Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Hidrobiológica
versión impresa ISSN 0188-8897
Hidrobiológica vol.17 no.1 Ciudad de México abr. 2007
Artículos
Antibiotics incorporation in Artemia franciscana nauplii, metanauplii, juveniles and adults, and their inhibitory action on Aeromonas hydrophila bacteria
Incorporación de antibióticos en nauplios, metanauplios juveniles y adultos de Artemia franciscana y su acción inhibitoria en la bacteria Aeromonas hydrophila
Germán Castro Mejía1, Thalía Castro Barrera1, José Luis Arredondo Figueroa2, Jorge Castro, Mejía1, and Ramón De Lara Andrade1
1 Laboratorio de Alimento Vivo, Departamento del Hombre y su Ambiente, División de Ciencias Biológicas y de la Salud, Universidad Autónoma Metropolitana Xochimilco. Calzada del Hueso 1100, Col. Villa Quietud, 04960 Mexico, D.F. e-mail: gecastro@correo.xoc.uam.mx
2 Planta Experimental de Producción Acuícola, Departamento de Hidrobiología, División de Ciencias Biológicas y de la Salud, Universidad Autónoma Metropolitana Iztapalapa. Avenida San Rafael Atlixco 186, Colonia Vicentina, Iztapalapa, 09340 Mexico, D.F. Apartado Postal 55-535.
Recibido: 09 de febrero de 2006
Aceptado: 19 de octubre de 2006
Abstract
The crustacean Artemia franciscana has been used as a drug carrier, mainly in its nauplius stage; however, the use of other developmental stages, i.e., metanauplius, juvenile, and adult, potentially allows treating diseases not only in fry but also in juveniles and adults. In the present work, we studied the incorporation of antibiotics in these stages to inhibit the growth of the bacterium Aeromonas hydrophila, which causes a high mortality in freshwater fishes. The antibiotics used were: chloramphenicol (base antibiotic), nitrofurantoin (Macrodantina®, 50 mg capsules) and ciprofloxacin (Ciproflox®, 250 mg capsules). Four wells were made in Petri dishes with Trypticase® soybean agar (TSA) and 2 mL of bacterial inoculum. The wells were used for each antibiotic and one for the control. A 1-mL sample of each Artemia stage, incorporated with an antibiotic, was placed in each well and incubated for 24 h at 37°C, measuring the inhibition halos thereafter. Results indicated that 4 h are needed for the nauplii to become saturated and for the metanauplii, juveniles, and adults to fill their digestive tract with the antibiotic. In nauplii, the three antibiotics produced inhibition halos; in metanauplii, ciprofloxacin produced the best result (22.57 mm); in juveniles, chloramphenicol (38 mm) and ciprofloxacin (33 mm) gave the best Results; in adults, the best Results were obtained also with chloramphenicol (33 mm) and ciprofloxacin (40 mm). Nitrofurantoin did not yield positive Results in metanauplii, juveniles, and adults, and because it is soluble in water, it is recommended to apply it in lipidic solutions to ease its incorporation. Results from this study allow us to establish the bases for the control and treatment of infectious diseases caused by the bacterium Aeromonas hydrophila through the use of commercial antibiotics, easily available in Mexico.
Key words: Artemia, Aeromonas hydrophila, antibiotics, bioencapsulation.
Resumen
El crustáceo Artemia franciscana se ha utilizado como transportador de medicamentos, principalmente en su estadio de nauplio; sin embargo, el uso de otros estadios de desarrollo, como por ejemplo, metanauplio, juvenil y adulto, ha permitido el tratamiento contra las enfermedades, no sólo en larvas, sino en juveniles y adultos. En el presente trabajo, se incorporaron antibióticos en estos estadios para inhibir el crecimiento de la bacteria Aeromonas hydrophila, que causa altas mortalidades en peces de agua dulce. Los antibióticos probados fueron: cloramfenicol (base pura), nitrofurantoina (Macrodantina®, cápsulas de 50 mg) y ciprofloxacina (Ciproflox®, cápsula de 250 mg). En cajas de Petri con un medio de agar de soya Triptocaseína (TSA) y 2 ml de inóculo de la bacteria se hicieron cuatro pozos. Los pozos se utilizaron para cada uno de los antibióticos y uno para el control. Una muestra de 1 mL de cada estadio de Artemia con antibiótico incorporado, se colocaron en cada pozo y se incubaron por 24 h a 37 °C, y se midieron los halos de inhibición. Los resultados indican que se necesitan 4 h para que el nauplio se sature y los metanauplios, juveniles y adultos llenen su tracto digestivo con el antibiótico. En los nauplios, los tres antibióticos produjeron halos de inhibición; en los metanauplios, el ciprofloxacino dio los mejores resultados (22.57 mm); en juveniles, el cloramfenicol (38 mm) y el ciprofloxacino (33 mm) dieron los mejores resultados; en adultos, los mejores resultados se obtuvieron con cloramfenicol (33 mm) y ciprofloxacino (40 mm). La nitrofurantoina no dio resultados positivos en metanauplios, juveniles y adultos, debido a que es soluble en el agua, se recomienda aplicarlo en soluciones lipídicas para facilitar su incorporación. Los resultados de este estudio, nos permiten establecer las bases para el control y tratamiento de enfermedades infecciosas causadas por la bacteria Aeromonas hydrophila, usando antibióticos comerciales de fácil adquisición en México.
Palabras clave: Artemia, Aeromonas hydrophila, antibióticos, bioencapsulación.
Introduction
Chemotherapeutics directly applied to the water or administered through the feed are generally used in aquaculture to treat infectious diseases. In this two ways of application, part of the drug is lost in the water, inducing a pollution problem and, besides, the organism does not consume the adequate dose (Gapasin et al., 1996). Therefore, it has been necessary to find alternative ways to provide medication in this field.
It is important to develop methodologies that will allow using live feed as a carrier, not only of enriching substances but also of prophylactic and therapeutic substances, because, through this mechanism, treatment is not lost in the water and is more efficient as it is not dissolved (Mohney et al., 1990; Chérel & Nin, 1991), and allows the administration of the adequate dose needed by each species and in each developmental stage.
The crustacean Artemia franciscana (Kellogg, 1906) has been traditionally used as a drug carrier (Mohney et al., 1990; Nelis et al., 1991; Verpraet et al., 1992; Aguilar-Aguila et al., 1994; Dixon et al., 1995a,b; Touraki et al., 1996, 1999; Majack et al., 2000; Gómez-Gil et al., 2001; Cook & Rust, 2002) mainly in its nauplius stage because of its availability, easy handling, and small size. This stage is used as feed for diverse species in culture (Castro et al., 2003); however, using Artemia in other developmental stages, such as metanauplii, juveniles, and adults will allow treating infectious diseases not only in fish fry but also in juveniles and adults, even in brood stock, avoiding in this way the horizontal and vertical transmission of the pathogen, and the consequent economical losses due to mortality of organisms in culture caused by viruses, bacteria, fungi, and parasites.
The pathogenic bacterium Aeromonas hydrophila (Stainer, 1943) can cause high mortality in culture systems, inducing morphological and physiological changes in the organisms and, consequently, strong economical losses to aquaculture farmers (Trust, 1986; Alderman, 1988; Alderman & Hastings, 1998). Based on the after mentioned, the aim of this work was to investigate whether bioencapsulation of three different antibiotics in nauplii, metanauplii, juveniles, and adults of A. franciscana presented activity against A. hydrophyla.
Material and methods
Culture of A. franciscana. Organisms were cultured until the adult stage in three 200 L cylindrical, plastic containers, maintaining a salinity of 60 g/L and constant aeration. A one organism/mL density was kept in each container. Organisms were fed daily with 50 mL of a rice bran preparation during the first five days of life and thereafter with two liters of Tetraselmis spp. and Chlorella spp. at a relation of 1:1, until reaching the adult stage, which, by reproducing, provided the metanauplius and juvenile stages.
For the nauplii, we followed the decapsulation technique described by Sorgeloos et al. (1986) and modified by Castro and De Lara (1991). The eggs, without the chorion, where placed in a 3-L glass container provided with 3 L water at a salinity of 40 g/L, and constant aeration and controlled temperature (23 ± 2°C).
Culture of the bacterial strain. The bacterium used in this work was Aeromonas hydrophila (strain ATCC 7966, provided by the School of Chemistry, UNAM). The bacterial strain was kept refrigerated at -20°C, until used. When needed, it was thawed by keeping it at ambient temperature for 24 h to verify viability it was placed in a liquid Trypticase® soybean (TSB Bioxon, Mexico) medium and incubated for 24 h at 37°C according to Gherna's technique (1994). Bacterial mobility was verified with a microscope.
Antibiotics. We performed an antibiogram with 39 antibiotics that inhibit Aeromonas growth, from these we chose three that gave a larger than 10 mm inhibition halo, independently from the sanitary regulation of Food and Drug Administration (FDA) enforced in aquaculture. The three chosen antibiotics, either in their base form (pure) or in capsules were: chloramphenicol (base antibiotic), nitrofurantoin (Macrodantina®, 50 mg capsules), and ciprofloxacin (Ciproflox®, 250 mg capsules).
Standard calibration curve of the antibiotic vs. the inhibitory halo. For each antibiotic, a calibration curve was performed to determine the relation between the diameter of the inhibition halo and the antibiotic concentration. This relation was used to calculate the appropriate amount of antibiotic to be incorporated during the different developmental stages of A. franciscana. To obtain the calibration curve of the antibiotic concentration, sensidisks of 6 mm in diameter were used, one for each antibiotic (chloramphenicol, 30 µg; nitrofurantoin, 300 µg; ciprofloxacin, 5 µg). Sensidisks were cut in half, and one of the halves was cut anew in half to obtain three parts, then each part was weighed on an analytical-grade scale (Dr. H. Sandoval, 2003. Personal communication). To obtain the inhibition halos of each part of the sensidisk, the three parts were placed in Petri dishes containing a Trypticase® soybean agar (TSA) medium and 2 mL of the bacterial broth. Petri dishes were incubated for 24 h at 37°C and the inhibition halo was measured with a micrometer (Vernier). This was done in triplicate.
The calibration curve was done based on the concentration value in each part of the sensidisk and contrasting it against the inhibition halo area. The linear regression between these two values was expressed with the equation:
y (concentration) = a x (inhibition area) + b
where the value of a is the slope of the curve and b is the interception. To avoid errors in data, by means of the Excel® software the straight line to the interception was taken to the zero value. Estimation of antibiotic concentrations obtained in each bioassay was achieved according to the equation.
All the work from there on was performed under sterile conditions, using sterilized materials.
Incorporation time of antibiotics in A. franciscana. To determine the antibiotics incorporation into the digestive tract of Artemia, with a 90% survival, the concentrations of each antibiotic varied and the amounts chosen were: 1 g of chloramphenicol, 250 mg of cyprofloxacin (one capsule), and 100 mg of nitrofurantoin (2 capsules); each antibiotic concentration was dissolved in 500 mL of water at 40 g/L salinity, and was then filtered through a 53 µm mesh to obtain the size needed for its ingestion by Artemia (Gelabert, 2003). To each antibiotic solution, 1,000 metanauplii, 100 juveniles, and 100 adults were added separately. Organisms were observed under the microscope every 20 min to determine the time needed to fill the digestive tract with the antibiotic in more than 90% of them. Considering that nauplii are unable to ingest particles because their digestive tract is still closed, they were left in the solution until their bodies became impregnated with the drug, since nauplii can only ingest particles 8 h after eclosion, when their mouth and digestive tract have oponed (Coutteau & Sorgeloos, 1997).
Drug incorporation bioassays during the different A. franciscana developmental stages.
Nauplii and metanauplii. A total of 5,000 nauplii (10 organisms/ mL) was placed in a 500-mL flask with water at 40 g/L salinity, ambient temperature (23 ± 2°C), and constant aeration. This procedure was followed with each antibiotic. The antibiotic, at the previously indicated concentration, was added to each flask. Once the nauplii became impregnated with the drug, they were filtered and washed with freshwater and then manually ground in a mortar.
In 10 cm diameter Petri dishes with 50 mL TSA medium and 2 mL of bacterial inoculum, four small wells (1.0 X 0.5 cm or 1.5-6 mL) were made, one for each antibiotic and one for the witness. 1 mL of the ground nauplii was placed in each well. The Petri dishes were incubated for 24 h at 37°C, and the inhibition halos were measured.
For the metanauplii bioassay, 48 h old organisms were used; they were placed in the solution with the specific drug and left until at least 90% of them showed a full digestive tract. Procedures to determine incorporation of the antibiotic, and its effects were the same as for nauplii.
Juveniles and adults. To perform the bioassays with these two developmental stages, 200 juvenile organisms (10 days old) and 200 adults (15 days old) were removed from the culture. Each group was placed in 1-L flasks containing 500 mL of water at 40 g/L salinity; this was done for each antibiotic. Organisms were left 24 h without any feed, afterwards these stages were incorporated into the solutions containing each of the antibiotics at the mentioned concentrations. The organisms were observed through a microscope until 90% had a full digestive tract, then they were removed from the solution and washed with freshwater. Because juveniles and adults have sizes 300 to 500-times larger than nauplii and metanauplii we made groups of 5, 10, 15, 25, 50 individuals, as well as a control group of 50 individuals. Each group was placed in a mortar and manually ground. To determine the inhibition halo in these developmental stages the same technique used for nauplii and metanauplii was followed.
Statistical analysis. To make the antibiotics calibration curve all data were subjected to linear regression aimed at obtaining the relation between the inhibition halo diameter and the antibiotic concentration. The standard curve was made in triplicate.
Results
Antibiotics calibration curves. Table 1 shows the Results from the determination of inhibition of the three chosen antibiotics considering the size of the sensidisk (1, 1/2, and 1/4). The relation between the concentration of each antibiotic and the inhibition area is expressed as:
Y = 3.1912X+5E-14 for chloramphenicol (R2 = 0.9944)
Y = 24.517X+2E-13 for ciprofloxacin (R2 = 0.9616)
Y= 0.2832X-6E-14 for nitrofurantoin (R2 = 0.9944).
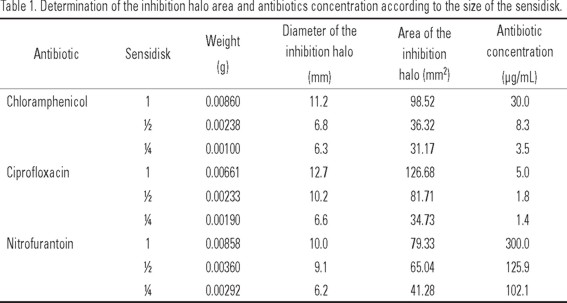
With these formulas, we calculated the concentration reached by each antibiotic incorporated into the A. franciscana nauplii, metanauplii, juveniles, and adults.
Antibiotics incorporation time into A. franciscana. In each of the developmental stages, the digestive tract became full in 3:30 to 4:00 h. No differences were observed in the filling time with any of the three antibiotics among the different developmental stages.
Incorporation bioassays during the different A. franciscana developmental stages
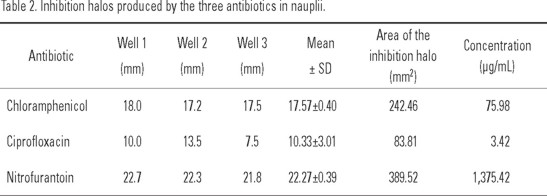
Nauplii. Results are shown in Table 2. The antibiotic with the largest inhibition halo was nitrofurantoin with an average value of 22.27 mm, yielding a concentration of 1,375.42 µg/mL. The other two antibiotics presented inhibition halos larger than 10 mm.
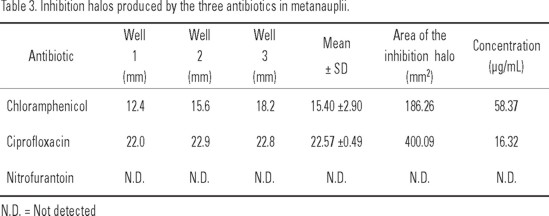
Metanauplii. Results are shown in Table 3. The antibiotic with the largest inhibition halo was ciprofloxacin with an average value of 22.57±0.49 mm yielding a concentration of 16.32 µg/mL, followed by chloramphenicol with 15.40 ± 2.90 mm; nitrofurantoin did not form an inhibition halo.
Juveniles. The bioassay in this stage was performed with 25 individuals for each antibiotic. Chloramphenicol and ciprofloxacin produced inhibition halos of 38 and 33 mm, respectively (355.39 µg/mL and 34.89 µg/mL). Nitrofurantoin did not form an inhibition halo (Table 4).

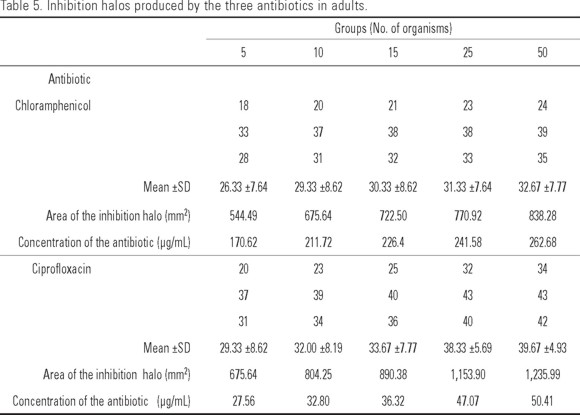
Adults. Table 5 shows the diameters of the inhibition halos obtained with the three antibiotics in the different A. franciscana groups. With chloramphenicol and ciprofloxacin an increase in the diameter of the inhibition halo was observed concomitantly with the increase in the amount of organisms, obtaining values from 26 to 33 mm for chloramphenicol (170.62 a 262.68 µg/mL) and 29 to 40 mm for ciprofloxacin (27.56 a 50.41 µg/mL). Nitrofurantoin showed no inhibition halo.
Discussion
Aeromonas hydrophila used did not show sensitivity to 36 of the 39 antibiotics specific for the inhibition of Aeromonas growth. Therefore, we chose chloramphenicol, ciprofloxacin, and nitrofurantoin that gave larger than 10-mm inhitibion halos, although two of these products do not comply with the sanitary regulation for their use in aquaculture, and the other is only recommended for human use (Hernandez, 2005) but they are easy to obtain in the pharmaceutical market in Mexico and their presentation in capsules eases their handling.
There is scarce bibliographic information on the use of these antibiotics being incorporated into A. franciscana, during its different developmental stages. For example, Léger and Sorgeloos (1992) used chloramphenicol in A. franciscana nauplii, enriching them during 6 h with a lipidic solution (SELCO); but there are no References to ciprofloxacin and nitrofurantoin incorporation in this developmental stage nor on their use in metanauplii and juveniles. In the adult stage, Dixon et al. (1995b) incorporated the antibiotic sarafloxacin, and Majack et al. (2000), Cook and Rust (2002), and Cook et al. (2003) used the antibiotic erythromycin, in three different chemical forms, obtaining a 75% survival in adults at a 67-79 µg/L concentration.
The technique used in this work to determine the relation between the inhibition area and the antibiotic concentration differs from the techniques described by Roque et al. (1998), Mohney et al. (1990), Majack et al. (2000), Gómez-Gil et al. (2001), and Cook et al. (2003). These authors performed dilutions of the antibiotics concentration, considering their known value, and used the plate diffusion technique to determine the inhibition areas. In the present work, a modification was performed to the plate diffusion technique, which consisted in using fractions of the sensidisks to determine the inhibition area; therefore, no proportion was observed in the diameter of the inhibition halo with respect to the antibiotic concentration. The obtained data allowed us to perform a regression that gave a correlation of R2 = 0.9 for the three antibiotics; hence, a linear response of the inhibition halo against the antibiotic concentration. Aguilar-Aguila et al. (1994) recorded a correlation value of R2 = 0.81 between the concentration of the antibiotic Romet-30 (trimethoprim + sulfamethoxazole) and the obtained inhibition area, using plate diffusion tests.
In this research we determined that a period of 4 h is more than enough to fill the digestive tract of more than 90% of A. franciscana metanauplii, juveniles, and adults, and to impregnate the body of the nauplii with chloramphenicol, and ciprofloxacin. Regarding nitrofurantoin, this yielded positive values only in the nauplii stage. Other authors, like Touraki et al. (1991; 1996), have indicated that the incorporation time in nauplii can vary from 1 to 8 h, depending on the substance being bioencapsulated. Dixon et al. (1995b) incorporated the antibiotic sarafloxacin at 10% + SELCO in a 6-h period; Mohney et al. (1990) and Touraki et al. (1999) incorporated Romet-30 (80% sulfadimethoxazole and 20% omethoprim) in a 4 h period; and Gómez-Gil et al. (2001) incorporated the antibiotic enrofloxacin in 4 h.
Regarding antibiotic incorporation in the adult stage, Majack et al. (2000), and Cook and Rust (2002) indicated that addition of the antibiotic erythromycin during periods of 14 until 18 h at a maximum induces a high mortality starting at 8 hours. Majack et al. (2000), using a 2.0 g/L erythromycin suspension, reached the maximal concentration in adult organisms in just 2 h.
Nauplii. The three antibiotics adhered to the nauplius and, hence, showed inhibition halos against the A. hydrophila bacterium. It is important to point out that this stage is the most used as drug carrier because of its easy handling. This might be a more realistic application approach, mainly because nauplii are used as feed for some larval stages of economically important fishes in Mexico.
Metanauplii. In contrast to the nauplius, in this stage the digestive tract is already complete and is able to incorporate small particles. Gelabert (2003) indicates that in small A. franciscana (0.8 to 3.2 mm) organisms, the particles filtration process is more efficient than in later stages; Wilson (1989) states that the intense metabolic activity during this stage fosters the filtration activity. This behavior was observed when the antibiotics chloramphenicol and ciprofloxacin were given, as these are not completely soluble and their particles were assimilated by metanauplii that gave inhibition halos, whereas nitrofurantoin, which is soluble, did not induce an inhibition halo.
Juveniles and adults. In these two stages, the morphological development of the organisms is complete; the thoracic appendages (thoracopods) play an important role in particles filtration, so that if the antibiotic is soluble, like nitrofurantoin, it does not incorporate easily into the organism, and therefore no inhibitory effect on the bacterium is manifested. As in metanauplii, the antibiotics chloramphenicol and ciprofloxacin yielded large inhibitory halos in these two developmental stages resulting from their retention in the organism's body.
When working with antibiotics, it is necessary to know the maximal amount tolerated by the carrier organism, in this case A. franciscana, to be able to recommend an adequate dose. When using the antibiotics chloramphenicol (base antibiotic) and ciprofloxacin (Ciproflox®, 250 mg capsules), commercial presentation, it is recommended to make 50% dilutions, because the commercial doses were not tolerated by this species, presenting a high mortality at 4 h of bioencapsulation. The antibiotic nitrofurantoin (Macrodantina, 50 mg capsules), although having effects in Artemia nauplii, did not yield positive Results in the metanauplii, juvenile, and adult stages, hence it must be tested at higher doses or the drug must be emulsified.
Although further research on the incorporation of drugs in more advanced developmental stages of A. franciscana is still needed, this study provides original and practical information that can be used in aquaculture activities, specifically for the prevention and treatment of diseases caused by the bacterium A. hydrophila, as well as on the use of easily available commercial antibiotics.
Although the objective of the present work was fulfilled, as we determined the incorporation time of the studied antibiotics into the different developmental stages of Artemia, as well as their survival and the effect on the Aeromonas hydrophila bacterium; we consider that it will be necessary to test other antibiotics recommended by FDA to be used in aquaculture and to assess the feasibility of the technique used in the present work.
References
AGUILAR-AGUILA, A., A. TEJEDA-MANSIR & A. RUIZ-ENRÍQUEZ. 1994. Using brine shrimp as a drug carrier for therapeutic applications in aquaculture. Aquaculture Engineering 13: 301-309. [ Links ]
ALDERMAN, D.J. 1988. Fisheries chemotherapy a review. In: Muir, J.F. & R.J. Roberts (Eds.). Recent Advances in Aquaculture. Vol. 3. Croom Helm. London, United Kingdom and Timber Press, Portland, Oregon, pp. 1-61. [ Links ]
ALDERMAN, D.J. & T.S. HASTINGS. 1998. Antibiotic use in aquaculture: development on antibiotic resistance-potential for consumer health risks. Journal of Food Science Technology 33: 139-155. [ Links ]
CASTRO, B.T., M.J. CASTRO & M.G. CASTRO. 2003. Artemia. In: B.T. Castro (Ed.). Alimento vivo para organismos acuáticos. AGT Editor, S.A. México. pp. 67-81. [ Links ]
CASTRO, M.J. & DE LARA, A.R. 1991. Manual de técnicas para el manejo de quistes de Artemia sp. Manual. CBS: Universidad Autónoma Metropolitana-Xochimilco. 47 p. [ Links ]
CHEREL, P. & F. NIN. 1991. Antibiotherapy using biocarriers (Artemia salina) in hatcheries. In: Chemotherapy in Aquaculture: from theory to reality. Ed. British Crown. pp. 389-393. [ Links ]
COOK M.A. & M.B. RUST. 2002. Bioencapsulation of five forms of erythromycin by adult Artemia salina (L.). Journal of Fish Diseases 25: 165-170. [ Links ]
COOK, M.A., M.B. RUST, K. MESSEE, T. MAJACK & M.E. PETERSON. 2003. Uptake of erythromycin by first-feeding sockeye salmon, Oncorhynchus nerka (Walbaum), feed live or freeze-dried enriched adult Artemia or medicated pellets. Journal of Fish Disease 26: 277-285. [ Links ]
COUTTEAU, P. & P. SORGELOOS. 1997. Manipulation of dietary lipids, fatty acids and vitamins in zooplankton cultures. Freshwater Biology 38: 501-512. [ Links ]
DIXON, B.A., S.O. VAN POUCKE, D. KAWAHIGASHI, M. CHAIR, M. DEHASQUE, H.J. NELIS, P. SORGELOOS & A.P. DE LEENHEER. 1995A. In: P. Lavens, E. Jaspers y I. Roelants (Eds.). Bioencapsulation of antibacterial drugs in nauplii and adult brine shrimp, Artemia franciscana. Larvi 95. Fish & Shellfish Larviculture Symposium. European Aquaculture Society. Special Publications. No. 24. Genth, Belgium, pp. 508-510. [ Links ]
DIXON, B.A., S.O. VAN POUCKE, M. CHAIR, M. DEHASQUE, H.J. NELIS, P. SORGELOOS & A.P. DE LEENHEER. 1995B. Bioencapsulation of the antibacterial drug sarafloxacin in naupliii of the brine shrimp Artemia franciscana. Journal of Aquatic Animal Health 7: 42-45. [ Links ]
GAPASIN, R.S.J., H.J. NELIS, M. CHAIR & P. SORGELOOS. 1996. Drug assimilation in the tissue of European sea bass (Dicentrarchus labrax) fry delivered orally through bioencapsulation. Journal of Applied Ichtyology 12: 39-42. [ Links ]
GELABERT, F. R. 2003. Bioencapsulation in Artemia: II. Influences of the particle concentration in the enrichment process. Aquaculture 216:143-153. [ Links ]
GHERNA, R.L. 1994. Culture preservation. In: P. Gerhardt, R. G. E. Murray, W. A. Wood & N. R. Krieg (Eds.). Methods for General and Molecular Bacteriology, pp. 278-292. American Society Microbiology. Washington, DC. 278-290 [ Links ]
GÓMEZ-GIL, B., J. CABANILLAS-RAMOS, S. PAEZ-BRAMBILLA & A. ROQUE. 2001. Standardization of the bioencapsulation of enrofloxacin and oxytetracycline in Artemia franciscana Kellogg, 1906. Aquaculture 196:1-12. [ Links ]
HERNANDEZ, S.P. 2005. Responsible use of antibiotics in aquaculture. FAO Fisheries Technical Paper. No. 469. Rome. 97 p. [ Links ]
LÉGER, P. & P. SORGELOOS. 1992. Optimized feeding regimes in shrimp hatchery. In: A.W. Fast & L.J. Lester (Eds.) Marine shrimp culture: principles and practices. Chapter 9. Elsevier Science Publishers. U.S.A. pp. 225-244. [ Links ]
MAJACK, T.J., M.B. RUST, K.C. MASSEE, G.W. KISSIL, R.W. HARDY & M.E. PETERSON. 2000. Bioencapsulation of erythromycin using adult brine shrimp, Artemia franciscana (Latreille). Journal of Fish Disease 23: 71-76. [ Links ]
MOHNEY, L., D. LIGHTNER & R. WILLIAMS. 1990. Bioencapsulation of therapeutic quantities of the antibacterial Romet-30 in nauplii of the Brine shrimp Artemia and in the Nematode Panagrellus redivivus. Journal of the World Aquaculture Society 21(3): 186-191. [ Links ]
NELIS, H.J., F.LEGER, P. SORGELOOS & P. DE LEENHEER. 1991. Liquid chromatographic determination of efficacy of incorporation of Trimethoprim and Sulfamethoxazole in Brine Shrimp (Artemia spp.). Used for prophylactic chemotherapy of fish. Antimicrobial Agents and Chemotherapy 35 (12): 2486-2489. [ Links ]
ROQUE, A., J.F. TURNBULL & A. GOMEZ-GIL. 1998. Delivery of bioencapsulated oxytetracycline to the marine shrimp Penaeus monodon. Journal of the World Aquaculture Society 29: 249-251. [ Links ]
SORGELOOS, P. P. LAVENS, P. LÉGER; P. TACKAERT & W. VERSICHELE. 1986. Manual for the culture and use of brine shrimp Artemia in aquaculture. Manual prepared for the Belgian Administration for Development Cooperation and the Food and Agriculture Organization of the United Nations. Artemia Reference Center. Faculty of Agriculture, State University of Ghent, Belgium. 319 p. [ Links ]
TOURAKI, M., P. RIGAS, P. PERGANTAS, T. ABATZOPOULOS & C. KASTRITSIS. 1991. Optimizing bioencapsulation of the antibiotics trimethoprim and sulfametroxazole in Artemia nauplii. In: Lavens, P., Sorgeloos P.M., Jaspers, E. & F. Ollevier (Eds.). Larvi. Fish and Crustacean Larviculture Symposium. Special Publication No. 15. European Aquaculture Society, Ghent, Belgium, pp. 415-417. [ Links ]
TOURAKI, M., S. MOURELATOS, G. KARAMANLIDOU, S. KALAITZOPOULOU & C. KASTRITSIS. 1996. Bioencapsulation of chemotherapeutics in Artemia as a means of prevention and treatment in infection diseases of marine fish fry. Aquaculture Engineering 15: 133-147. [ Links ]
TOURAKI, M., I. NIOPAS & C. KASTRITSIS. 1999. Bioaccumulation of trimethoprim, sulfamethoxazole and N-Acetyl-Sulfamethoxazole in Artemia nauplii and residual kinetics in seabass larvae after repeated oral dosing of medicated nauplii. Aquaculture 175: 15-30. [ Links ]
TRUST, T.J. 1986. Pathogenesis of infectious diseases of fish. Annual Review of Microbiology 40: 479-502. [ Links ]
VERPRAET, R., M. CHAIR, P. LÉGER, H. NELIS, P. SORGELOOS & A. DE LEENHEER. 1992. Live-food mediated drug delivery as a tool of disease treatment in larviculture. The enrichment of therapeutics in rotifers and Artemia nauplii. Aquaculture Engineering 11: 133-139. [ Links ]
WILSON, J. A. 1989. Fundamentos de Fisiología Animal. Limusa y McMillan, México, 984 pp. [ Links ]














