Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Hidrobiológica
versão impressa ISSN 0188-8897
Hidrobiológica vol.16 no.1 Ciudad de México Abr. 2006
Artículos
Antibiotic and heavy metal resistance of Aeromonas hydrophila isolated from charal (Chirostoma humboldtianum, Valenciannes, 1835)
Resistencia a antibióticos y metales pesados en Aeromonas hydrophila aisladas de charal (Chirostoma humboldtianum, Valenciannes, 1835)
Gloria Luz Paniagua, Eric Monroy, Magdalena Perches, Erasmo Negrete, Octavio García y Sergio Vaca
Facultad de Estudios Superiores Iztacala, UNAM. Av. de los Barrios 1, Los Reyes Iztacala, Tlalnepantla 54090, Edo. de México, México. E-mail: vsergio@servidor.unam.mx
Recibido: 27 de mayo de 2005
Aceptado: 24 de octubre de 2005
ABSTRACT
Antibiotic and heavy metal susceptibilities of twenty Aeromonas hydrophila strains, isolated from the gastrointestinal tract of the charal (Chirostoma humboldtianum), an autochthonous Mexican fish, were analyzed. All strains produced β-lactamase and were resistant to penicillin and dicloxacillin, showing single peak for minimum inhibitory concentration (MIC) distributions at 2000-4000 µg/mL and 500-1000 µg/ml, respectively. Ampicillin MIC distribution was bimodal with 20% resistant strains (125-250 µg/ml) and 80% highly resistant ones (500-4000 µg/ml). All strains were susceptible to ceftriaxone (MIC= 3.9 µg/ml) and all but one were susceptible to cefuroxime (3.9 µg/ml and 62.5 µg/ml). All strains had a single MIC distribution pattern for lead (800-3200 µg/ml), and mercury (20 µg/ml) and were considered resistant and susceptible to these ions, respectively. Fifteen percent of the isolates were resistant to arsenite (MIC= 400-800 µg/ml) and all were susceptible to silver (MIC= 1.25-2.5µg/ml), chromate (MIC= 93.5-375 µg/ml), and zinc (MIC=21.25-42.5 µg/ml).
Key words: Aeromonas, heavy metal resistance, Chirostoma.
RESUMEN
Se analizó la susceptibilidad a antibióticos β-lactámicos y a metales pesados en 20 cepas de Aeromonas hydrophila, aisladas del tracto gastrointestinal de charal (Chirostoma humboldtianum), un pez autóctono mexicano. Todas las cepas produjeron β-lactamasa, fueron resistentes a penicilina y dicloxacilina, y presentaron distribuciones unimodales para la concentración mínima inhibitoria (CMI) de 2000-4000 µg/ml y 500-1000 µg/ml, respectivamente. La distribución de la CMI de ampicilina fue bimodal con 20% de cepas resistentes (125-250 µg/ml) y 80% altamente resistentes (500-4000 µg/ml). Todas las cepas fueron sensibles a ceftriaxona (CMI= 3.9 µg/ml) y todas, excepto una, fueron sensibles a cefuroxima (3.9 µg/ml y 62.5µg/ml). Las 20 cepas mostraron distribuciones unimodales de las CMI de plomo (800-3200 µg/ml) y mercurio (20 µg/ml) y fueron consideradas resistentes y sensibles, respectivamente, a estos iones. Quince por ciento de las cepas fueron resistentes a arsenito (CMI= 400-800 µg/ml) y todas fueron sensibles a plata (CMI=1.25-2.5 µg/ml), cromato (CMI=93.5-375 µg/ml) y zinc (CMI= 21.25-42.5 µg/ml).
Palabras clave: Aeromonas, resistencia a metales pesados, Chirostoma.
INTRODUCTION
Aeromonas hydrophila is a Gram negative bacterium widely distributed in freshwater environments (Holmes et al., 1996). It is a well-known fish (Hazen et al., 1978; Joseph & Carnahan, 1994; Austin & Adams, 1996) and human pathogen (Altwegg & Geiss, 1989). Since some strains of Aeromonas are enteropathogens possessing a range of virulence factors (enterotoxins, cytotoxins, haemolysins, and invasive ability), infected fish may be vehicles of human infection (Cahill, 1990; Kirov, 1993). Antibiotic-resistant A. hydrophila from clinical and environmental sources have been reported (Gosling, 1986; Chang & Bolton, 1987; Koehler & Ashdown, 1993; Yamaguchi et al., 1999; Thayumanavan et al., 2003) and some are heavy metal resistant also (Miranda &Castillo, 1998).
Chirostoma humboldtianum is a Mexican endemic fish belonging to the Atherinopsidae family (Dyer & Chernoff, 1996). It is a wild non-cultivated fish widely consumed in Mexico since long before the Spanish Conquest (Sierra & Sierra, 1977), for which, to our knowledge, there is no microbiological studies published. In this paper we report the isolation of thirty bacterial strains: Aeromonas hydrophila (20), Hafnia alvei (5), Enterobacter cloacae (2), Enterobacter amnigenus (1), Citrobacter freundii (1), and Klebsiella spp. (1) from the charal (Chirostoma humboldtianum) and the A. hydrophila susceptibility to five antibiotics and to six heavy metals.
MATERIALS AND METHODS
Bacteria isolation and identification. Fifty two fishes were collected during September of year 2000 and March 2001, from the San Felipe Tiacaque freshwater pond at Ixtlahuaca, Edo. de Mexico, Mexico. Fishes were collected in sterile polyethylene bags and brought to the laboratory in an ice chest. Samples were processed within 2 h of collection. Extraction of the intestinal tract content of the fishes was done as suggested in Thoesen (1994). First the excess of mucus was removed by cleaning the ventral surface area using a sterile paper towel, then, a 70% ethanol solution was spread over, and dried with a paper towel. After this, by using sterile surgical scissors and a scalpel, an incision was performed starting from the ventral wall towards the anus. The digestive tract was gently held using sterile forceps. Intestinal content was aseptically swabbed using sterile cotton buds, inoculated into brain hearth infusion broth (BHI, Bioxon, Mexico) and incubated at 25°C for 24 h. Afterwards, aliquots of culture were streaked on eosin methylene blue agar and sheep blood agar (Bioxon, Mexico) and incubated at 25°C for 24 h. Thirty strains were isolated and they were identified as A. hydrophila (20), Hafnia alvei (5), Enterobacter cloacae (2), Enterobacter amnigenus (1), Citrobacter freundii (1), and Klebsiella spp. (1) by the API-20E system (bioMérieux, France). Aeromonas hydrophila isolates were selected for further characterization.
MIC of heavy-metals determination. The minimal inhibitory concentration (MIC) of toxic metals in nutrient agar (Bioxon, Mexico) was determined by an agar dilution procedure, as previously described (Vaca et al., 1995). In brief: Each bacterial strain was grown in nutrient broth at 25°C for 24 h with agitation. Cultures were diluted 1:3 in the wells of a Steers replicator and inoculated on nutrient agar plus serial double dilutions of each metal. Ions tested, all from Merck, were as follows: lead [Pb (CH3COO) 2], chromate (K2CrO4), zinc (ZnCl2), silver [(CH3COO)Ag], mercury (HgCl2), and arsenite (NaAsO2)]. MIC breakpoints (µg/ml) for considering a bacterial isolate as susceptible (S) or resistant (R) were those reported in Vaca et al. (1995): lead S < 800, R >800; chromate S <750, R >750; mercury S <54, R >54; Nakahara et al. (1977): arsenite S <400, R >400; zinc S <170, R >170; and Gupta et al. (1998): silver S ≤ 34, R > 34.
MIC of β-lactams determination. MIC of β-lactam antibiotics were determined in Mueller-Hinton according with the guidelines of the NCCLS (1997). β-lactams tested, all from Sigma-Aldrich, were: ampicillin, penicillin G procainic, dicloxacillin, cefuroxime, and ceftriaxone. Ampicillin plus sulbactam (2:1) was from Glaxo.
MIC breakpoints (µg/ml) for considering a bacterial isolate as susceptible (S) or resistant (R) were those recommended by the NCCLS (1997): penicillin S ≤ 0.12, R ≥ 0.25; dicloxacillin, ampicillin, cefuroxime S ≤ 8, R ≥ 32; ceftriaxone S ≤ 8, R ≥64.
β-lactamase activity was detected by hydrolysis of the chromogenic cephalosporin nitrocefin (BBL) (O'Callaghan et al., 1972).
RESULTS
All strains produced β-lactamases and were resistant to penicillin and dicloxacillin, showing single peak MIC distributions at 2000-4000 µg/ml (Fig. 1A) and 500-1000 µg/ml (Fig. 1C), respectively. Ampicillin MIC distribution was bimodal (Fig. 1E) with 20% resistant strains (MIC= 125-250 µg/ml) and 80% highly resistant ones (MIC= 500-4000 µg/ml). Strains showed three distinguishable susceptibilities to the combination ampicillin plus sulbactam (Fig. 1B). Thirty percent of the strains had a 3.9 µg/ml ampicillin plus sulbactam MIC (96.7-99.9 % lower than their corresponding ampicillin MIC), whereas 45% of the strains showed a 31.3 µg/ml ampicillin plus sulbactam MIC (87.5-98.4 % lower than their ampicillin MIC) and 25% had a 125-250 µg/ml ampicillin plus sulbactam MIC (75-87.5% lower than their ampicillin MIC). All strains were susceptible to ceftriaxone (Fig. 1D) and all but one were susceptible to cefuroxime (Fig. 1F).

All strains were resistant to lead (MIC=800-3200 µg/ml, Fig. 2A) and susceptible to chromate (MIC≤ 375 µg/ml), silver (MIC≤ 2.5 µg/ml), mercury (MIC=20 µg/ml) and zinc (MIC≤ 42.5 µg/ml) (Fig. 2 C-F). Susceptibility to arsenite showed a bimodal MIC distribution with a peak of 15% resistant strains (MIC=400-800 µg/ml), and 85% of them susceptible (MIC=200 µg/ml Fig. 2B).
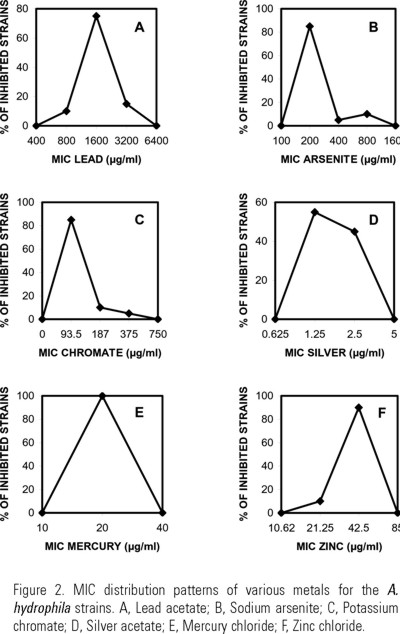
DISCUSSION
Chirostoma humboldtianum is an endemic Mexican fish, widely consumed by low-statuses Mexican people, for which no microbiological studies have been published. We have found that these fishes harbored β-lactamase producer A. hydrophila strains resistant to penicillin, dicloxacillin, and ampicillin (Fig. 1 A, C, E). Since ampicillin MIC was 75-99.9% lowered by sulbactam (Fig. 1B) these results suggest us that β-lactamases produced by the strains probably are conventional spectrum enzymes, which do not confer resistance to cephalosporins (Payne et al., 1994). This conclusion was further supported by the ceftriaxone and cefuroxime susceptibility of the strains (Fig. 1 D, F). These results are similar to those found by Castro-Escarpulli et al. (2003) for 82 Aeromonas spp. strains isolated from frozen tilapia (Oreochromis niloticus niloticus) purchased in local markets of Mexico City; all strains were resistant to penicillin and ampicillin, but sensitive to cefuroxime.
All A. hydrophila strains reported here were resistant to lead, showing a single-peak MIC distribution pattern (800-3200 µg/ml, Fig. 2A). This result is in good agreement with similar single-peak MIC distributions for Pb found by Nakahara et al. (1977) in clinical Gram-negative bacterial strains (at 1600-3200 µg/ml) and Vaca et al. (1995) in a group of strains (at 800-1600 µg/ml), mostly Gram-positive, isolated from soil adjacent to a busy traffic high way from Mexico City highly polluted by automobile exhausts containing Pb (9.6-29.3 ppm) derived from gasoline.
The presence of toxic inorganic ions in the environment usually inhibits the exposed microorganisms but may also select variants able to tolerate high concentrations of the ions (Doelman, 1987).
Although in this work concentrations of heavy metals present in the water or in the fishes were not measured, the fact that all A. hydrophyla strains isolated were lead-resistant suggests that bacterial populations present at this pond had been subject to a strong selection by lead-containing pollutants. The mechanisms of bacterial resistance to lead are complex and they are not due to a single point mutation. Lead-resistant bacteria have been isolated from metal polluted soils; resistance is due to the extracellular exclusion (Pseudomonas marginalis), or to the intracellular accumulation (Citrobacter freundii) of the metal (Roane, 1999). ATPases responsible for expulsion of lead have been described in Staphylococcus aureus and Escherichia coli (Rensing et al., 1998). However, the first detailed mechanism of resistance to lead described so far is the one reported for Ralstonia metallidurans CH34 (formerly Alcaligenes eutrophus CH34) (Borremans et al., 2001). In R. metallidurans, resistance to lead is conferred by the pbr locus, which is an operon, inducible by lead, divergently transcribed that includes five structural and one regulatory gene. The products of these genes participate in the capture, expulsion and intracellular accumulation of lead (Borremans et al., 2001).
Bacterial antibiotic and heavy metal resistance determinants commonly reside on plasmids, and selective pressure for one resistance indirectly leads to selection for the others, as the responsible genes are linked.
Since many low-statuses farmer people consume these fish and usually swim in this freshwater pond, contact with the antibiotic and heavy metal resistant Aeromonas posess an important human health risk. In addition, passage of these bacteria from fish to humans would permit the horizontal gene transfer from Aeromonas to other bacteria harbored by the exposed persons.
References
ALTWEGG, M. & H.K. GEISS. 1989. Aeromonas as a human pathogen. Critical Reviews in Microbiology 16: 253-286. [ Links ]
AUSTIN, B. & C. ADAMS. 1996. Fish pathogens. In: Austin, B., M. Altwegg, P.J. Gosling, & S. Joseph (Eds.). The genus Aeromonas, John Wiley & Sons, New York, pp. 197-243. [ Links ]
BORREMANS, B., J.L HOBMAN, A. PROVOST, N.L. BROWN & D. VAN DER LELIE. 2001. Cloning and functional analysis of the pbr lead resistance determinant of Ralstonia metallidurans CH34. Journal of Bacteriology 183:5651-5658. [ Links ]
CAHILL, M.M. 1990. Virulence factors in motile Aeromonas species. Journal of Applied Bacteriology 69: 1-16. [ Links ]
CASTRO-ESCARPULLI, G., M.J. FIGUERAS, G. AGUILERA-ARREOLA, L. SOLER, E. FERNÁNDEZ-RENDÓN, G.O. APARICIO, J. GUARRO & M.R. CHACÓN. 2003. Characterization of Aeromonas spp. isolated from frozen fish intended for human consumption in Mexico. International Journal of Food Microbiology 84: 41-49. [ Links ]
CHANG, B.J. & S.M. BOLTON. 1987. Plasmids and resistance to antimicrobial agents in Aeromonas sobria and Aeromonas hydrophila clinical isolates. Antimicrobial Agents and Chemotherapy 31: 1281-1282. [ Links ]
DOELMAN, P. 1987. Resistance of soil microbial communities to heavy metals. In: Jensen, V., A. Kjoller & L.H. Soresen (Eds.). Microbial Communities in Soil. Elsevier, New York, pp. 369-383. [ Links ]
DYER, B.S. & B. CHERNOFF 1996. Phylogenetic relationships among atheriniform fishes (Teleostei:Atherinomorpha). Zoological Journal of the Linnean Society 117: 1-69. [ Links ]
GOSLING, P.J. 1986. Biochemical characteristics, enterotoxigenicity and susceptibility to antimicrobial agents of clinical isolates of Aeromonas species encountered in the western region of Saudi Arabia. Journal of Medical Microbiology 22: 51-55. [ Links ]
GUPTA, A., M. MAYNES & S. SILVER. 1998. Effects of halides on plasmid-mediated silver resistance in E. coli. Applied and Environmental Microbiology 64: 5042-5045. [ Links ]
HAZEN, T.C., C.B. FLIERMANS, R.P. HIRSCH & G.W. ESCH. 1978. Prevalence and distributions of Aeromonas hydrophila in the United States. Applied and Environmental Microbiology 36: 731-738. [ Links ]
HOLMES, P., L.M. NICOLLS & D.P. SARTORY. 1996. The ecology of mesophilic Aeromonas in the aquatic environment. In: Austin, B., M. Altwegg, P.J. Gosling & S. Joseph (Eds.) The genus Aeromonas, John Wiley & Sons, New York, pp. 127-150. [ Links ]
JOSEPH, S.W. & A. CARNAHAN. 1994. The isolation, identification, and systematics of the motile Aeromonas species. Annual Review of Fish Diseases 4: 315-343. [ Links ]
KIROV, S.M. 1993. The public health significance of Aeromonas spp. in foods. International Journal of Food Microbiology. 20: 179-198. [ Links ]
KOEHLER, J.M. & L.R. ASHDOWN. 1993. In vitro susceptibilities of tropical strains of Aeromonas species from Queensland, Australia, to 22 antimicrobial agents. Antimicrobial Agents and Chemotherapy 37: 905-907. [ Links ]
MIRANDA, C. D. & G. CASTILLO. 1998. Resistance to antibiotic and heavy metals of motile aeromonads from Chilean freshwater. The Science of the Total Environment 224: 167-176. [ Links ]
NAKAHARA, H., T. ISHIKAWA, Y. SARAI, I. KONDO & S. MITSUHASHI. 1977. Frequency of heavy-metal resistance in bacteria from inpatients in Japan. Nature 266: 165-167. [ Links ]
NATIONAL COMMITTEE FOR CLINICAL LABORATORY STANDARDS. 1997. Performance Standards for Antimicrobial Disc Susceptibility Tests: Approved Standards NCCLS Document M2-A6. Villanova, PA, USA. [ Links ]
O'CALLAGHAN, C.H., A. MORRIS, S.M. KIRBY & S.H. SHINGLER. 1972. Novel method for detection of beta-lactamase by using a chromogenic cephalosporin substrate. Antimicrobial Agents and Chemotherapy 1: 283-288. [ Links ]
PAYNE, D.J., R. CRAMP, D.J. WINSTANLEY & D.J.C. KNOWLES. 1994. Comparative activities of clavulanic acid, sulbactam, and tazobactam against clinically important β-lactamases. Antimicrobial Agents and Chemotherapy 38: 767-772. [ Links ]
RENSING, C., Y. SUN, B. MITRA & B.P. ROSEN. 1998. Pb(II)-translocating P-type ATPases. Journal of Biological Chemistry 273: 32614-32617. [ Links ]
ROANE, T.M. 1999. Lead resistance in two isolates from heavy metal-contaminated soils. Microbial Ecology 37: 218-224. [ Links ]
SIERRA, C.J. & J. SIERRA. 1977. Reseña histórica de la pesca en México. Edición del Departamento de Pesca. México, 99 p. [ Links ]
THAYUMANAVAN, T., G. VIVEKANANDHAN, K. SAVITHAMANI, R. SUBASHKUMAR & R. LAKSHMANAPERUMALSAMY. 2003. Incidence of haemolysin-positive and drug-resistant Aeromonas hydrophila in freshly caught finfish and prawn collected from major commercial fishes of coastal South India. FEMS Immunology and Medical Microbiology 36: 41-45. [ Links ]
THOESEN, J.C. 1994. Suggested procedures for the detection and identification of certain finfish and shellfish pathogens. Fish Health Section, American Fisheries Society. [ Links ]
VACA, S., R. MIRANDA & C. CERVANTES. 1995. Inorganic-ion resistance by bacteria isolated from a Mexico City freeway. Antonie van Leeuwenhoek 67: 333-337. [ Links ]
YAMAGUCHI, K., D. MATHAI, D.J. BIEDENBACH, M.T. LEWIS, A.C. GALES & R. JONES. 1999. Evaluation of the in vitro activity of six broad spectrum beta-lactam antimicrobial agents tested against over 2,000 clinical isolates from 22 medical centers in Japan. Diagnostic Microbiology and Infectious Disease 34: 123-134. [ Links ]














