortúa–Bores, J. M. Ezquerra–Brauer, M. G. Salazar–García, L. E. Cruz–Suárez, H. Nolasco & R. Civera–Cerecedo. 2006. Nutritional value of cowpea (Vigna ungui–culata L. Walp) meals as ingredients in diets for Pacific white shrimp (Litopenaeus vannamei Boone). Food Chemistry 97: 41–49. [ Links ]
Russell, P. L. & B. O. Juliano. 1983. Differential scanning calorimetry of rice starches. Starch/Stärke 11: 382–386. [ Links ]
Tacon, A. G. J. 1987. The Nutrition and Feeding of Farmed Fish and Shrimp—A Training Manual. 1. The Essential Nutrients. Food and Agriculture Organization of the United Nations, GCP/RLA/075/ITA, Brazil, 117 p. [ Links ]
Venero, J. A., D. A. Davis & C. Lim. 2008. Use of plant protein sources in crustacean diets. pp. 163–203. In. C. Lim, C. D. Webster and C. S. Lee. Alternative Protein Sources in Aquaculture Diets. The Haworth Press, New York. [ Links ]
Yáñez–Farías, G. A., J. G. Moreno–Valencia, M. R. Falcón–Villa & J. M. Barrón–Hoyos. 1997. Isolation and partial characterization of starches from dry beans (Phaseolus vulgaris) and chickpeas (Cicer arietinum), grown in Sonora, México. Starch/ Stärke 49: 341–345. [ Links ]














