Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Hidrobiológica
versión impresa ISSN 0188-8897
Hidrobiológica vol.17 no.3 Ciudad de México dic. 2007
Artículos
Growth and accessory pigments to chlorophyll a ratios of Thalassiosira pseudonana (Bacillariophyceae) cultured under different irradiances
Crecimiento y proporciones de pigmentos accesorios/clorofila a de Thalassiosira pseudonana (Bacillariophyceae) cultivada bajo diferentes irradianzas
Enrique Valenzuela-Espinoza1, Roberto Millán-Núñez2*, Charles C. Trees3, Eduardo Santamaría-del-Ángel2 and Filiberto Núñez-Cebrero1
1 Instituto de Investigaciones Oceanológicas.
2 Facultad de Ciencias Marinas. Universidad Autónoma de Baja California. Apartado postal 453, Ensenada, Baja California, México. *Corresponding author. e-mail address: rmillan@uabc.mx
3 Center for Hydro-Optics and Remote Sensing. San Diego State University. San Diego, California 92120.
Recibido: 8 de enero de 2007
Aceptado: 14 de septiembre de 2007
Resumen
Se estudió el efecto de diferentes irradianzas en el crecimiento y proporción de pigmentos accesorios/clorofila a en Thalassiosira pseudonana cultivada durante cinco días bajo cuatro irradianzas (50, 150, 300 y 750 µmol quanta m-2 s-1) en medio f/2. Se determinó diariamente el crecimiento y la composición de pigmentos para cada tratamiento. La densidad inicial promedio para los distintos tratamientos fue 1.15 ± 0.057 × 105 cél ml-1, la cual se incrementó a 1.21 ± 0.012 106 cél ml-1 en los primeros dos días de cultivo y no hubo cambios significativos entre irradianzas. Las tasas de crecimiento disminuyeron con los números finales de células siendo similares entre tratamientos a excepción de la irradiación más baja, que aumentó su densidad celular. Las concentraciones de clorofila a y fucoxantina mostraron diferencias estadísticas (p < 0.001) entre los diferentes niveles de luz. Estas concentraciones fueron siempre mayores en bajas irradianzas. La diadinoxantina disminuyó ante el incrementó de la irradianza, lo contrario ocurrió con la diatoxantina. Las proporciones fucoxantina/clorofila a no fueron significativamente afectadas por el nivel de irradianzas (p = 0.444), pero sí por el tiempo de cultivo (p = 0.003). Las proporciones diatoxantina/clorofila a se incrementan con la irradianza y tiempo de cultivo, con valores mayores en altas irradianzas, mientras que, las porporciones diadinoxantina/clorofila a sólo aumentaron en 750 µmol quanta m-2 s-1. Se concluye que variaciones en la intensidad de luz no modifican la densidad celular de T. pseudonana, pero si tienen un efecto significativo en proporciones de pigmentos accesorios/clorofila a.
Palabras clave: Crecimiento microalgal, proporciones de pigmentos, Thalassiosira pseudonana, irradianzas.
Abstract
This study investigated how different light conditions affect the growth and accessory pigment to chlorophyll a ratios in Thalassiosira pseudonana. The microalga was grown for five days under four irradiances (50, 150, 300 and 750 µmol quanta m-2 s-1) with f/2 medium. Daily growth and pigment composition were determined for each treatment. Initial mean cellular density for all treatment was 1.15 ± 0.057 × 105 cell ml-1, which increased the first two days of culture to 1.21 ± 0.012 x 106 cell ml-1 on average and did not show significant changes among irradiances. Growth rates decreased with the final cell numbers being similar among treatments except for the lowest irradiance, which increased their cellular density. Chlorophyll a and fucoxanthin concentrations showed statistically significant differences (p < 0.001) among the different levels of light. These concentrations were always higher at low than at high irradiances. For diadinoxanthin the concentrations decreased as the irradiance increased, which was contrary to what occurred with diatoxanthin. Fucoxanthin to chlorophyll a ratio was not significantly affected by the irradiance level (p = 0.444), but did change during time under culture (p = 0.003). Diatoxanthin to chlorophyll a ratios increased among different irradiances and with time, with higher values at high irradiances, whereas, diadinoxanthin to chlorophyll a ratios only increased at 750 µmol quanta m-2 s-1. It is concluded that variations in light intensity did not change the cellular densities of T. pseudonana but did have significant effects on accessory pigment to chlorophyll a ratios.
Keys words: Microalgal growth, pigments ratios, Thalassiosira pseudonana, irradiance.
Introduction
Thallasiosira pseudonana Hasle et Heimdal is a marine centric diatom with a cell width of 4-5 µm (Andersen et al., 1997) and frequently occurs in neritic waters. It has chlorophyll a and the unique accessory pigment fucoxanthin which can vary as a function of light, nutrient status and specific growth rate. This species is generally cultured to produce food for marine invertebrate larvae and often variations in nutrients modify the growth rate (Parslow et al., 1984; Thompson, 1999), biochemical and pigment composition (Sciandra et al., 2000; Vonshak & Torzillo, 2004). A better understanding of pigment composition of T. pseudonana under different culture conditions is needed in order to know their effect on total chlorophyll a and pigment ratios in this specific taxonomic group. It is also important to take into account that environmental changes in laboratory take place at different time scales with respect to natural environment systems, where seasonal cycles are varying according to the climatic and geographical location. These conditions not only affect pigment content of phytoplankton but also its taxonomic composition in the field.
Previous studies have examined the interaction between nitrogen and/or carbon limitation on specific growth rate of T. pseudonana and chlorophyll a: carbon ratio with changes in the cells nutritional status (Clark, 2001). Also, others have considered the effects of temperature on growth rate, cell composition, nitrogen metabolism, light limitation and influence of CO2 in the cellular metabolism of T. pseudonana (Stramski, et al., 2002; Berges et al., 2002; Claquin et al., 2002; Bucciarelli & Sunda, 2003). In addition, chemotaxonomic studies have been done to understand the contribution of different algal groups to the accessory pigments to cholorophyll a ratios in light and nutrient limitation conditions (Goericke & Montoya, 1998). Due to the importance of the phytoplankton dynamics in the face of abiotic factors, recent studies have evaluated the response of different phytoplankton taxa to total chlorophyll a in natural samples as well as in phytoplankton cultures (Stramski et al., 2002; Henriksen et al., 2002; Platt et al., 2003). These studies showed that pigment composition is affected by nutrient and light regimes and so it can not be assumed that natural populations have similar responses to those found for species grown in culture. It is important to consider that in productive waters, the algae may be present at such concentrations that self-shading may limit their growth, and this also modifies their pigment concentration (Kirk, 1994). In this respect, the present study evaluated the response of one algal species to variation of light conditions and their effect on the accessory pigments to chlorophyll a ratios under laboratory conditions.
Materials and methods
Cultures. The centric diatom Thalassiosira pseudonana was obtained from the culture collection of the Instituto de Investigaciones Oceanológicas of the Universidad Autónoma de Baja California, México and grown in f/2 medium (Guillard, 1975). Laboratory experiments were carried out with four different light conditions (50, 150, 300 and 750 µmol quanta m-2 s-1) and one concentration of NaNO3/NaH2PO4 (883/36.3 µM) to evaluate the effects of irradiance on growth and accessory pigments to chlorophyll a ratios of T. pseudonana in batch cultures.
Cultures were started in Erlenmeyer flasks, using 200 ml of media with duplicates for each treatment. Culture media were autoclaved at 120 °C; 1.05 Kg cm-2 of pressure for 15 minutes and then each flask was inoculated with 10 ml of T. pseudonana with concentration of 1.48 ± 0.001 x 106 cells ml-1. These cultures were grown at 150 µmol quanta m-2 s-1, with four transfers to new sterile medium, to acclimatize the cells to light and nutrient conditions. The light was provided by fluorescent lamps (day-light 75 W). An experimental range of irradiances, varying from a minimum of 50 to a maximum of 750 µmol quanta m-2 s-1 (measured with a PAR scalar irradiance meter model QSL-100, and 4Π sensor from Biospherical Instruments were carried out in Fernbach flasks (2.9 L of medium, n = 2 for each treatment). These were sterilized in the same way as described above. A volume of 200 ml of culture from the previous level Erlenmeyer was added to each Fernbach under controlled conditions and initial average cell densities of 1.15 ± 0.057 x 105 cells ml-1. The culture temperature was 21.7 ± 1 °C, salinity was 33. The pH was measured daily with a pH meter (Altex) and adjusted within an interval of 7.3 to 8.5 by CO2 addition with a flow rate of 270 ml min-1. Cell density was assessed daily with a Coulter (Beckman Coulter) particle counter Multisizer 3 by duplicate. Specific growth rate (μ) day-1 was calculated as follows:
µ = ln (N1) - ln (N0) / t1 - t0,
Where µ is specific growth rate (day-1), N1 and N0 are the final and initial cellular densities and t1 and t0 are the final and initial time, respectively.
Pigment analysis. For chlorophyll a and carotenoid pigment analysis 10 ml samples were filtered daily through 25 mm GF/F glass filters for all experimental conditions. Filters were frozen and stored in liquid nitrogen and analyzed at Center for Hydro-Optics and Remote Sensing (CHORS) at San Diego State University. Prior to analysis, the filters were thawed, placed in 4 ml 100% acetone, sonicated for 10 seconds and stored in the freezer for 24 hours. After that, the samples were centrifuged to 2000-x g for 5 minutes and then refiltered through 0.2 µm filters. From the acetone extract 100 µL were injected into a HPLC system according to the method described by Trees et al. (2000).
Statistical analysis. A two way analysis of variance was used to determine the effects of irradiance and culture time on pigment content and pigment ratios. To determine statistically significant differences of the independent variables (p <0.05), multiple comparison procedures (Tukey test) were performed.
Results
Cellular density of T. pseudonana increased during the first two days of culture, although significant changes were not found among the different irradiances. During the experiments, differences in cellular density were observed, but they were not statistically significant different (p = 0.179) among the various light levels. Within each light condition, for the different days of culturing there was a statistically significant difference (p ≤ 0.001). Likewise, the maximum average cellular densities were found for the fourth day in all light conditions, except for the 50 µmol quanta m-2 s-1, which registered an increase in their cellular density on the fifth day (Fig. 1). The final cell numbers were similar in all treatments. At irradiances of 50 and 150 µmol quanta m-2 s-1, the growth rates were 1.5 and 1.4, respectively, but these declined the next day to average rates of 0.7 and 0.8 per day (Table 1). The same responses were observed during these periods for 300 and 750 µmol quanta m-2 s-1 and were essentially identical (1.6 for both treatments), decreasing to 0.7 per day (Table 1). From the third to fourth day, specific growth rates decreased during the slower growth phase indicating that cultures were beginning their stationary growth phase. After that, cellular density decreased in all experiments except for the 50 µmol quanta m-2 s-1 (Fig. 1; Table 1).


The pigments measured in T. pseudonana can be differentiated into photosynthetic and photoprotective in regard to their function in photosynthesis (Jeffrey & Vesk, 1997). The pigment concentrations showed a wide range of variation, mainly related to irradiance levels. Both chlorophyll a and the photosynthetic carotenoid, fucoxanthin showed statistically significant differences (p <0.001) among light levels and culture times. In addition, their concentrations were always higher at low irradiancies (Table 2) and increased with cellular densities and culture times. Maximum values of chlorophyll a (2265.9 ± 72.2 µg L-1) and fucoxanthin (544 ± 35.4 µg L-1) were generally observed during the fourth day of experiment. After this period, these pigment concentrations decreased. The photoprotective carotenoids, diadinoxanthin and diatoxanthin showed statistically significant differences (p <0.001; p <0.007) among the different light levels and culture times respectively. These carotenoids were found at smaller concentrations than the other pigments. The concentration of accessory pigment diadinoxanthin decreased when the irradiance increased, while the opposite trend occurred with diatoxanthin (Table 2).
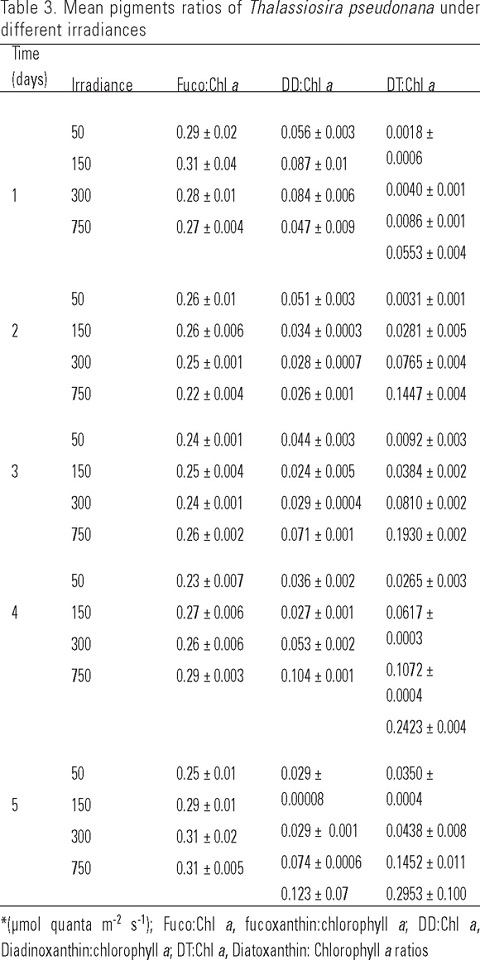
When T. pseudonana was grown at four irradiances it was observed that the ratios of cellular fucoxanthin to total chlorophyll a content decreased in the second and third days of the experiments (Table 3). Fucoxanthin to chlorophyll a ratios were not significantly affected by the level of irradiance (p =0.444). However, these ratios were significantly affected throughout the culturing period (p =0.003). The mean ratios at irradiances of 50 and 150 µmol quanta m-2 s-1 were 0.26 ± 0.007 and 0.28 ± 0.009 whereas at 300 and 750 µmol quanta m-2 s-1 their mean ratios were identical (0.27 ± 0.009). On the other hand, diatoxanthin to chlorophyll a ratios increased among different irradiances and with culture time, with higher values at high irradiances, whereas, diadinoxanthin to chlorophyll a ratios only increased at 750 µmol quanta m-2 s-1 (Table 3). Within the different light levels and culturing periods, diatoxanthin to chlorophyll a ratios showed significant differences (p ≤0.001). Their concentration increased (Table 3) with higher values at high irradiances than those occurring at low irradiances.
Discussion
Growth rates of T. pseudonana indicated that irradiances did not have significant effects on growth during the first two days of the culture experiment (Fig. 1, Table 1). An explanation is that the irradiances in the cultures would have been attenuated due to the fast growth and increased turbidity of the cultures as cellular densities increase. Brown et al. (1996) showed that irradiances were reduced by 20-25% at late-logarithmic phase and by 30-40% at stationary phase during the experiment. On the other hand, the highest growth rates of T. pseudonana grown under 24:0 h L:D have been documented to range from 1.9 to 2.1 at 125 and 330 µmol photon m-2 s-1 and temperatures of 17.5-15°C respectively (Thompson et al., 1990; Sakshaug et al., 1987). In this study the highest mean growth rates were recorded in the first culture day and ranged from 1.4 to 1.6 among various irradiances (Table 1). These results are consistent with the finding of Stramski et al. (2002) who observed that growth rate of T. pseudonana at low irradiance was reduced to 0.64 compared to second day at high irradiance. In addition, the cultures of T. pseudonana grown at 300 µmol quanta m-2 s-1 had a faster growth rate than those cultures grown at other irradiances, even though the difference in the growth rate between low and high irradiances was relatively small. This indicates that T. pseudonana is an excellent microalga for the mass culture in aquaculture, because of their ability to tolerate a wide range of growth irradiances and generate high cellular density in a short period of time. In addition, in aquaculture culture it is necessary to take into account that chlorophyll a concentrations change with the irradiances and therefore modify the phytoplankton biomass.
At the level of the individual pigments, chlorophyll a concentrations were higher at low irradiances than at high irradiances (Table 2) due to the physiological response of T. pseudonana to light limitation (Goericke & Montoya, 1998). Likewise the other major pigment, fucoxanthin, also increased at low irradiancies (Table 2), and the covariance of chlorophyll a and fucoxanthin throughout the culture time may be related to their similar physiological response to light conditions. Also, an increase in fucoxanthin is indicative of light limitation due to self-shading and modification of their pigment concentration. In addition, this light-harvesting pigment collects light from the prevailing field at specific wavelengths and transfers the absorbed energy to the reaction center of chlorophyll a, which directly participates in photosynthesis (Kirk, 1994).
Diadinoxanthin increased at the lowest irradiance, whereas in other irradiances it decreased (Table 2). Diatoxanthin was higher than diadinoxanthin at high irradiances and increased continuously up to the third day of culturing. The differences between diadinoxanthin and diatoxanthin content in T. pseudonana are to be attributed to the excessive light levels that could limit their photosynthesis rate by inducing photoinhibition. Cartotenoids participate in the photoadaptative response through diadino-diatoxanthin xanthophyll cycle that is triggered by light causing changes in pigment concentrations in response to the prolonged light periods. This also have been observed by Rmiki et al. (1996) who found that diatoxanthin content of phytoplankton algae showed important variations along inshore-offshore transects and their diatoxanthin to chlorophyll a ratios varied from 0.002 to 0.074 at irradiances of 300 and 550 µE m-2 s-1 in inshore and offshore respectively. Another estimate of variations in the pigment content of T. pseudonana is the fucoxanthin to chlorophyll a ratio which showed a remarkable similarity among the different light intensities. This may be due to the fact that photosynthetically active pigments covary with chlorophyll a and possibly with irradiance as described by Goericke and Montoya (1998) and Schlüter et al. (2006). In addition, when cultures of T. pseudonana were exposed to high irradiances the ratios of diadinoxanthin to chlorophyll a showed a decrease in the second day at the highest irradiance (Table 3), while diatoxanthin to chlorophyll a ratio was almost three-fold higher at higher irradiance (Table 3). These changes in pigments ratios are related to protecting the cell against photo-oxidation in response to high levels of oxygen which is an inhibitor of photosynthesis and can also cause photorespiration modifying the biochemical composition. The results presented here also have been observed by others (Schlüter et al., 2000; Descy et al., 2000) and suggests that change in accessory pigments per cell is indicative of photoacclimatation processes as a response to the light climate and interconversion of pigment takes place depending on the culture irradiance level.
It is conclude that the concentration of individual pigments showed statistically significant differences (p < 0.001) among the different light levels and culturing times. Fucoxanthin to chlorophyll a ratios were not significantly affected by the irradiance level, but these were significantly affected by the culture period. Diatoxanthin to chlorophyll a ratio s increased among different irradiances and with culture time, with higher values at high irradiances, whereas, diadinoxanthin to chlorophyll a ratios only increased at 750 µmol quanta m-2 s-1. Also, variations in light intensity did not change the cellular densities of T. pseudonana but did have significant effects on accessory pigment to chlorophyll a ratios.
Acknowledgements
This research comprised part of the project "Production of Microalgae" at the Instituto de Investigaciones Oceanológicas of the Universidad Autónoma de Baja California, with support from the UABC. The authors are grateful to CONACYT for to the first author during his graduate studies. Special thank to the Center for Hydro-Optics and Remote Sensing from San Diego State University for processing HPLC samples.
References
ANDERSEN, R. A., S. L. MORTON & J. P. SEXTON. 1997. CCMP-Provasoli-Guillard national center for culture of marine phytoplankton. Journal of Phycology 33 (6): 3-74. [ Links ]
BERGES, J. A., D. E. VARELA & P. J. HARRISON. 2002. Effects of temperature on growth rate, cell composition and nitrogen metabolism in the marine diatom Thalassiosira pseudonana (Bacillariophyceae). Marine Ecology Progress Series 225: 139-146. [ Links ]
BROWN, M. R., G. A. DUSTAN, S. J. NORWOOD & K. A. MILLER. 1996. Effects of harvest stage and light on the biochemical composition of the diatom Thalassiosira pseudonana. Journal Phycology 32: 64-73. [ Links ]
BUCCIARELLI, E. & W. G. SUNDA. 2003. Infuence of CO2, nitrate, phosphate, and silicate limitation on intracellular dimethylsulfoniopropionate in batch cultures of the coastal diatom Thalassiosira pseudonana. Limnology and Oceanography 48 (6): 2256-2265. [ Links ]
CLAQUIN, P., V. MARTIN-JÉZÉQUEL, J. C. KROMKAMP, M. J. W. VELDHUIS & G. W. KRAAY. 2002. Uncoupling of silicon compared with carbon and nitrogen metabolism and role of the cell cycle in continuous cultures of Thalassiosira pseudonana (Bacillariophyceae) under light, nitrogen, and phosphorus control. Journal Phycology 38: 922-930. [ Links ]
CLARK, D. R. 2001. Growth rate relationships to physiological indices of nutrient status in marine diatoms. Journal Phycology 37: 249-256. [ Links ]
DESCY, J. P., H. W. HIGGINS, D. J. MACKEY, J. P. HURLEY & T. M. FROST. 2000. Pigment ratios and phytoplankton assessment in northern Wisconsin lakes. Journal Phycology 36: 274-286. [ Links ]
GOERICKE, R. & J. P. MONTOYA. 1998. Estimating the contribution of microalgal taxa to chlorophyll a in the field—variations of pigments ratios under nutrient-and light-limited growth. Marine Ecology Progress Series 169: 97-112. [ Links ]
GUILLARD, R. R. L. 1975. Culture of phytoplankton for feeding marine invertebrates. In: Smith, W.L. & M.H. Chanley (Eds.). Culture of marine invertebrates animals. Plenum Publishing Corp. New York, pp. 29-60. [ Links ]
HENRIKSEN, P., B. RIEMANN, H. KASS, H. MUNK SØRENSEN & H. L. SØRENSEN. 2002. Effects of nutrient-limitation and irradiance on marine phytoplankton pigments. Journal of Plankton Research 24 (9): 835-858. [ Links ]
JEFFREY, S. W. & M. VESK. 1997. Introduction to marine phytoplankton and their pigment signatures. In: S.W. Jeffrey, R.F.C. Mantoura & S.W. Wright (Eds.). Phytoplankton Pigments in Oceanography. UNESCO, Paris, pp. 37-84. [ Links ]
KIRK, J. T. O. 1994. New York, NY, USA. Light and photosynthesis in aquatic ecosystems. 2nd Ed. Cambridge University Press. 509 p. [ Links ]
PARSLOW, J. S., P. J. HARRISON & P. A. THOMPSON. 1984. Satured uptake kinetics: trasient response of the marine diatom Thalassiosira pseudonana to ammonium, nitrate, silicate or phosphate starvation. Marine Biology 83: 51-59. [ Links ]
PLATT, T., S. SATHYENDRANATH, A. M. EDWARS, D. S. BROOMHEAD & O. ULLOA. 2003. Nitrate supply and demand in the mixed layer of the ocean. Marine Ecology Progress Series 254: 3-9. [ Links ]
RMIKI, NOUR-EDDINE, C. BRUNET, J. CABIOCH & Y. LEMOINE. 1996. Xanthophyll-cycle and photosynthetic adaptation to environment in macroalgae and microalgae. Hydrobiologia 326/327: 407-413. [ Links ]
SAKSHAUG, E., S. DEMERS & C. M. YENTSCH. 1987. Thalassiosira oceanica and T. pseudonana: two different photoadaptational responses. Marine Ecology Progress Series 41: 275-282. [ Links ]
SCHLÜTER, L., F. MØHLENBERG, H. HAVSKUM & S. LARSEN. 2000. The use of phytoplankton pigments for identifying and quantifying phyto-plankton groups in castal areas: testing the influence of light and nutrients on pigment/chlorophyll a ratios. Marine Ecology Progress Series 192: 49-63. [ Links ]
SCHLÜTER, L., T. L. LAURIDSEN, G. KROGH & T. JØRGENSEN. 2006. Identification and quantification of phytoplankton groups in lakes using new pigment ratios - a comparison between pigment analysis by HPLC and microscopy. Freshwater Biology 51: 1474-1485. [ Links ]
SCIANDRA, A., L. LIZZARA, H. CLAUSTRE & M. BABIN. 2000. Responses of growth rate, pigment composition and optical properties of Cryptomonas sp. to light and nitrogen stresses. Marine Ecology Progress Series 201: 107-120. [ Links ]
STRAMSKI, D., A. SCIANDRA & H. CLAUSTRE. 2002. Effects of temperature, nitrogen, and light limitation on the optical properties of the marine diatoms Thalassiosira pseudonana. Limnology and Oceanography 47 (2): 392-403. [ Links ]
THOMPSON, P. 1999. The response of growth and biochemical composition to variations in daylength, temperature, and irradiance in the marine diatom Thalassiosira pseudonana (Bacillariophyceae). Journal Phycology 35: 1215-1223. [ Links ]
THOMPSON, P. A., P. J. HARRISON & J. N. C. WHYTE. 1990. Influence of irradiance on the fatty acid composition of phytoplankton. Journal Phycology 26: 278-288. [ Links ]
TREES, C. C., R. R. BIGIDARE, D. N. KARL & L. VAN HEUKELEM. 2000. Fluorometric chlorophyll a: sampling, laboratory methods, and data analysis protocols. In: G.S. Fargion & J.L. Muller (Eds.). Ocean Optics Protocols for Satellite Ocean Color Sensor Validation. Revision 2, Chapter 14, NASA TM 2000-209966, Goddard Space Fligth Center, Greenbelt, MD, pp. 162-169. [ Links ]
VONSHAK, A. & G. TORZILLO. 2004. Great Britain, United Kingdom. Environmental stress physiology. In: Amos Richmond (Ed.). Handbook of microalgal culture: Biotechnology and Applied Phycology. Blackwell Publishing Company. pp. 57-82. [ Links ]














