Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Hidrobiológica
versión impresa ISSN 0188-8897
Hidrobiológica vol.17 no.2 Ciudad de México ago. 2007
Artículos
Patterns of nutrient exchange in a riverine mangrove forest in the Shark River Estuary, Florida, USA
Patrones de intercambio de nutrients en un bosque de manglar riverino en Shark River Estuary, Florida, USA
Victor H. Rivera-Monroy1a, Kim de Mutsert1, Robert R. Twilley1, Edward Castañeda-Moya1, Melissa M. Romigh2 and Stephen E. Davis, III2
1 Wetland Biogeochemistry Institute, Department of Oceanography and Coastal Sciences, Louisiana State University, Baton Rouge, LA 70803 USA.
2 Department of Wildlife and Fisheries Sciences, 2258 TAMU, Texas A & M University, College Station, TX 77843 USA.
Recibido: 11 de mayo de 2006
Aceptado: 22 de marzo de 2007
Abstract
This study aimed to evaluate tidal and seasonal variations in concentrations and fluxes of nitrogen (NH4+, NO2+NO3, total nitrogen) and phosphorus (soluble reactive phosphorus, total phosphorus) in a riverine mangrove forest using the flume technique during the dry (May, December 2003) and rainy (October 2003) seasons in the Shark River Estuary, Florida. Tidal water temperatures during the sampling period were on average 29.4 (± 0.4) oC in May and October declining to 20 oC (± 4) in December. Salinity values remained constant in May (28 ± 0.12 PSU), whereas salinity in October and December ranged from 6-21 PSU and 9-25 PSU, respectively. Nitrate + nitrite (N+N) and NH4+ concentrations ranged from 0.0 to 3.5 µM and from 0 to 4.8 µM throughout the study period, respectively. Mean TN concentrations in October and December were 39 (±0.8) µM and 37 (±1.5) µM, respectively. SRP and N+N concentrations in the flume increased with higher frequency in flooding tides. TP concentrations ranged between 0.2-2.9 µM with higher concentrations in the dry season than in the rainy season. Mean concentrations were <1. 5 µM during the sampling period in October (0.75 ± 0.02) and December (0.76 ± 0.01), and were relatively constant in both upstream and downstream locations of the flume. Water residence time in the flume (25 m2) was relatively short for any nutrient exchange to occur between the water column and the forest floor. However, the distinct seasonality in nutrient concentrations in the flume and adjacent tidal creek indicate that the Gulf of Mexico is the main source of SRP and N+N into the mangrove forest.
Key words: Mangroves, nitrogen, phosphorus, flume, Everglades National Park.
Resumen
El objetivo de este estudio fue evaluar los cambios en las concentraciones y flujos de nitrógeno (NH4+, NO2+NO3, nitrogeno total) y fósforo (fósforo reactivo soluble, fósforo total) asociados al regimen climatico y de marea en un manglar riverino, usando la técnica del flume durante las epocas seca (Mayo, Diciembre) y de lluvias (Octubre) del 2003 en Shark River Estuary. La temperatura promedio de la columna de agua fue 29.4 (± 0.4) ºC durante Mayo y Diciembre, y disminuyó a 20 (± 4) ºC en Diciembre. Los valores de salinidad permanecieron constantes durante Mayo (28 ± 0.12 PSU), mientras que en Octubre y Diciembre variaron entre 6-21 PSU y 9-25 PSU, respectivamente. Las concentraciones de nitrato + nitrito (N+N) y amonio (NH4+) fluctuaron entre 0.0±3.5 µM y 0.0-4.8 µM, respectivamente. Las concentraciones de nitrógeno total en Octubre y Diciembre fueron en promedio 39 (± 0.8) µM y 37 ± 1.5) µM, respectivamente. Las concentraciones de fósforo reactivo soluble y N+N en el flume incrementaron con mayor frequencia durante los períodos de marea alta. Las concentraciones de fósforo total variaron entre 0.2-2.9 µM, con máximos valores durante la época seca. En promedio, las concentraciones de fósforo total fueron <1.5 µM durante Octubre (0.75 ± 0.02) y Diciembre (0.78 ± 0.01), y se mantuvieron relativamente constantes en ambos extremos del flume. El tiempo de residencia delagua en el flume (25 m2) fue relativamente corto para permitir algún intercambio de nutrientes entre la columna de agua y el suelo del manglar. Sin embargo, la marcada estacionalidad en las concentraciones de nutrientes en el flume y en el canal mareal adjacente a éste, indican que el Golfo de México es la principal fuente de fósforo reactivo soluble y N+N hacia el bosque de manglar.
Palabras clave: Manglares, nitrógeno, fósforo, flume, Everglades National Park.
Introduction
Mangrove forests are one of the most dominant vegetation communities in the Everglades National Park (ENP) with an extension of 144,447 ha (Welch et. al., 1999). Although this is the largest mangrove area in the continental USA, there are large information gaps regarding factors regulating productivity and spatial distribution of these coastal wetlands. Current landscape level restoration efforts in south Florida (CERP, 2004) will shift surface regional water flows that may impact the temporal and spatial distributions of mangrove forests in the coastal transition zone. It is not clear if changes in hydroperiod, salinity, and potential nutrient inputs (e.g., nitrogen and phosphorus) will significantly modify critical biogeochemical processes that control productivity patterns, not only within mangrove forests, but also in adjacent estuarine and coastal waters.
Although mangrove research in south Florida has historically provided significant conceptual models to explain how mangrove forest structure responds to different hydrological and edaphic conditions (sensu Lugo & Snedaker, 1974), there are large information gaps on functional aspects related to nutrient cycling. For instance, few studies have underscored critical ecological processes such as denitrification, nitrogen fixation (Pelegri et. al., 1997; Pelegri & Twilley, 1998), phosphorus sedimentary processes (Chen & Twilley, 1999), and mangrove-water column nutrient exchange (Childers et al., 1999; Davis et. al., 2003) despite their role in maintaining primary productivity rates and regulating the coupling between mangrove forests and estuarine waters. Furthermore, the functional role of mangrove forests in south Florida as potential sources, sinks, or transformers of nutrients is still not clear (Davis et al., 2001a, Davis et al., 2001b, Sutula et al., 2001).
Methodological limitations have also hampered comparisons among mangrove systems since material exchange is actually occurring at spatial and temporal scales that are not sampled with present designs in flux measurements (Dittmar & Lara, 2001a). As a consequence, the nutrient outwelling concept for mangrove-dominated estuaries in the tropics has not yet been resolved due to methodological differences (Dittmar & Lara, 2001a). To specifically quantify material fluxes at the mangrove forest-tidal creek interface, Rivera-Monroy et. al. (1995) modified the flume methodology to estimate fluxes of organic and inorganic nitrogen and total suspended sediments in a fringe mangrove forest. The flume method was initially developed for intertidal marshes to evaluate material fluxes across the marsh surface at field scales (10-30 m) using a flume constructed along the intertidal zone of marsh surface (Childers, 1994; Childers et. al., 1999). This method is a variation of a hypsometric technique to study exchange of materials at the boundary of mangrove wetland and coastal waters (Twilley, 1985; Woodroffe, 1985). To date, the flume technique has been only applied in mangrove forests in Mexico (Rivera-Monroy et. al., 1995), along Taylor River in Florida (Davis et. al., 2001a), and in Colombia (Rivera-Monroy et. al., unpublished results).
We used the flume technique to evaluate nitrogen (N) and phosphorus (P) fluxes in Shark River estuary, one of the largest estuaries in the southwest Florida coast. Discharge of fresh water from Shark River Slough to the estuary follows patterns of seasonal and inter-annual rainfall, and historical changes have occurred in these flows due to human land use in the Everglades watershed (Light & Dineen, 1994). Freshwater diversions into Shark River estuary are planned to restore regional historical flows, but no upstream restoration measures have been implemented. We designed a flume to assess the role of riverine mangrove forests including: 1) sink or source of inorganic (NH4+, NO2-+NO3-) and total nitrogen (TN), soluble reactive phosphorus (SRP) and total phosphorus (TP), 2) the seasonality of nutrient fluxes, and 3) the applicability of the flume technique in a riverine mangrove forest. We hypothesized that mangrove forests in this coastal region import TP and SRP and inorganic nitrogen. Because P is a limiting nutrient in this mangrove forest, we assumed that the dominant source is tidal flooding in contrast to remineralization from organic matter in the soil.
Materials and methods
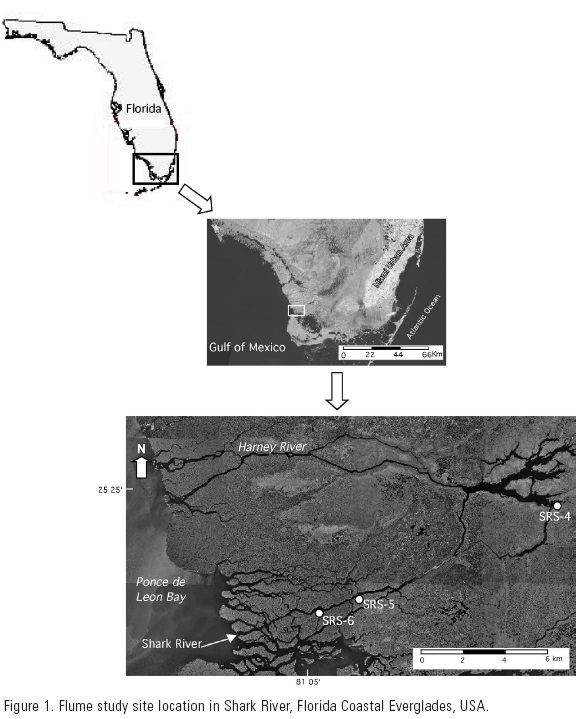
This study was conducted along Shark River estuary approximately 4 km from the estuary mouth (Fig.1). The climate of the area is subtropical moist, with distinct wet (June-November) and dry (December-May) seasons. Shark River is ~14 km long and is on average 70 m wide. The forested area along the river is comprised of the mangrove species Rhizophora mangle (L.), Laguncularia racemosa (Gaertn), and Avicennia germinans (L.) Stearn, although L. racemosa is the dominant species in terms of basal area indicating the effect of previous large-scale disturbances (e.g., hurricanes) in the region (Smith et al., 1994; Chen & Twilley, 1999). On average, mangrove trees in these forests are 10-14 m tall. Woody production ranges from 0.06-4.8 kg tree-1 yr -1 (Chen & Twilley, 1999) and litter production varies from 8-11 Mg ha-1 yr-1 (Castañeda-Moya et al., in preparation). Tides are semi-diurnal with a mean tidal range of 0.5 m. South Florida is a carbonate environmental setting where limestone bedrock is covered with a thick layer (> 3m) of wetland peat (Light and Dineen, 1994).The flume was installed at the boundary between a tidal creek creek (12.5m long, 2 m wide) and the mangrove forest approximately 20 m inland from the creek mouth where a water quality long-term sampling station is located (SRS-6, 25.36463 °N and 81.07795 °W) (Fig. 1). This water quality station is part of the Florida Coastal Everglades, Long Term Ecological Research (FCE-LTER) sampling network (http://fcelter.fiu.edu/maps). The flume consisted of clear, corrugated fiberglass panels (each 1 m height and 2.7 m long) that form two vertical walls 2 m apart and 12.5m long. The flume was open to surface water at each end to allow exchange from a tidal creek to the interior forest. Panels were removed after each sampling period to avoid long-term panel effects such as shading, edge scouring, and wrack accumulation (Childers & Day, 1988). Nutrient concentrations were monitored at each end of the flume and at the mouth of the tidal creek. Duplicate water samples were collected simultaneously at these three locations at 30-minute intervals during three to five consecutive tides during the dry season (May 23-27; December 14-17) and the rainy season (October 7-9) in 2003. Portable auto-samplers (ISCO) were installed at both ends of the flume and in the tidal creek to collect these water samples so as to minimize disturbance to the forest surface during each sampling event. As part of the FCE-LTER program, water levels were continuously monitored along Shark River and inside the forest (approximately 70 m inland from Shark River) with acoustic water level recorders; stage heights were recorded every 30 min at one end of the flume. Salinity and temperature were also monitored in the tidal creek at 30-minute intervals during each sampling period using a pre-calibrated mini-sonde (Hach-Hydrolab MiniSonde 4a).Water samples were retrieved every 12 h, packed in ice and transported to the laboratory where they were filtered using 47 mm Whatman GF/F material. Filtration was performed within 24 h of collection, and both filtered and unfiltered samples were refrigerated at 4 ºC in 125 ml plastic bottles. Water samples were analyzed for inorganic nitrogen (NH4+, NO2- and NO3-) and SRP concentrations in the laboratory using a Lachat auto-analyzer and standard techniques (Strickland & Parsons, 1972; Parsons et. al., 1984). Unfiltered samples were analyzed for TN and TP also using a Lachat auto-analyzer after persulphate oxidation (D'Elia et. al., 1977; Valderrama, 1981).
Soil elevation within the flume was surveyed in August 2003 to characterize the microtopography. These data along with the water level data were used to calculate the volume of the flume and the changes in water volume during a tide. Fluxes were estimated based on changes in water volume together with measurements of nutrient concentrations at each end of the flume per unit time (Wolaver et al., 1985). Net areal flux was calculated following modifications by Rivera-Monroy et al. (1995) to the general methodology developed by Childers & Day (1988):

Nutrient content (mg) was pooled into upstream and downstream values and compared using paired t-test (SAS, 2004) to test if fluxes were significantly different from zero with each individual full-tide (Rivera-Monroy et al., 1995). Similarly, nutrient content for ebb and flood half-tides were tested for significance with a paired t-test since water masses flowing through a flume during each half-tide might be independent (Childers & Day, 1988; Rivera-Monroy et al., 1995). Seasonal variation of nutrient concentrations was evaluated by calculating means and standard errors from the total number of samples taken for each analyte during ebb and flood tide for each full tide within each sampling date.
Results
Salinity and nutrient concentrations. Frequency and duration of inundation in the flume was variable in all seasons and was correlated with the semi-diurnal tide observed at the mouth of the creek and topography of the flume. The flume was only partially flooded during the daily second tide in May and December (Fig. 2). Creek water temperatures throughout the sampling period averaged 29.4 (-0.4) °C in May and October, declining to 20 °C (-4) in December (Fig. 3). Salinity showed a distinct unimodal pattern in October and December indicating stronger salinity gradients in the estuary, as result of larger freshwater inputs into Shark River estuary. Ebb waters draining from the forest interior had higher salinity than flood waters in this season. Throughout May (dry season) salinity remained relatively constant (28 -0.12 PSU) during the 5-day sampling period, whereas salinity in October and December ranged from 6-21 PSU and 9-25 PSU, respectively (Fig 3).
NO2- + NO3- (N+N) concentrations in the flume and the creek ranged from 0.0-3.5 µM throughout the study period. There was a distinct pattern in May and October when N+N concentrations were significantly higher during flood tide indicating a potential import to the forest. This pattern is more conspicuous in May than in October and December when peak concentrations were below 2.5 µM and 1.5 µM, respectively. In December, average concentrations (0.6 ± 0.6 µM) were relatively similar among tides. The relationship between changes in water level and N+N concentrations inside the flume during flooding conditions showed a larger input of N+N in the rainy season (i.e., October; Fig. 4). In contrast to N+N, NH4+ concentrations were more variable in the flume than in the creek and ranged between 0-4.8 µM. Maximum values were measured in October in both the flume (3.4 ± 1.8 µM) and creek (4.8 ± 0.05 µM). Minimum mean concentrations were measured in the flume during May. As observed for NH4+, TN concentrations were also low throughout May in contrast to higher mean concentrations in October and December (Fig. 4). Concentrations in May averaged 12 ± 0.5 µM in the creek and flume, although there was a significant change in concentrations on the last sampling day (May 27), when concentrations increased significantly to =35 µM in the forest side of the flume during ebb tide (Fig. 4). In comparison, average concentrations in October and December were 39 ± 0.8 µM and 37 ± 1.5 µM, respectively. In these last sampling periods there were opposite trends when TN concentrations generally declined in October but increased in December in both creek and flume stations.
SRP concentrations increased in the flume and creek stations as frequency of flood tides increased (Fig. 5). This pattern was more evident in the first 3 days of the sampling period in May when there was a significant correlation between flood tide frequency and an increase in SRP concentration in the flume (Fig. 6B). Overall SRP concentrations were similar in the flume and the creek. TP concentrations ranged from 0.2-2.9 µM with higher concentrations in the dry season than in the rainy season. Mean concentrations were <1. 5 µM during October (0.75 ± 0.02) and December (0.76 ± 0.01), and remained relatively constant in upstream and downstream locations of the flume.

Fluxes. Net fluxes were calculated only when both sides of the flume were flooded either during the flood or ebb tide (nine tides total) (Table 1). Despite observed seasonal changes in concentrations in the flume, material fluxes in sequential tides were not significantly different (Table 1). Most of the net fluxes were close to zero particularly in the case of SRP, N+N, TP, and NH4+.
Discussion
Although our study did not show significant exchange of organic and inorganic nitrogen and phosphorus in a riverine mangrove forest, there was a distinct seasonality in nutrient concentrations at the boundary between the mangrove forest and Shark River estuary. The lack of measurable fluxes indicates that water residence time in the flume was relatively short for any nutrient exchange to occur between the water column and the forest floor. This was confirmed when nutrient content was pooled into upstream and downstream values for each flume experiment and compared using paired t-test (diff=0, P values ranged from 0.1 to 0.7). In all cases when whole tides were registered, nutrient concentrations were similar at both ends of the flume during both flood and ebb tides (e.g., Fig. 4). This result suggests that the flume dimensions (24 m2) used in this study are not adequate to measure nutrient exchange in this forest due to the tidal signature at this location. Thus, at this sampling spatial scale it is not possible to evaluate if this riverine mangrove forest is a net sink or source of nitrogen and phosphorus.
Based on temporal trends in nutrient concentrations in the flume and at the mouth of the tidal creek, we observed an apparent uptake of N=N and SRP into the mangrove forest at larger spatial scales (>24 m2). One indication of this process is the significant (p < 0.05) correlation between changes in water level and nutrient concentrations during flooding tides in the flume (Figures 6A and 6B). Frequency and duration of inundation in this mangrove site was higher than values observed in the middle region of the estuary (SRS-5) and upstream (SRS-4) during 2003 (Fig. 1). On annual basis, the mangrove forest at the SRS-6 site was inundated 69% of the time, whereas mangroves in SRS-4 and SRS-5 were inundated 49% and 54% percent of the time, respectively (Fig. 7).

Low concentrations occurred during ebb tides indicating that nutrient behavior may be linked to different biological process inside the mangrove forest (e.g., plant uptake, immobilization, denitrification). Lower concentrations in ebb tide waters may result from longer residence times of water parcels "trapped" (Wolanski & Ridd, 1986; Struve et al., 2003) in the interior forest, which can cause a decoupling between tidal cycles and an increase in residual drainage fluxes (e.g., Whitinget al., 1989). Apparently, this decoupling is stronger during the dry season when water exchange between the forest and Shark River is driven by tidal forcing. In contrast, tidal forcing plus rainfall reduces the residence time of residual water in the interior of this mangrove forest. Longer residence times inside the mangrove forest are expected in the dry season, particularly in periods between neap and spring tides. This climatic control of water residence times and correspondent control of internal biogeochemical processing is one of the functional characteristics of coastal Everglades (Davis et al., 2001a; Childers et al., 2006) and other tropical estuaries (Eyre & Balls, 1999).
Import of N+N into the mangrove sediments using the flume technique has been reported in fringing mangroves in similar phosphorus-limited karstic settings. For example, a study in Terminos Lagoon, Mexico reported a net import of N+N into a fringe forest from both the adjacent creek (0.08 g m-2 y-1) and a basin forest (0.004 g m-2 y-1) (Rivera-Monroy et al., 1995). Also, Davis et al. (2001a) using flow-through flumes found that a fringe mangrove zone located in lower Taylor River, Florida was a small sink of nitrogen (0.015 g N m-2 yr-1), while the entire cross section of tidal creek was a larger sink (2.19 g N m-2 yr-1). Furthermore, a benthic flux study using batch cores in Shark River, and performed close to the flume location at SRS-6, reported significant N+N uptake by the mangrove sediments ranging from 0.27 to 0.45 mg N m-2 hr-1 (Childers et al., 1999). Further studies will be needed to determine the fate of this inorganic nitrogen inside the mangrove forest. We hypothesize that most of this inorganic nitrogen is retained in the sediments (probably by bacteria and fungi on particles) and not lost via denitrification as has been found in other mangrove forests (Rivera-Monroy & Twilley, 1996; Alongi et al., 2002; Alongi et al., 2004; Alongi et al., 2005).
SRP concentrations also increased as the flume and creek were inundated during flooding tides. This apparent import of P into the mangrove forest is in agreement with previous work showing a P-fertility gradient with higher soil P concentrations close to the mouth of Shark River (Chen & Twilley, 1999) where our flume was located. Higher concentrations in this region are a result of marine inputs of P from the Gulf of Mexico.
This marine source of P, relative to inputs from upstream sources (i.e., riverine), is quite unique for estuaries. Due to this inversion in P sources, Shark River has been characterized as an "upside-down" estuary (Childers et al., 2006). TP concentrations in south Florida estuaries adjacent to the Gulf of Mexico are on average approximately 0.5 µM in contrast to oligotrophic areas upstream where concentrations are <0.25 µM (Childers et al., 2006). Our results confirm this pattern as TP concentrations in the flume and creek were on average 0.75 µM over the sampling period and SRP concentrations showed a seasonal gradient related to tidal inundation of the mangrove forest during all seasons, particularly in the dry season (May). Indeed, the constant salinity value (28 PSU) throughout the sampling period in May and the strong association between water levels and SRP concentrations indicates that marine waters are a source of SRP. It has been hypothesized that mineral inputs to the mouth of Shark River estuary from the Gulf of Mexico (rather than upland inputs) control the patterns of mangrove structure and productivity along this estuary (Chen & Twilley, 1999).
TP and TN concentrations measured in this study were within the range reported for Shark River (Childers et al.,2006). TP has less seasonal variability along the estuary where mean concentrations range from 0.25 to 0.75 µM and increase down-estuary with the highest concentrations at the SRS-6 site (Childers et al., 2006). In contrast to TP, TN concentrations are consistently low in the downstream reach of Shark River and significantly lower than those measured in freshwater sites upstream (>75 µM). During our study, we measured one of the lowest mean average concentrations in May (16 µM), probably due to low freshwater input as result of low rainfall upstream in the Shark River Slough during 2003.
Our results underscore the complex biogeochemical processes involved in controlling the net exchange of nutrients at the boundary between mangrove forest and estuarine waters, and stress the need to measure these exchanges at multiple scales. Discerning the role of mangroves as sink, source or transformers of nutrients is challenging since few studies are actually available across different geomorphological settings to help determine general patterns in different type of mangroves (Lugo & Snedaker, 1974; Twilley, 1995). Despite the large extent of mangrove forests throughout tropical and subtropical latitudes (1.7 x 105 km2, Valiela et al., 2001) there are a limited number of studies evaluating nutrient fluxes in mangrove forests. Historically, material exchange in mangrove-dominated systems has been evaluated using an "Eulerian" approach (e.g., Boto & Wellington, 1988; Wattayakorn et al., 1990; Dittmar & Lara, 2001b; 2001a; Dittmar et al.,, 2001) where material fluxes are estimated along tidal creeks and embayments over several tidal cycles. This approach estimates annual exchanges between large mangrove areas and the estuary through the product of water discharge and material concentration over a tidal cycle to obtain net fluxes. However, results from these studies integrate biogeochemical processes in the forest, tidal creeks, and the coastal boundary system thus making it difficult to separate the effect of the mangrove forest from the water column on these nutrient exchanges.
Despite the lack of significant net fluxes, the flume methodology allowed us to evaluate the hydrologic coupling between Shark River and the adjacent mangrove forest. The flume technique is considered an in situ, system level measurement of nutrient exchange in mangroves (Davis et al., 2001b) and has proved useful in micro-tidal environments (i.e., Rivera-Monroy et al.,1995). Thus, to correctly evaluate the role of mangroves as sinks, sources, or transformers of nutrients, a clear partition of nutrient fluxes should be established in Shark River from the mangrove forest into larger bodies of water as water travels through the landscape. Our study showed the utility of the flume technique as a sampling tool and underscored the need to include a larger sampling area to account for the frequency and duration of inundation in this semi-diurnal micro-tidal riverine mangrove forest. We are now implementing sampling protocols to combine larger flume sampling areas and the relative small network of tidal creeks extending up to 30-40 m inside the forest, which are a common feature in mangrove forests in Shark River.
Acknowledgements
We thank Justin Baker (UL-Lafayette) and Dan Bond (LSU) for their help in flume design and construction. We also greatly appreciate the support and assistance provided by Dan Childers (Florida International University, FIU), Ken Krauss (USGS-Lafayette), Carlos Coronado-Molina (SFWMD), Sharon Ewe (FIU), the Wetland Ecosystems Lab at FIU, and Rachel Butzler (TAMU). Special thanks to Douglas Morrison and Leslie Patterson from the Florida Bay Interagency Science Center-Everglades National Park for logistical support during the study. This material is based upon work supported by the National Science Foundation under Grant No. 9910514.
References
ALONGI, D. M., J. PFITZNER, L. A. TROTT, F. TIRENDI, P. DIXON & D. W. KLUMPP, 2005. Rapid sediment accumulation and microbial mineralization in forests of the mangrove Kandelia candel in the Jiulongjiang Estuary, China. Estuarine, Coastal and Shelf Science 63: 605-618. [ Links ]
ALONGI, D. M., A. SASEKUMAR, V. C. CHONG, J. PFITZNER, L. A. TROTT, F. TIRENDI, P. DIXON & G. J. BRUNSKILL. 2004. Sediment accumulation and organic material flux in a managed mangrove ecosystem: estimates of land-ocean-atmosphere exchange in peninsular Malaysia. Marine Geology 208: 383-402. [ Links ]
ALONGI, D. M., L. A. TROTT, G. WATTAYAKORN & B. F. CLOUGH. 2002. Below-ground nitrogen cycling in relation to net canopy production in mangrove forests of southern Thailand. Marine Biology 140: 855-864. [ Links ]
BOTO, K. G. & J. T. WELLINGTON. 1988. Seasonal Variations in concentrations and fluxes of dissolved organic and inorganic materials in a tropical, tidally-dominated, mangrove waterway. Marine Ecology Progress Series 50: 151-160. [ Links ]
CERP. 2004. Annual Report Comprehensive Everglades Restoration Plan. South Florida Water Management District, West Palm Beach. [ Links ]
CHEN, R. & R. R. TWILLEY. 1999. Pattern of mangrove forest structure and soil nutrient dynamics along the Shark River Estuary, Florida. Estuaries 22: 955-970. [ Links ]
CHILDERS, D. L., J. N. BOYER, S. E. DAVIS, C. J. MADDEN, D. T. RUDNICK & F. H. SKLAR. 2006. Relating precipitation and water management to nutrient concentrations in the oligotrophic "upside-down" estuaries of the Florida Everglades. Limnology and Oceanography 51: 602-616. [ Links ]
CHILDERS, D. L., S. E. DAVIS, R. R. TWILLEY & V. H. RIVERA-MONROY. 1999. Wetland-water column interactions and the biogeochemistry of estuarine-watershed coupling around the Gulf of Mexico. In: Bianchi, T. S., Pennock J. R. & Twilley, R. R. (Eds.). Biogeochemistry of Gulf of Mexico Estuaries. John Wiley & Sons, New York, pp. 211-235. [ Links ]
CHILDERS, D. L. 1994. Fifteen years of marsh flumes: A review of marsh-water column interactions in southeastern USA estuaries. In: Mitsch, W. J. (Ed.). Global Wetlands: Old World and New. Elsevier, Amsterdam, pp. 277-293. [ Links ]
CHILDERS, D. L. & J. W. DAY. 1988. A flow-through flume technique for quantifying nutrient and material fluxes in microtidal estuaries. Estuarine, Coastal and Shelf Science 27: 483-494. [ Links ]
DAVIS, S. E., C. CORONADO-MOLINA, D. L. CHILDERS & J. DAY. 2003. Temporally dependent C, N, and P dynamics associated with the decay of Rhizophora mangle L. leaf litter in oligotrophic mangrove wetlands of the southern Everglades. Aquatic Botany 75: 199-215. [ Links ]
DAVIS, S. E., D. L. CHILDERS, J. W. DAY, D. T. RUDNICK & F. H. SKLAR. 2001a. Nutrient dynamics in vegetated and unvegetated areas of a southern Everglades mangrove creek. Estuarine, Coastal and Shelf Science 52: 753-765. [ Links ]
DAVIS, S. E., D. L. CHILDERS, J. W. DAY, D. T. RUDNICK & F. H. SKLAR. 2001b. Wetland-water column exchange of carbon, nitrogen, and phosphorus in a southern Everglades dwarf mangrove. Estuaries 24: 610-622. [ Links ]
D'ELIA, C. F., P. A. STEUDLER & N. CORWIN. 1977. Determination of total nitrogen in aqueous samples using persulphate digestion. Limnology and Oceanography 22: 760-764. [ Links ]
DITTMAR, T. & R. J. LARA. 2001a. Do mangroves rather than rivers provide nutrients to coastal environments south of the Amazon River? Evidence from long-term flux measurements. Marine Ecology Progress Series 213: 67-77. [ Links ]
DITTMAR, T. & R. J. LARA. 2001b. Driving forces behind nutrient and organic matter dynamics in a mangrove tidal creek in North Brazil. Estuarine, Coastal and Shelf Science 52: 249-259. [ Links ]
DITTMAR, T., R. J. LARA & G. KATTNER. 2001. River or mangrove? Tracing major organic matter sources in tropical Brazilian coastal waters. Marine Chemistry 73: 253-271. [ Links ]
EYRE, B. & P. BALLS. 1999. A comparative study of nutrient behavior along the salinity gradient of tropical and temperate estuaries. Estuaries 22: 313-326. [ Links ]
LIGHT, S. S. & J. W. DINNEN. 1994. Water control in the Everglades: a historical perspective. In: Davis, S.M, Ogden, J. C. (Eds.). Everglades: The Ecosystem and its Restoration. St. Lucie Press, Delray Beach, Florida, pp. 47-84. [ Links ]
LUGO, A. E. & S. C. SNEDAKER. 1974. The ecology of mangroves. Annual Review of Ecology and Systematics 5: 39-64. [ Links ]
PARSONS, T. R., Y. MAITA & C. M. LALLI. 1984. A manual of chemical and biological methods for seawater analysis. Pergamon Press, New York [ Links ]
PELEGRI, S. P., V. H. RIVERA-MONROY & R. R. TWILLEY. 1997. A comparison of nitrogen fixation (acetylene reduction) among three species of mangrove litter, sediments, and pneumatophores in south Florida, USA. Hydrobiologia 356: 73-79. [ Links ]
PELEGRI, S. P. & R. R. TWILLEY. 1998. Heterotrophic nitrogen fixation (acetylene reduction) during leaf-litter decomposition of two mangrove species from south Florida, USA. Marine Biology 131: 53-61. [ Links ]
RIVERA-MONROY, V. H., J. W. DAY, R. R. TWILLEY, F. VERA-HERRERA & C. CORONADO-MOLINA. 1995. Flux of nitrogen and sediment in a fringe mangrove forest in Terminos Lagoon, Mexico. Estuarine, Coastal and Shelf Science 40: 139-160. [ Links ]
RIVERA-MONROY, V. H. & R. R. TWILLEY. 1996. An analysis of shrimp pond dynamics in three semi-intensive farms along the Caribbean coast of Colombia: Basis for a dynamic simulation model, University of Southwestern Louisiana, INVEMAR, CENIACUA. [ Links ]
SAS JMP. 2004. JMP Statistics and graphics guide, Version 5, Release 5.1.2. Statistical Analysis System, Cary, North Carolina. [ Links ]
SMITH, T. J. I., M. B. ROBBLEE, H. R. WANLESS & T. W. DOYLE. 1994. Mangroves, hurricanes, and lightning strikes. Bioscience 44: 256-262. [ Links ]
STRICKLAND, J. D. H. & T. R. PARSONS. 1972. A practical handbook of sea-water analysis. Fisheries Research Board of Canada 167: 1-310. [ Links ]
STRUVE, J., R. A. FALCONER & Y. WU. 2003. Influence of model mangrove trees on the hydrodynamics in a flume. Estuarine, Coastal and Shelf Science 58: 163-171. [ Links ]
SUTULA, M., J. DAY, J. CABLE & D. T. RUDNICK. 2001. Hydrological and nutrient budgets of freshwater and estuarine wetlands of Taylor Slough in southern Everglades, Florida (U.S.A.). Biogeochemistry 56: 287-310. [ Links ]
TWILLEY, R. R. 1985. The exchange of organic carbon in basin mangrove forests in a southwest Florida estuary. Estuarine, Coastal and Shelf Science 20: 543-557. [ Links ]
TWILLEY, R. R. 1995. Properties of mangroves ecosystems and their relation to the energy signature of coastal environments. In: Hall C. A. S. (Ed.). Maximum Power: The ideas and applications of H. T. Odum. Colorado Press, Colorado, pp. 43-62. [ Links ]
VALDERRAMA, J. C. 1981. The simultaneous analysis of total nitrogen and total phosphorus in natural-waters. Marine Chemistry 10: 109-122. [ Links ]
VALIELA, I., J. L. BOWEN & J. K. YORK. 2001. Mangrove forests: One of the world's threatened major tropical environments. BioScience 51: 807-815. [ Links ]
WATTAYAKORN, G., E. WOLANSKI & B. KJERFVE. 1990. Mixing, trapping and outwelling in the Klong Ngao mangrove swamp, Thailand. Estuarine, Coastal and Shelf Science 31: 667-688. [ Links ]
WELCH, R., M. MADDEN & R. DOREN. 1999. Mapping the Everglades. Photogrammetric Engineering and Remote Sensing 65: 163-170. [ Links ]
WHITING, G. J., H. N. MCKELLAR, J. D. SPURRIER & T. G. WOLAVER. 1989. Nitrogen exchange between a portion of vegetated salt marsh and the adjoining creek. Limnology and Oceanography 34: 463-473. [ Links ]
WOLANSKI, E. & P. RIDD. 1986. Tidal mixing and trapping in mangrove swaps. Estuarine, Coastal and Shelf Science 23: 759-771. [ Links ]
WOLAVER, T. G., G. WHITING, B. KJERFE, J. SPURRIER, H. MCKELLAR, R. DAME, T. CHRZANOWSKI, R. ZINGMARK & T. WILLIAMS. 1985. The flume design: a methodology for evaluating material fluxes between a vegetated salt marsh and the adjacent tidal creek. Journal of Experimental Marine Biology and Ecology 91: 281-291. [ Links ]
WOODROFFE, C. D. 1985. Studies of a mangrove basin, Tuff Crater, New Zealand. III. The flux of organic and inorganic particulate matter. Estuarine, Coastal and Shelf Science 20: 447-462. [ Links ]














