Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Hidrobiológica
versión impresa ISSN 0188-8897
Hidrobiológica vol.17 supl.1 Ciudad de México jul. 2007
Population dynamics of Brachionus calyciflorus and Brachionus havanaensis (Rotifera) on mixed diets with Microcystis aeruginosa and green algae
Dinámica poblacional de Brachionus calyciflorus y Brachionus havanaensis (Rotifera) en una dieta mixta de Microcystis aeruginosa y alga verde
A. F., Alva-Martínez1, S.S.S. Sarma2 and S. Nandini3
1 Doctoral Programme, Universidad Autónoma Metropolitana, Campus Xochimilco, Calzada del Hueso No. 1100, Villa Quietud, C.P. 04960, México City, México. afam99@yahoo.com
2 Laboratorio de Zoología Acuática, División de Investigación y Posgrado, Edificio UMF, Universidad Nacional Autónoma de México, Campus Iztacala, AP 314, CP 54090, Tlalnepantla, Edo. de México. México. FAX: +52 55 5623 1256.
3 UIICSE, División de Investigación y Posgrado, Universidad Nacional Autónoma de México, Campus Iztacala, AP 314, CP 54090, Tlalnepantla, Edo. de México. México.
Recibido: 16 de octubre de 2005
Aceptado: 12 de septiembre de 2006
Abstract
The effect of the cyanobacterium Microcystis aeruginosa was evaluated as a diet, separately and together with one of the two edible algal species (Chlorella vulgaris or Scenedesmus acutus) at different proportions (0, 25, 50, 75 or 100% on the basis of biomass) on the population growth of two rotifers Brachionus calyciflorus and Brachionus havanaensis. Population growth curves of B. calyciflorus and B. havanaensis cultured on M. aeruginosa alone, or in combination with one of the two algal species decreased with increasing proportion of cyanobacteria in the diet. In treatments containing exclusive algal diets (either Chlorella or Scenedesmus) and those with low proportion of Microcystis, B. havanaensis was more abundant than B. calyciflorus. However, both rotifer species died in less than two weeks on an exclusive diet of M. aeruginosa. Peak population densities (mean ± standard error) of B. calyciflorus grown on an exclusive diet of Chlorella or Scenedesmus were 47 ± 6 and 15 ± 1 ind. ml-1, respectively. Corresponding values for B. havanaensis were much higher (203 ± 21 and 187 ± 2 ind. ml-1). Regardless of the rotifer species and the diet type and composition, the population growth rates (r) were inversely related to increasing proportion of M. aeruginosa in the diet.
Key words: Microcystis, algae, Rotifera, population growth.
Resumen
En este trabajo se evaluó a la cianobacteria Microcystis aeruginosa como dieta, por separado y junto con una de dos especies comestibles de algas (Chlorella vulgaris o Scenedesmus acutus) en diferentes proporciones (0, 25, 50, 75 o el 100 % de la biomasa) sobre el incremento poblacional de dos especies de rotíferos. Brachionus calyciflorus y Brachionus havanaensis. Las curvas del incremento poblacional de B. calyciflorus y B. havanaensis empleando M. aeruginosa sola, o en la combinación con una de las dos especies de algas como alimento, disminuyeron con la proporción creciente de la cianobacteria en la dieta. En tratamientos que contienen exclusivamente dietas de algas (Chlorella o Scenedesmus) y aquellas con proporción baja de Microcystis, B. havanaensis fueron más abundantes que B. calyciflorus. Sin embargo, ambas especies de rotíferos murieron en menos de dos semanas con una dieta exclusiva de M. aeruginosa. Las densidades de población máximas (media ± error estándar) de B. calyciflorus cultivado sobre una dieta exclusiva de Chlorella o Scenedesmus fue 47 ± 6 y 15 ± 1 ind. ml-1, respectivamente. Los valores correspondientes para B. havanaensis fueron más altos (203 ± 21 y 187 ± 3 ind. ml-1). Sin tener en cuenta la especie del rotífero y el tipo de dieta y composición, las proporciones de aumento de la población (r) estuvieron inversamente relacionadas con la proporción creciente de M. aeruginosa en la dieta.
Palabras clave: Microcystis, alga, Rotifera, crecimiento poblacional.
Introduction
Microcystis aeruginosa is a highly toxic cyanobacteria affecting various groups of animals ranging from zooplankton to mammals (Whitton & Potts, 2000). In tropical freshwater bodies, Microcystis is common, and at times reaches very high abundances leading to decreased Secchi transparency (< 10 cm), thus preventing the growth of other photosynthetic groups such as green algae (Ha et al., 1999). The inability of zooplankton to feed on Microcystis is generally related to the presence of toxins and its colonial nature. Large sizes of the colonies, up to 1000 µm diameter, often cause mechanical problems for feeding by zooplankton (Nandini, 2000). The presence of toxins is often seasonal and related to zooplankton density (Park et al., 1993). Certain species such as Bosmina longirostris among cladocerans are specialist feeders and can thus avoid the adverse impact of toxic cyanobacteria while others such as Daphnia are generalists and suffer greatly during cyanobacterial blooms (Lampert, 1981; Fulton, 1988). Similar observations on other zooplankton species, especially on rotifers, are rather scarce.
Among freshwater zooplankton, rotifers are often more diverse and numerically more abundant than crustaceans (Nogrady et al., 1993). In addition, Microcystis infected ponds often contain a large number of rotifers but limited number of cladocerans, which suggests some possible coexistence of rotifers with cyanobacteria (Ramírez-García et al., 2002). Alternatively, rotifers similar to copepods may avoid direct consumption of Microcystis but feed on decomposing cyanobacteria (Liu et al., 2002). Laboratory studies, however, have indicated that a few rotifer species indeed feed on cyanobacteria (Nandini & Rao, 1998). Therefore the extent to which some common rotifer species use cyanobacteria as diet remains unclear.
In nature, Microcystis is often the dominant genus in waterbodies, co-occurring with other algal species (Ramírez-García et al., 2002). Moreover, seasonal changes in nature may also contribute varying proportions of Microcystis with edible phytoplankton such as green algae (Gomez & Bauer, 1998). Thus, zooplankton inhabiting Microcystis-dominated waterbodies probably feed on mixed diets (Alva-Martínez et al., 2004). It is known that below threshold levels, microcystin present in Microcystis does not show deleterious effects on zooplankton populations (Vezie et al., 1998). Further, M. aeruginosa also contains fairly good percentage of non-toxic proteins (up to 60%), which may be metabolized by zooplankton provided that microcystin levels are low (Bickel et al., 2000).
Life table demography and population growth studies are often used to evaluate the effects of different toxins on various zooplankton species (Mangas-Ramírez et al., 2004). In life table studies, though it is possible to obtain data related to survival and reproduction in an age-specific manner, the possible role of adaptation of the test population cannot be evaluated simultaneously, since the new born individuals are eliminated from the experimental jars (Krebs, 1985). Population growth studies provide such a possibility. The population growth approach also permits to quantify peak population abundances, which are sensitive indicators in ecotoxicological assessments (García et al., 2004).
Rotifers have been extensively used in several bioassay studies due to their wide distribution, life history characteristics such as a short life-span and high growth rates and the ease of maintenance under laboratory conditions (Snell & Janssen, 1995). Among the various species, Brachionus calyciflorus is recognized by The Society of Environmental Toxicology and Chemistry (SETAC) as a bioassay organism. Studies on Brachionus havanaensis have a more local interest since it is a commonly found rotifer in several Mexican freshwater bodies and has often been documented in the presence of cyanobacterial blooms (Nandini et al., 2005).
While exhaustive information is available on the chemical and the biological nature of microcystin, the main toxic substance in M. aeruginosa (Whitton & Potts, 2000), the role of cyanobacteria in mixed diets together with other edible algae, on the population growth of zooplankton is less well known. It has been documented that when used alone as a diet, M. aeruginosa does not support the population growth of several species of zooplankton including rotifers and cladoceran (Nandini & Rao, 1998). The aim of the present work was to evaluate the role of Microcystis aeruginosa as a diet, separately and together with one of the two edible algal species (Chlorella vulgaris or Scenedesmus acutus) on the population growth of B. calyciflorus and B. havanaensis.
Materials and methods
The rotifers B. calyciflorus and B. havanaensis were originally isolated from the principal Virgilio Uribe Canal, Mexico City. Clonal populations were established using a single parthenogenetic individual from each of the two rotifer species. Mass cultures were obtained using the single-celled Chlorella vulgaris (strain CL-V-3, CICESE, Ensenada, Mexico) or Scenedesmus acutus (Strain No. 72, UTEX, USA) as diets. For experiments as well as for maintaining rotifer mass cultures we used reconstituted moderately hard water (EPA medium). This medium was prepared by dissolving 0.9 g NaHCO3, 0.6 g CaSO4, 0.6g MgSO4 and 0.04g KCl in one liter of distilled water (Weber, 1993). Both C. vulgaris and S. acutus were separately batch-cultured using Bold-basal medium (Borowitzka & Borowitzka, 1988).
For feeding rotifers in mass culture tanks or for the experiments, log phase alga was harvested, centrifuged at 4000 rpm for five minutes and resuspended it in distilled water. The stock algal density was estimated using a haemocytometer from which the chosen food level was derived by diluting with EPA medium.
Microcystis aeruginosa was collected every alternate day from the waterbody Virgilio Uribe in Mexico City (19° 17' 31'' N 99° 06' 14'' W Google earth, 2006) where it was always present. In order to avoid mechanical problems of consumption by the rotifers, we disintegrated the colonies using a sonicator (Branson Sonic Power Co., Dunbury, Connecticut, U.S.A.). In general, we followed the recommended procedures to ensure that no cell lysis occurred and that no colonies were present (Box, 1981; Alva-Martínez et al., 2004). The diameter a single cell of M. aeruginosa was (4.5 ± 0.5 µm); slightly smaller than Chlorella vulgaris (5.5 ± 0.5 µm). The density of sonicated M. aeruginosa was also estimated using haemocytometer.
It is possible to culture M. aeruginosa under laboratory conditions. However, the toxicity of the laboratory cultured M. aeruginosa is often lower than that of field collected cyanobacteria. This is because the toxicity of cyanobacteria is an inducible response to zooplankton grazing (Jang et al., 2003).
The population growth experiments for both rotifer species were conducted simultaneously under similar test conditions (temperature 23 ± 1ºC, pH: 7.1-7.6; continuous but diffused fluorescent illumination and food density of 1.0 X 106 cells ml-1 (dry weight, 5.8 µg ml-1) of Chlorella vulgaris or its equivalent biomass of S. acutus). Microcystis aeruginosa (single-celled form) was also offered at a density of 1.0 X 106 cells ml-1. Algal and cyanobacterial diets were prepared daily following the procedure mentioned above. For each rotifer species 50 ml transparent jars containing 25 ml of medium with chosen diet density and type were used. Three diets were offered to B. calyciflorus or B. havanaensis (C. vulgaris, S. acutus and M. aeruginosa) in different combinations but with similar biomass, based on dry weights (see Alva-Martínez et al., 2004): Chlorella vulgaris only (= 100% C. v.); Scenedesmus acutus only (= 100% S. a.); Microcystis aeruginosa only (= 100% M. a.); 75% C. v. + 25% M. a.; 50% C. v. + 50% M. a.; 25% C. v. + 75% M. a.; 75% S. a. + 25% M. a.; 50% S. a. + 50% M. a. and 25% S.a. + 75% M. a.
For each treatment three replicates were used. Into each of the 54 test jars (2 rotifer species X 3 replicates X 9 diets combinations) containing specified diet type and combination, one of the two rotifer species was introduced at an initial density of 1 ind. ml-1, using a finely drawn Pasteur pipette under a stereomicroscope (SMZ645, Nikon, Japan) at 20X magnification.
Following initiation of the growth experiment, we daily enumerated the rotifers in each test jar either individually or using two to three aliquots of one to five ml, depending on the population density. Following quantification of population density, the population from each jar were transferred to fresh containers with the appropriate diet type and combination. The growth experiments were terminated after three weeks by which time, the rotifer populations in the test jars began to decline.
Peak density and growth rates (r) were obtained using one of the two following equations, depending on the nature of growth curve for each replicate (Krebs, 1985):
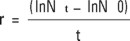
Where Nt and No are the final and initial population densities and t is the time or
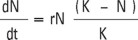
Where, K is the carrying capacity.
For each rotifer species, data of the peak population abundance and the population growth were analysed using the Analysis of Variance (ANOVA) for quantifying the differences among the treatments (Sokal & Rohlf, 2000).
Results
Population growth curves of B. calyciflorus and B. havanaensis fed on M. aeruginosa alone, or in combination with either Chlorella or Scenedesmus (Figs. 1 and 2) showed that in general, increase in the proportion of cyanobacteria in the diet resulted in decreased population growth of the rotifer species. In treatments containing exclusive algal diets (either Chlorella or Scenedesmus) and those with low proportion of Microcystis, B. havanaensis was more abundant than B. calyciflorus. However, both the rotifer species died in less than two weeks on an exclusive diet of M. aeruginosa.
Peak population densities (mean ± standard error) of B. calyciflorus grown on an exclusive diet of Chlorella or Scenedesmus were 47 ± 6 and 15 ± 1 ind. ml-1, respectively. Corresponding values for B. havanaensis were much higher (203 ± 21 and 187 ± 2 ind. ml-1). Regardless of the rotifer species and the diet type and composition, the population growth rates (mean ± standard error) varied from + 0.36 ± 0.01 to -0.27 ± 0.02 d-1. For both rotifer species there was an inverse linear relationship between a greater proportion of M. aeruginosa in the diet and the growth rate (Fig. 3). With 75% or 100% of M. aeruginosa in the diet neither test species showed an increase in their population density.
Statistically, peak abundances and the rates of population increase of both the rotifer species were significantly affected by the diet composition (P<0.01, one-way ANOVA, Table 1).

Discussion
Microcystis aeruginosa used here is toxic to zooplankton as has been shown by Alva-Martínez et al., (2004). This was also evident in this work since neither B. calyciflorus nor B. havanaensis grew when cultured exclusively on M. aeruginosa. Ideally separation and quantification of microcystin from M. aeruginosa would permit toxicity evaluations with greater precision. However, these procedures are expensive and are not feasible in many laboratories. In the present work, we did not quantify microcystin. However, Lampert (1981) has mentioned an indirect method of distinguishing toxic and non-toxic strains of M. aeruginosa. If the population of zooplankton species dies within 72 hours when exposed to cyanobacterial diet while the same does not experience mortality for the same duration in a medium free from any diet, then the cyanobacterial strain in question must be treated as toxic. In the present work, mortalities of the test zooplankton species were observed when exposed to 100% M. aeruginosa. Further, an increase in the concentration of M. aeruginosa in the diet, also resulted in lower growth of both rotifer species. M. aeruginosa is toxic to many species of rotifers: Keratella (Sartonov, 1995), B. calyciflorus (Nandini & Rao, 1998); Hexarthra mira (Nandini & Rao 1998) and B. havanaensis (present work). However, when combined with an edible alga, there is some possibility of its assimilation without being adversely affected by its toxins. For example, Alva-Martínez et al., (2004) have cultured Daphnia pulex using mixed diet of M. aeruginosa with green algae. They found that the addition of M. aeruginosa at a low level (25% on the basis of biomass) to the algal diet actually enhanced the population growth of D. pulex than when grown on algae only. In the present study, it was not observed improved population growth of either rotifer species when grown on mixed diets containing M. aeruginosa and algae, when compared to those grown on algae only. However, inclusion of algae with M. aeruginosa allowed better population growth of B. calyciflorus as well as B. havanaensis, as compared to those cultured exclusively on the cyanobacteria.
Most brachionid species generally complete one population cycle (initial log phase, exponential phase and the stabilization or the retardation stages) in less than 3 weeks. When cultured on green algae at 20-25 ºC, B. calyciflorus usually reaches peak population in less than two weeks, while B. havanaensis may need a few days more (Pavón-Meza et al., 2004). However, when cultured on diets containing toxic substances or in the presence of toxicants such as pesticides and heavy metals, the pattern of population growth changes considerably (Alva-Martínez et al., 2004; García et al., 2004). These changes are reflected in one or more of the following characteristics: a) enhanced lag period, b) lower peak abundances and c) declined population growth rates (Mangas-Ramírez et al., 2004). In the present study, we observed all of them in many treatments, especially for B. havanaensis.
In the population growth studies, peak population abundance and the rate of population increase are among the important variables greatly influenced by the presence of toxic substances, either in the medium or in the diet (Sarma et al., 2005). Peak population densities indicate the abundances that can be supported by the given test conditions. Decrease in survival and reproduction of a zooplankton species is eventually reflected in peak abundances (Krebs, 1985). In addition, peak abundances also sum up, if any, the role of adaptation of the test populations during the experimental period. For example, when B. calyciflorus and B. havanaensis were exposed to M. aeruginosa (with and without the presence of algae), reproduction did occur. Thus, the individuals born during the experimental period may have adapted to the test conditions. If individuals of different generations and age groups of a species adapt to test conditions, then positive population growth rates may result. From the data we gathered for B. calyciflorus and B. havanaensis, both these rotifer species failed to adapt to conditions of an exclusive diet of M. aeruginosa or when present it was present in a high proportion (75% on the basis of biomass). The adaptation of zooplankton to M. aeruginosa apparently differs among different species. For example, in certain cladoceran species: Ceriodaphnia cornuta (Nandini, 2000); Daphnia magna (Gustafsson & Hansson, 2004), there is some tendency of adapting to a diet of M. aeruginosa while in others (e.g., D. pulex: Alva-Martínez et al., 2004), it is not apparent. The occurrence of both B. calyciflorus and B. havanaensis in waterbodies infected with M. aeruginosa (Nandini et al., 2005), suggests possible utilization of some cyanobacteria by these rotifer species, though our study could not establish this. This may have been because the duration of exposure to M. aeruginosa was not long enough as it was in the case of D. pulex (Alva-Martínez et al., 2004).
The rate of population increase in Brachionus spp. when grown on green algal diets varies from 0.12 to 1.5 d-1, depending on the food concentration and temperature (Sarma et al., 2001). In the present study, in treatments containing exclusive algal diets, the population growth rates of both the rotifer species were within the range reported in literature under comparable test conditions. Negative (r) values are common for species under toxicant stress as shown in many organisms (reviewed in Forbes & Calow, 1999). B. calyciflorus and B. havanaensis had negative growth rates when the diet contained M. aeruginosa only a high proportion of it (75%) in mixed diets with algae. Decrease in (r) values of both the rotifers species with increasing proportion of M. aeruginosa clearly reflected the use of this parameter as a sensible variable in ecotoxicological studies (Forbes & Calow, 1999).
In conclusion, the data of the present study showed that both B. calyciflorus and B. havanaensis did not grow well when M. aeruginosa was employed as an exclusive diet or together with green algae at high proportion. It also suggests the possible use of M. aeruginosa (at low proportion (25%) together with an edible algae) as a diet for growing B. calyciflorus and B. havanaensis; but priority it should be confirmed that the accumulated cyanotoxins, if any, have no long term deleterious effects on these rotifer species.
Acknowledgements
We are grateful to Maria Elena Castellanos Páez and María de Jesús Ferrara Guerrero UAM-Xochimilco for discussion during the preparation of this manuscript. We are also thankful to three anonymous reviewers for improving our presentation. AFAM thanks CONACyT for a doctoral scholarship (Ref. 176190).
References
ALVA-MARTÍNEZ, A.F., S.S.S. SARMA & S. NANDINI. 2004. Population growth of Daphnia pulex (Cladocera) on a mixed diet (Microcystis aeruginosa with Chlorella or Scenedesmus). Crustaceana 77: 973-988. [ Links ]
BICKEL, H., S. LYCK & H. UTKILEN. 2000. Energy state and toxin content experiments on Microcystis aeruginosa (Chroococcales, Cyanophyta). Phycologia 39: 212-218. [ Links ]
BOROWITZKA, M.A. & L.J. BOROWITZKA. 1988. Microalgal biotechnology. Cambridge University Press, United Kingdom, 477 pp. [ Links ]
BOX, J.D. 1981. Enumeration of cell concentrations in suspensions of colonial freshwater microalgae, with particular reference to Microcystis aeruginosa. Brazilian Phycological Journal 16: 153-164. [ Links ]
FORBES, V.E. & P. CALOW. 1999. Is the per capita rate of increase a good measure of population-level effects in ecotoxicology? Environmental Toxicolology and Chemistry 18: 1544-1556. [ Links ]
FULTON, R.S. III. 1988. Resistance to blue-green algal toxins by Bosmina longirostris. Journal of Plankton Research 10: 771-778. [ Links ]
GARCÍA, G.G., S. NANDINI & S.S.S. SARMA 2004. The effect of cadmium on the population dynamics of Moina macrocopa and Macrothrix triserialis (Cladocera). Bulletin of Environmental Contamination and Toxicology 72: 717-724 [ Links ]
GOMEZ, N. & D.E. BAUER. 1998. Phytoplankton from the Southern Coastal Fringe of the Rio de la Plata (Buenos Aires, Argentina). Hydrobiologia 380: 1-8. [ Links ]
GUSTAFSSON, S. & L.A. HANSSON. 2004. Development of tolerance against toxic cyanobacteria in Daphnia. Aquatic Ecology 38:37-44 [ Links ]
HA, K., E.A. CHO, H.W. KIM & G.J. JOO. 1999. Microcystis bloom formation in the lower Nakdong River, South Korea: Importance of hydrodynamics and nutrient loading. Marine and Freshwater Research 50: 89-94. [ Links ]
JANG, M.H., K. HA, , G.J. JOO, & N. TAKAMURA, 2003. Toxin production of cyanobacteria is increased by exposure to zooplankton. Freshwater Biology 48: 1540-1550. [ Links ]
KREBS, C.J. 1985. Ecology: The Experimental Analysis of Distribution and Abundance. Third Edition. Harper and Row, New York, 800 pp. [ Links ]
LAMPERT, W. 1981. Inhibitory and toxic effects of blue-green algae on Daphnia. Internationale Revue gesamten Hydrobiologie 66: 285-298. [ Links ]
LIU, H., P. XIE, F. CHEN, H. TANG & L. XIE. 2002. Enhancement of planktonic rotifers by Microcystis aeruginosa blooms: An enclosure experiment in a shallow eutrophic lake. Journal of Freshwater Ecology 17: 239-248. [ Links ]
MANGAS-RAMÍREZ, E., S.S.S. SARMA & S. NANDINI. 2004. Recovery patterns of Moina macrocopa exposed previously to different concentrations of cadmium and methyl parathion: life table demography and population growth studies. Hydrobiologia 526: 255-265. [ Links ]
NANDINI, S. & T.R. RAO. 1998. Somatic and population growth in selected cladoceran and rotifer species offered the cyanobacterium Microcystis aeruginosa as food. Aquatic Ecology 31: 283-298. [ Links ]
NANDINI, S. 2000. Responses of rotifers and cladocerans to Microcystis aeruginosa (Cyanophyceae): A demographic study. Aquatic Ecology 34: 227-242. [ Links ]
NANDINI, S., S.S.S. SARMA & P. RAMÍREZ-GARCÍA. 2005. Seasonal variations in the species diversity of planktonic rotifers in Lake Xochimilco, Mexico. Journal of Freshwater Ecology 20: 287-294. [ Links ]
NOGRADY, T., R.L. WALLACE & T.W. SNELL. 1993. Rotifera. SBP Academic Publishers, The Hague, 142 p. [ Links ]
PARK, H.D., M.F. WATANABE, K.I. HARADA, M. SUZUKI, H. HAYASHI & T. OKINO. 1993. Seasonal variations of Microcystis species and toxic heptapeptide microcystins in Lake Suwa. Environmental Toxicology and Water Quality 8: 425-435. [ Links ]
PAVÓN-MEZA, E.L., S.S.S. SARMA & S. NANDINI. 2004. Combined effects of food (Chlorella vulgaris) concentration and temperature on the population growth of Brachionus havanaensis (Rotifera: Brachionidae). Journal of Freshwater Ecology 19: 521-530. [ Links ]
RAMÍREZ-GARCÍA, P., S. NANDINI, S.S.S. SARMA, E. ROBLES-VALDERRAMA, I. CUESTA & D. HURTADO-MARIA. 2002. Seasonal variations of zooplankton abundance in the freshwater reservoir Valle de Bravo (Mexico). Hydrobiologia 467: 99-108. [ Links ]
SARMA, S.S.S., P.S. LARIOS-JURADO & S. NANDINI. 2001. Effect of three food types on the population growth of Brachionus calyciflorus and Brachionus patulus (Rotifera: Brachionidae). Revista de Biología Tropical 49: 75-82. [ Links ]
SARMA, S.S.S, H.F. NUÑEZ-CRUZ & S. NANDINI. 2005. Effects on the population dynamics of Brachionus rubens (Rotifera) caused by mercury and cadmium administered through medium and algal food (Chlorella vulgaris). Acta Zoologica Sinica 51: 46-52. [ Links ]
SARTONOV, A. 1995. Effects of Microcystis aeruginosa on interference competition between Daphnia pulex and Keratella cochlearis. Hydrobiologia 307: 117-126. [ Links ]
SNELL, T.W. & C.R. JANSSEN. 1995. Rotifers in ecotoxicology: A review. Hydrobiologia 313/314: 231-247. [ Links ]
SOKAL, R.R. & F.J. ROHLF. 2000. Biometry. W. H. Freeman and Company, San Francisco, 887 pp. [ Links ]
VEZIE, C., L. BRIENT, K. SIVONEN, G. BERTRU, J.C. LEFEUVRE & M. SALKINOJA-SALONEN. 1998. Variation of microcystin content of cyanobacterial blooms and isolated strains in Lake Grand-Lieu (France). Microbial Ecology 35: 126-135. [ Links ]
WEBER, C.I. 1993. Methods for measuring the acute toxicity of effluents and receiving waters to freshwater and marine organisms. 4th ed. United States Environmental Protection Agency, Cincinnati, Ohio, EPA/600/4-90/027F, 293 p. [ Links ]
WHITTON, B.A. & M. POTTS. 2000. The ecology of cyanobacteria: Their diversity in space. Kluwer Academic Press, Dordrecht, The Netherlands, 704 p. [ Links ]














