Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Hidrobiológica
versão impressa ISSN 0188-8897
Hidrobiológica vol.16 no.3 Ciudad de México Dez. 2006
Artículos
Use of constructed wetlands for the treatment of water from an experimental channel at Xochimilco, Mexico
Empleo de humedales artificiales para el tratamiento de aguas de un canal experimental de Xochimilco, México
Patricia Martínez-Cruz1, Armando Hernández-Martínez 1, Ruth Soto-Castor2, Alfonso Esquivel Herrera2 and Jesús Rangel Levario3
1 Laboratorio de Biotecnología, Departamento Sistemas Biológicos, CBS., Universidad Autónoma Metropolitana-Xochimilco. Calz. del Hueso 1100, Col. Villa Quietud, C.P., México, D.F. E-mail address: pmartine@correo.xoc.uam.mx
2 Laboratorio de Ecología Microbiana, Departamento El Hombre y su Ambiente, CBS., Universidad Autónoma Metropolitana-Xochimilco. Calz. del Hueso 1100, Col. Villa Quietud, C.P., México, D.F.
3 Departamento Métodos y Sistemas, CyAD, Universidad Autónoma Metropolitana-Xochimilco. Calz. del Hueso 1100, Col. Villa Quietud, C.P., México, D.F.
Recibido: 28 de junio de 2005
Aceptado: 13 de marzo de 2006
ABSTRACT
Two constructed wetlands were designed and their performance in the treatment of water from Cuemanco, in an experimental channel at Xochimilco, Mexico was assessed. One of them employed emergent macrophytes (Scirpus americanus) and the other one used floating macrophytes (the duckweed Lemna gibba). The system was stabilized for five months. Water quality was improved after 220 days of treatment, as organic matter (assessed as DQO) was removed from 43.38% to 58.52% by employing L. gibba and S. americanus, respectively. Nutrient removal significatively (P < 0.05) differed between the two treatments and the control, as 86.35% and 80.33% of ammonia, 89.47% and 90.23% of nitrite, and 38.44% and 50.20% of reactive phosphorus were removed by S. americanus and L. gibba, respectively. On the other hand, water pH changed from alkaline values to neutrality after the treatment, an important issue as this water is employed for cultured land irrigation.
Key words: Chinampas, constructed wetlands, Lemna gibba, Scirpus americanus, nutrient removal.
RESUMEN
En este estudio se diseñaron y evaluaron dos sistemas de humedales artificiales: uno utilizando macrofitas enraizadas (Scirpus americanus) y el otro con plantas acuáticas flotantes (Lemna gibba), para el tratamiento de las aguas de un canal experimental de Cuemanco en Xochimilco-México. El uso de estas plantas acuáticas autóctonas de la Zona Chinampera de Xochimilco, mejora la calidad del agua del Canal de Cuemanco cuando ésta es tratada durante 220 días después de un periodo de adaptación de 5 meses, ya que fue posible remover la materia orgánica (DQO) en un 48.38% y 58.52% con L. gibba y S. americanus respectivamente. La concentración de nutrientes con los sistemas experimentales disminuyó significativamente con respecto al control (P < 0.05), logrando remociones de 86.35% y 80.33% del nitrógeno amoniacal, 89.47% y 90.23% del nitrito, 38.44% y 50.20% del fósforo reactivo, con S. americanus y L. gibba, respectivamente. Por otro lado, el empleo de estos sistemas disminuye el pH alcalino de las aguas del canal generando valores neutros que son importantes ya que esta agua suele utilizarse para el riego de las chinampas.
Palabras clave: Chinampas, humedales artificiales, Lemna gibba, Scirpus americanus, remoción de nutrientes.
INTRODUCCIÓN
At present, Xochimilco's lacustrine zone comprises a network of channels as well as some small lagoons that, along with the chinampas, conform a unique ecosystem which has served for centuries as a means of communication and a source of aquatic resources, while its waters have been used for irrigation. Especially important are the cultures on the chinampas, artificial islands made by interweaving twigs and branches of trees locally known as ahuejotes, along with reeds and bulrushes and filled with sediment from the lake's bottom; in ancient times this agrosystem produced high yields. Xochimilco is located at the southern part of Mexico's Basin, in the outskirts of Mexico City (Quiroz, 1980, Juárez-Figueroa et al., 2003).
Before the 1930's it was an open water lake but its dessication rate accelerated from this time on, as water from its sources was diverted for human consumption in Mexico City. This, along with chinampa enlargement and change of land use for urbanization, resulted in the transformation of an open lake to a channel network. As more water was pumped into Mexico City (about 50% of the water from underground sources) from this zone, water levels at the channels alarmingly diminished and this loss was compensated by pumping water from the water treatment plant at Cerro de la Estrella into Xochimilco (Mazari et al., 2000).
This plant processes water from Iztapalapa, a municipality east of Mexico City that produces household as well as industrial wastewater; this results in faecal matter and inorganic salts along with toxic chemicals being thus introduced into the Xochimilco ecosystem. The decomposition of organic matter depletes oxygen from the water receiving it (Mason, 1995), while phosphates and other salts provoke phytoplankton blooms, and toxic chemicals such as heavy metals have given rise to irreversible effects on the flora and fauna of the channels (Balanzario, 1982; Báez et al., 1975, Quiroz & Miranda, 1994). According to Cairns (2001) nutrient excess, along with the input of high concentrations of chemical compounds from industrial, agricultural and household sources, produce a chemical stress on natural water reservoirs that affects the natural ecosystem processes and, in the particular case of Xochimilco, has resulted in diminished biodiversity and has made its water, flora and fauna resources unsuitable for human consumption, due to the accumulation of toxic pollutants, especially heavy metals. The presence of these latter in the water of the channels is due to the input of insufficiently treated wastewater from household and industrial sources through the effluent from treatment facilities managed by the local government. The most important of these treatment plants is located at Cerro de la Estrella; from 1971 to 1993 it produced 2 m3/s of secondary effluent and since 1994 its production grew to 4 m3/s of tertiary treated effluent. Nevertheless, this plant is not designed for heavy metal treatment and insufficiently designed for nutrient salt removal; thus for 22 years this plant has discharged semi-treated water that contains arsenic, cadmium, chrome, mercury, lead, zinc as well as organic and inorganic toxic compounds (DGCOH, 1993).
As a result, a series of studies have been undertaken to assess the degree of this pollution and to serve as a basis for the development of proposals for the restoration of Xochimilco. One of the possible solutions could be through the use of aquatic macrophytes in constructed wetlands (Tchobanoglous, 1997; Zhu & Sikora, 1995; Comin et al., 1997; Abbisy & Mandi, 1999; Giersberg et al., 1986), as these water treatment systems have been found to either eliminate or accumulate pollutants in a natural, clean, affordable and effective way, and there are a number of studies dealing with their physiology, depuration mechanisms and potential for use (Reddy & DeBusk, 1987) or bioeconomic assessments related to their effective life spans (Steer et al., 2003).
Typically, these systems are mono or polycultures of vascular plants in shallow ponds or raceways receiving wastewater that, by being kept in contact with plants, is cleared from pollutants through several mechanisms (Reddy & DeBusk, 1987). In systems based on emergent macrophytes, an important part of the depuration process is accomplished by microorganisms associated to the root system that uptake some of these pollutants as a nutrient source or otherwise transform them (Hoagland et al., 2001). Subsurface flow systems are constructed wet-lands where wastewater flows horizontally through channels containing the support medium and emergent macrophytes; other systems employ floating or submerged macrophytes (Tchobanoglous, 1997; Finlayson & Chick, 1983; Miranda-Ríos & Luna-Pabello, 2001).
Constructed wetlands are an alternative to conventional water treatment facilities, mainly in rural areas where the cost of connecting to wastewater treatment plants can be prohibitive; their construction and operating costs are lower, their operation is relatively simpler and their mean life span is about 20 years but require more land than conventional plants; they generate useful by-products such as biomass and are attractive to wildlife, especially waterfowl (Steer et al., 2003; Seoánez, 1999). Biochemical oxygen demand (BOD), chemical oxygen demand (COD) and fecal coliforms can be removed nearly to 100%; nevertheless, nitrogen and phosphorus removal fluctuates and on occasions can be low (Gray et al., 2000).
Other considerations for plant selection must also be taken into account, such as plant sensitivity to detrimental factors in the treated water, such as heavy metals. Miranda et al. (2000) determined that Lemna gibba rapidly uptakes cadmium and lead from polluted water and thus attains a saturation state which also results in chlorophyll a and b decrease of more than 50% at the third day of growing under experimental conditions.
Nevertheless, this species appears to be growing successfully at several localities of Xochimilco; this may be due to the fact that these metals are concentrated mainly at the sediment and their concentrations in water are low (Bojórquez & Amaro, 2003).
The present study assesses the efficiency of constructed wet-lands for pollutant removal from the water of the an experimental Canal de Cuemanco, at Xochimilco. A subsurface flow system was compared to a floating macrophyte system, employing bulrushes (Scirpus americanus) and duckweed (Lemna gibba), respectively.
MATERIALS AND METHODS
Reservoir construction. Two 135-liter polypropilene containers were used; to these we adapted tubing at its upper and lower ends for wastewater input and sampling, respectively. These models were installed at CIBAC (Centro de Investigaciones Biológicas y Acuícolas de Cuemanco), located at the eastern margin of the Virgilio Uribe olympic rowing facilites and west of the Canal de Cuemanco, México.
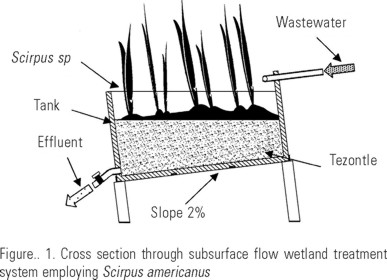
Constructed subsurface flow wetland. Tezontle (a porous volcanic rock) was fragmented and sorted, selecting 1-2 cm-diameter particles. These were added to the first container up to 2/3 of its volume and served as a support for bulrush roots (Scirpus americanus) (González, 1990; Zavaleta-Beckler & Ramos-Espinosa, 1999) previously collected from the Canal de Cuemanco and rinsed with clean water (Fig. 1).
Floating macrophytes system. The other container was filled to its upper rim with water from the Canal de Cuemanco and duckweed (Lemna gibba) obtained at the channel was placed at its surface, totally covering it (Fig. 2), these presented the characteristic purple spots above and purple coloration beneath their fronds (Novelo y Lot, 1990); some of the fronds were slightly swollen and so not as flat as those of Lemna obscura (=Lemna minor) and their presence in Cuemanco further confirms their identity, as Novelo & Lot (1990) reported L. gibba in perturbated environments with high nutrient concentrations. These plants were compared to the botanical specimens from the Producción Agrícola y Animal Department collection, Universidad Autónoma Metropolitana Xochimilco.
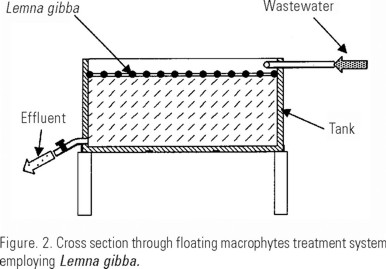
As a control, a pond was excavated, its walls and floor were lined with plastic foil and filled with water from the channel (about 300 liters) in order to determine the evolution of water conditions with no macrophyte treatment.
Adaptation and treatment. Both macrophyte systems were adapted to the treatment conditions for five months prior to the experiments by removing 1/3 of the water from each of the reactors every week and replacing it with water from the Canal de Cuemanco. Treatment began after this conditioning and lasted for 220 days (from late April to November, 2001); both biorreactors were fed with water from the channel, by replacing one third of the water volume with water from Canal Nacional per week. Every seven days, duplicate samples were obtained from the Canal Nacional (dynamic control), from the experimental pond (static control) and from the output from the bulrush and duckweed systems. These samples were immediately placed on ice after their collection and protected from direct sunlight to be transported to the laboratory (in about 20 minutes).
Physical and chemical analysis. Water temperature was directly measured with a capillary mercury thermometer, ±0.05°C precision. Chemical oxygen demand (COD), pH, and ammonia, nitrite and phosphate concentrations were assessed following the standard methods in APHA, AWWA & WPCF (1995).
Statistical analysis. The data were analyzed using repeated measures ANOVA to look for significant differences between treatments and within treatments, over time. Differences of means were evaluated for significance by Tukey's HSD (Honest Significant Difference) test (P < 0.05) for homogeneous variances. Calculations were performed with STATISTICA® software (Statsoft, 1999).
RESULTS AND DISCUSSION
The analysis of variance for the water parameters, according to the treatments, indicated that water temperature did not differ among treatments and the differences found for all of the other parameters are thus not due to variations in temperature, but to the treatment employed, or between control and treatment conditions. Pairwise comparisons between treatments for the variables here considered, as obtained from Tukey's HSD test are presented at Fig. 3. This figure confirms that temperature did not differ between any of the treatments. Phosphate and COD showed differences between the experimental treatments and the controls, as well as among the different treatments, but no differences between the static and the dynamic control, while the rest of the parameters (nitrite, ammonia and pH) differed both between the experimental treatments and between both controls. All measured variables showed variations along the year in all systems studied, and this agrees with the results by Forbes et al., (2005), who found that water treatment through wetlands usually show high variability, especially regarding phosphate retention. The irregular behavior detected in the present work is probably due to the presence of a complex microbial community -which comprises cyanobacteria, autotrophic and heterotrophic bacteria, fungi and protozoa- which also contributes to the degradation of certain compounds (Quiroz & Miranda, 1984).
Removal of chemical oxygen demand. As shown Fig. 4b, organic matter concentrations, expressed as COD can be reduced by both macrophyte systems; this removal reached average levels of 48.38% and 58.52% with Lemna gibba and Scirpus americanus, respectively. These values agree with the results by Juwarkar et al. (1995) who employed Typha latifolia and thus removed from 28 to 41% of COD, while Steinmann et al. (2003) removed 37.6% of COD with a macrophyte wetland. Nevertheless, Paing y Voisin (2005) attained 92% COD removal with bulrush, even though this was accomplished only after two or three years had elapsed. Uhi & Dittmer (2005) obtained 84% decrease in COD by treating water with bulrush for 10 years, while Molle et al., (2005) were able to increase the efficiency of this process up to 90%. According to the Tukey's HSD test (Fig.3b) significant differences are found between the control and experimental systems, but no differences between both experimental systems. Thus, both macrophytes are able to induce the decay of organic matter into energy and inorganic metabolic by-products through chemical oxidation. Results by Abissy & Mandy (1999) who obtained an organic matter removal of 91% in arid zone studies with a system planted with Typha latifolia and 83% in unplanted systems in a 20-month study, and those by Sikora et al. (1995) who obtained 90% removal with Scirpus spp. and Typha latifolia, show that higher efficiencies can be attained after longer periods.
Nitrite removal. In Table 1, we see that nitrite attained removal percentages of 90.23% and 89.47% for L. gibba and S. americanus, respectively, compared to the dynamic control. This suggests that nitrites are rapidly oxidized to nitrate which is readily uptaken by macrophytes as a nitrogen source, in agreement to Quiroz et al., (1982), who found that hydrophytes such as Lemnaceae and Scirpus olneyi, among others, have a high ability for nitrogen absortion from the environment. Miranda et al., (2000), confirmed that absortion and adsortion of cadmium and lead occur in L. gibba submitted for 13 days to to these metals. Even though the controls showed high variability, (Fig. 4f), according to the Tukey's HSD test (Fig. 3f) significant differences are found between these and the macrophyte systems, but there was no difference between L. gibba y S. americanus. Our nitrite removal results are higher than those by Shutes et al. (2005), who obtained nitrite removal up to 65.5% with a joint Typha and Phragmities system through five consecutive years, while Uhi & Dittmer (2005) accomplished 95.9% nitrite removal by employing bulrush in a 10-year treatment which suggests that similar efficiencies can be attained by our system, providing that a similar period has elapsed.
Temperature. In accordance with Fig. 3a, we did not find statistically significant differences between any of the experimental systems or controls employed, but we found important seasonal variation: average temperature for the period from May to August was 23°C for all the systems, while from September to February et decreased to an average of 13°C for all of the systems (Fig. 4a). This agrees with results by Talling (1990) and Andersson et al., (2005), neither of whom found statistically significant variation for this parameter.
Reactive phosphorus removal. Phosphate removal is especially important in freshwater systems in order to minimize eutrophication, as phosphorus is commonly the limiting factor for production. Our results showed high reactive phosphate concentrations for all systems considered from April to May (Fig. 4e). As these are the driest moths, there is apparently a concentration effect because of water evaporation. Afterwards, from June to early November phosphate diminished due to dilution by rainfall and maximum removal percentages of 50.20% and 38.44% for L. gibba and S. americanus, respectively, were attained as compared to the dynamic control (Table 1). According to the statistical analysis, phosphate removal differs significantly when the experimental systems are compared with the controls but the difference is not statistically significant when both experimental systems are compared (Fig. 3e); apparently, phosphorus removal is limited by the capacity of the media to adsorb, bind, or precipitate the incoming phosphorus (Arias & Brix, 2005), and there are even some researchers, Leader et al., (2005) who report phosphate removal up to 98% by employing co-treatment systems involving calcium or iron. Martin & Gerald (1994) removed 69.5% with a system that combined floating and emergent macrophytes. Richardson (1985) believed that emergent macrophytes were capable of higher phosphate absorption, due to rhizosphere activity, but warned that this system saturates and is able to release the absorbed phosphate if plants are not regularly harvested. We believe that chemical precipitation of reactive phosphate can also occur and thus prevent higher total phosphate removal, as Martin & Gerald (1994) found that phosphorus absortion by plants occurs slowly and only for soluble phosphate compounds. On the other hand, Andersson et al., (2005) obtained variable phosphate removal (30 to 90%) for seven years, depending on the macrophyte system employed.
Ammonia removal. Table 1 show that the highest removal percentages for this nutrient were 86.35% for S. americanus and 80.33% for L. gibba, compared to the dynamic control, and this implies statistically significant differences between experimental systems and this control, even though there are irregular behavior in dynamic control (Fig. 4c). Nevertheless, no statistically significant differences between experimental systems and the static control were found (Fig. 3c). These differences might be due to the fact that ammonia-excreting organisms, such as fish, amphibia and zooplankton occur at the Canal de Cuemanco, where even the excreta of urea by waterfowl can be hydrolized to ammonia, while this phenomenon did not occur at the pond (static control). Macronutrient (nitrogen, phosphorus and potassium) removal has also been demonstrated for Ludwigia peploides when used for phytoremediation of natural ecosystems for preventing the eutrophization of epicontinental aquatic systems (Wang et al., 2002). Our removal figures are higher than those obtained by Abissy & Mandi (1999), who removed 17% and 31% of ammonia by employing Typha latifolia and Juncus sibilanus, respectively, Shutes et al., (2005) who also removed 31% of ammonia with a joint Typha latifolia. and Phragmites australis system and are also higher than those by Schulz et al. (2003), who attained removal percentages from 64.1% to 73.8% with emergent macrophytes. Nevertheless, higher removal percentages are possible through the use of systems including floating as well as emergent macrophytes such as those reported by Martin & Gerald (1994) and Zhu & Sikora (1995), who achieved removal percentages of 98.3% and 95-100%, respectively.
Other macrophytes also bioaccumulate heavy metals such as cadmium and lead, as occurs with several Azolla species, which include A. microphylla, A. filiculoides and A. pinnata, as has been proved for wetlands. These species also have the ability to increase their tissues and thus to grow rapidly when some nitrogen compunds are available, according to Anju et al.,(2004).
The results here presented prove that macrophytes bioaccumulate nitrogen and phosphorus compounds and this ability can result in their potential use as soil conditioners, germination promoters or as fodder.
pH. Water from the Canal de Cuemanco tends to be alkaline throughout the year (Fig. 4d) due to salinization of the chinampa's soil, which has caused diminishing productivity of crops; these results agree with previous reports by Quiroz & Miranda (1994). Körner et al. (2001) found that Lemna gibba does not grow when pH is above 9.8, depending on temperature. During the present study we found that our macrophyte systems neutralized pH to average values of 6.88 and 7.64 for S. americanus and L. gibba, respectively, and pH differences between experimental and control systems are thus statistically significant and also between S. americanus and L. gibba systems (Fig. 3d). The values obtained fall within the range permitted by the Norma Oficial Mexicana NOM-CCA031-ECOL-1993. Our results agree with those by Hench et al. (2003), who obtained values from 7.1 to 6.5 by treating with reeds, bulrushes and duckweed.
The main conclusions that can be drawn from this experiment were: Excepting water temperature and ammonia, the differences between the controls and the macrophyte systems began to be noticeable from the second half of June and this suggests that warm temperatures (which at the zone occur from March to mid June) favor the development of the root system.
Our constructed wetlands both appear to be efficient for organic matter (COD), ammonia and reactive phosphate removal, as shown by Tukey's HSD analysis, when comparing these systems to controls.
Macrophytes drive water pH to a neutral range, especially in the case of S. americanus and this may have beneficial effects for the aquatic biota and also for crops, as water from the channels is employed for watering them.
Presently, it cannot be established yet which system of the two is more efficient, as statistical analysis did not show significant differences between them.
It would be desirable to continue the treatment for a longer period in order to increase the development of the root system of S. americanus and to obtain a better evaluation of seasonal differences, at least for three years.
These results point out that L. gibba-based and S. americanus-based water treatment systems are a feasible alternative for the treatment of water from the Canal de Cuemanco. Another advantage to be taken into account is the fact that these macrophytes are native to the region and that their use would also contribute to biodiversity conservation of this ecosystem.
ACKNOWLEDGEMENTS
The authors acknowledge Dr. Virginia Graüe, excoordinator of CIBAC for her support to field research in that center, and are grateful to industrial designers Alejandro Pichardo and Rafael Segura for their support to the design and construction of the experimental prototypes, and to Elida Urbina for her collaboration in the monitoring activities and sample processing. Isabel Pérez Montfort corrected the English version of the manuscript.
REFERENCES
ABBISY, M. & L. MANDI 1999. Comparative Study of wastewater purification efficiencies of two emergent helophytes: Typha latifolia and Juncus subilagus under arid climate. Water Science Technology 39(10-11):123-128. [ Links ]
ANJU, A. A. SOOD & P.K. SINGH. 2004. Hyperaccumulation of cadmium and nickel by Azolla species. Indian Journal of Plant Physiology. 9(3): 302-304. [ Links ]
APHA-AWWA-WPCF 1995. Standard methods for the examination of water and wastewater. 19th edition. American Public Health Association, Washington, D.C. pp. 1-44, 1-45, 2-88, 2-89, 4-145 a 4-148. [ Links ]
ANDERSSON, J.L., S. KALLNER & K.S.TONDERSKI. 2005. Free water surface wetlands for wastewater treatment in Sweden; nitrogen and phosphorus removal. Water Science Technology 51(9):39-46. [ Links ]
ARIAS, C.A. & H. BRIX. 2005. Phosphorus renoval in constructed wetlands: can suitable alternative media be identified?. Water Science & Technology 51(9):267-273. [ Links ]
BÁEZ, P.A., R. BELMONT R & O. GONZÁLEZ. 1975. Modificación de la calidad de las aguas del Lago de Xochimilco por el uso de aguas negras en su recargo. Primer Congreso Ibero del Medio Ambiente. Madrid, España, pp. 1055-1069. [ Links ]
BALANZARIO, Z.J. 1982. Contaminación de los canales de Xochimilco y repercusión en las actividades económicas. Boletín de la Sociedad Mexicana de Geografía y Estadística. UNAM, pp. 284. [ Links ]
BOJÓRQUEZ, C.L. & E.J.M. AMARO. 2003. Caracterización múltiple de la calidad del agua de los canales de Xochimilco. In Stephan-Otto, E. (ed.) El Agua en la Cuenca de México. Sus Problemas Históricos y Perspectivas de Solución. UAM-Xochimilco - Patronato del Parque Ecológico de Xochimilco A.C. Mexico. 1:281-302. [ Links ]
CAIRNS, J. 2001. The role of reservoirs in sustainable use of the planet. Hydrobiologia 547:61-67. [ Links ]
COMÍN, F.A., J.A. ROMERO, V. ASTORGA & C. GARCÍA. 1997. Nitrogen removal and cycling in restored wetlands used as filters of nutrients for agricultural runoff. Water Science Technology 35(5): 255-261. [ Links ]
DGCOH, 1993. Diagnóstico de la Calidad FQB del Sistema de Canales de Xochimilco. Sec. Gral. de Obras, Depto del Distrito Federal. México (mimeo) [ Links ]
FORBES, M.G., K.L. DICKSON, F. SALEH, W.T. WALLER, R.D. DOYLE & P. HUDAK. 2005. Recovery and Fractionation of Phosphorus retsained by lightweight expanded shale and masonry sand used as media in subsurface flow treatments wetlands. Environment Science and Technology 39: 4621-4627. [ Links ]
FINLAYSON, C.M. & A.J. CHICK. 1983. Testing the potential of aquatic plants to treat abattoir effluent. Water Research 17(4): 415-422. [ Links ]
GIERSBERG, R.M., V.B. ELKINS, S.R. LUION & L. GOLDAM. 1986. Role of aquatic plants in wastewater treatment by artifical wetlands. Water Research 20(3): 363-368. [ Links ]
GONZÁLEZ, E.M.S. 1990. Cyperaceae. In: Rzedowski J. & G.C. Rzedowski (eds.) Flora Fanerogámica del Valle de México. V. III Monocotyledoneae. Instituto de Ecología. Centro Regional del Bajío. Pátzcuaro, México. pp. 174-235. [ Links ]
GRAY, S., J. KINROSS, P. READ & A. MARLAND. 2000. The nutrient assimilative capacity of maerl as a substrate in constructed wetland systems for waste treatment. Water Research 34(8): 2183-2190. [ Links ]
HENCH, K.R., G.K. BISSONNETTE, A.J. SEXSTONE, J.G. COLEMAN, K. GARBUTT & J.G. SKOUSEN. 2003. Fate of physical, chemical and microbial contaminants in domestic wastewater following treatments by small constructed wetlands. Water Research 37: 921-927. [ Links ]
HOAGLAND, C.R., L.E. GENTRY. M.B. DAVID & D.A. KOVACIC. 2001. Plant Nutrient uptake and Biomass Accumulation in a Constructed Wetland. Journal of Freshwater Ecology 16(4):527-540. [ Links ]
JUÁREZ-FIGUEROA, L.A., J. SILVA-SÁNCHEZ, F.J. URIBE-SALAS & E. CIFUENTES-GARCÍA. 2003. Microbiological indicators of water quality in the Xochimilco Canals, Mexico City. Salud Pública de México 45(5):389-395. [ Links ]
JUWARKAR A.S., B. OKE, A. JUWARKAR & S.M. PATNAIK. 1995. Domestic wastewater treatment trough constructed wetland in India. Water Science Technology 32(5):255-261. [ Links ]
KÖRNER, S., S.K. DAS, S. VEENSTRA & J.E. VERMAAT. 2001. The effect of pH variation at the ammonium/ammonia equilibrium in wastewater and its toxicity to Lemna gibba. Aquatic Botany 71:71-78. [ Links ]
LEADER, J.W.; K.R. REDDY & A.C. WILKIE. 2005. Optimization of low-cost phosphorus removal from wastewater using co-treatments with constructed wetlands. Water Science Technology 51(9):283-290. [ Links ]
MARTIN, C.D. & A.M. GERALD. 1994. Nutrient reduction in an in-series constructed wetland system treating landfill leachate. Water Science Technology 29(4):267-272. [ Links ]
MASON, C.F. 1995. Biology of Freshwater Pollution. 3rd ed. Blackwell Science. London, pp. 48. [ Links ]
MAZARI-HIRIART, M., E. CIFUENTES, E. VELÁZQUEZ & J. CALVA. 2000. Microbiological groundwater quality and health indicators in Mexico, City. Urban Ecosystems 4:91-103. [ Links ]
MIRANDA, G.; A. QUIROZ & M. SALAZAR. 2000. Cadmium and lead removal from water by the duckweed Lemna gibba L.(Lemnaceae). Hidrobiologica UAM-Iztapalapa. Mexico. 10(1):7-12. [ Links ]
MIRANDA-RÍOS, M. & V.M. LUNA-PABELLO. 2001. Estado del Arte y Perspectivas de Aplicación de los Humedales Horizontales de Flujo Horizontal en México. Serie: Tratamiento Biológico de Aguas Residuales, Facultad de Química, Universidad Nacional Autónoma de México. México. pp. 121. [ Links ]
MOLLE, P.; A. LIÉNARD, C. BOUTIN, G. MERLIN & A. IWEMA. 2005. How to treat raw sewage with constructed wetlands: an overview of the French systems. Water Science Technology 51(9):11-21. [ Links ]
NOM-CCA031-ECOL-1993. Establece los límites máximos permisibles de contaminantes en las descargas de aguas residuales provenientes de la industria, actividades agroindustriales, de servicios y el tratamiento de aguas residuales en los sistemas de drenaje y alcantarillado urbano o municipal. Diario Oficial de la Federación, 18 de octubre de 1993. [ Links ]
NOVELO, A. & A. LOT. 1990. Lemnaceae. In Rzedowski J. & G.C. Rzedowski (eds.) Flora Fanerogámica del Valle de México. V. III Monocotyledoneae. Instituto de Ecología. Centro Regional del Bajío. Pátzcuaro, México. pp. 240-247. [ Links ]
PAING, J. & J. VOISIN. 2005. Vertical flow constructed wetlands for municipal wastewater and septage treatment in French rural area. Water Science Technology 51(9):145-155. [ Links ]
QUIROZ, F.A., M.G. MIRANDA & A. LOT. 1982. Uso potencial de algunas hidrófitas como abono verde en la zona chinampera de Xochimilco. Biótica 7(4):631-633. [ Links ]
QUIROZ, F.A. & M.G. MIRANDA. 1984. Determinación del aporte total de nitrógeno y fósforo al sedimento en los canales de Mixquic por la comunidad de lemnáceas. Biótica 9(4):429-432. [ Links ]
QUIROZ A. & G. MIRANDA. 1994. Heavy metals and macronutrients concentration in leaves and petioles of Nymphaea mexicana Zucc. In a polluted pound of Xochimilco, México. International Journal of Experimental Botany 55:83-87 [ Links ]
REDDY, K.R. & T.A. DEBUSK. 1987. State of the art utilization of aquatic plants in water pollution control. Water Science Technology 9(10):61-79. [ Links ]
RICHARDSON, C.J. 1985. Mechanisms controlling phosphorus retention capacity in freshwater wetlands. Science 228:1424-1427. [ Links ]
SCHULZ, C., J. GELBRECHT & B. RENNERT. 2003. Treatment of rainbow trout farm effluents in constructed wetland with emergent plants and subsurface horizontal water flow. Aquaculture 217:207-221. [ Links ]
SEOÁNEZ, C.M. 1999. Aguas residuales: tratamiento por humedales artificiales. Ediciones Mundi-Prensa, España, Madrid, pp. 51-57. [ Links ]
SHUTES, B., J.B. ELLIS, D.M. REVITT & L.N.L. ACHOLES. 2005. Constructed wetlands in UK urgan surface drainage systems. Water Science Technology 51(9):31-37. [ Links ]
SIKORA, F.J., Z. TONG, L.L. BEHRENDS, S.L. STEINBERG & H.S. COONROD. 1995. Ammonium removal in constructed wetlands with recirculating subsurface flow: removal rates and mechanisms. Water Science Technology 32(3):193-202. [ Links ]
STATISTICA® 1999. Software, Statistic Software package for MS Windows, 1999. [ Links ]
STEER, D., T. ASELTYNE & L. FRASER. 2003. Life-cycle economic model of small treatment wetlands for domestic wastewater disposal. Ecological economics 44:359-369. [ Links ]
STEINMANN, CH.R., S. WEINHART S. & A. MELZER. 2003. A combined system of lagoon and constructed wetland for an effective wastewater treatment. Water Research 37: 2035-2042. [ Links ]
TALLING J.F. 1990. Diel and seasonal energy transfer, storage and stratification in Africa reservoirs and lakes. Arch Hydrobiol Beih Ergebn Limnol. 33:651-660. [ Links ]
TCHOBANOGLOUS, G. 1997. Land-Based Systems, constructed wetlands and aquatic plant systems in the United States: an overview. Capítulo 6 in "Ecological Engineering for Wasterwater Treatment. 2a. edición. CRC Press, Inc. Pp.. 77-87. [ Links ]
UHI, M. & U. DITTMER. 2005. Constructed wetlands for CSO treatment: an overview of practice and research in Germany. Water Science Technology 51(9):23-30. [ Links ]
WANG, Q., Y. CUI, Y. DONG. 2002. Phytoremediation of Polluted Waters Potentials and prospects of Wetlands Plants. Acta Biotechnology 22(1):199-208. [ Links ]
ZAVALETA-BECKLER, P. & M.G. RAMOS-ESPINOSA. 1999. Flora de Xochimilco. Serie Académicos CBS Universidad Autónoma Metropolitana Xochimilco. (25):93. [ Links ]
ZHU T. & F. SIKORA. 1995. Ammonium and nitrate removal in vegetated and unvegetated gravel bed microcosm wetlands. Water Science Technology 32(3):219-228. [ Links ]














