Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Hidrobiológica
versão impressa ISSN 0188-8897
Hidrobiológica vol.15 no.3 Ciudad de México 2005
Artículo de revisión
Methanogenesis and methane oxidation in wetlands. Implications in the global carbon cycle
Metanogénesis y metano-oxidación en humedales. Implicaciones en el ciclo del carbono global
Rocio Torres-Alvarado1, Florina Ramírez-Vives2, Francisco José Fernández 2 e Irene Barriga-Sosa1
1 Departamento de Hidrobiología, Universidad Autónoma Metropolitana-Iztapalapa (UAM-I). Av. San Rafael Atlixco # 186.Col. Vicentina. A. P. 55 535. Ciudad de México. 09430, México.
2 Departamento de Biotecnología. Universidad Autónoma Metropolitana-Iztapalapa (UAM-I). Av. San Rafael Atlixco # 186.Col. Vicentina. A. P. 55 535. Ciudad de México. 09430, México.
Recibido: 25 de agosto de 2004
Aceptado: 10 de diciembre de 2004
Abstract
Wetlands are important ecosystems on the Earth. They are distinguished by the presence of water, saturated anoxic soils, and different kinds of vegetation adapted to this conditions. Organic matter in these environments is mineralized mainly in the sediments throughout anaerobic processes where sulfate reduction is one of the most important terminal stages of anaerobic decomposition in coastal wetlands, whereas methanogenesis is important in freshwater wetlands.
In this environments, methane, a greenhouse gas, is produced as a result of the activity of a large and diverse group of methanogenic bacteria (Domain Archaea). The generated methane can be diffused to the atmosphere or can be oxidized by several microorganisms under aerobic and anaerobic conditions, such microorganisms intercept and consume this gas diminishing its emission to the atmosphere.
The production and consumption of methane in wetlands involve complex physiological processes of plants and microorganisms, which are regulated by climatic and edaphic factors, mainly soil temperature and water table level. The interaction of these processes with heterogeneous environments results in large variations in the methane fluxes.
Because methane is an important gas that contribute with as much as 15% to the greenhouse effect, several studies had analyzed methane production and its emission from wetlands. These studies established that natural and agricultural freshwater wetlands represent approximately 40% of the sources of atmospheric methane. However, most of the ecological studies assessing the production, consumption, and emission of methane have been performed in boreal and temperate wetlands, yet there are few studies evaluating these activities in tropical wetlands, particularly in Brazil and Panama. In Mexico there are not studies contributing to this respect.
Key words: Wetlands, methanogenesis, methane oxidation, methane fluxes.
Resumen
Los humedales son ecosistemas importantes en la Tierra. Se caracterizan por la presencia de agua, suelos saturados anóxicos y diferentes clases de vegetación adaptada a estas condiciones. La materia orgánica en estos ambientes es mineralizada principalmente en los sedimentos a través de procesos anaeróbicos, siendo la sulfatorreducción la fase terminal más importante de la descomposición anaeróbica en los humedales costeros, mientras que la metanogénesis domina en los humedales dulceacuícolas.
En estos ambientes el metano, un gas invernadero, es producido como resultado de la actividad de un grupo diverso de bacterias metanogénicas (Dominio Archaea). El metano generado puede difundirse hacia la atmósfera o puede ser oxidado bajo condiciones aeróbicas y anaeróbicas por varios microorganismos, los cuales interceptan y consumen este gas disminuyendo su emisión a la atmósfera.
La producción y consumo del metano en los humedales involucra procesos fisiológicos complejos de plantas y microorganismos, los cuales son regulados por factores edafológicos y climáticos, principalmente temperatura y nivel del manto freático. Las interacciones de estos procesos con los ambientes heterogéneos da por resultado grandes variaciones en los flujos del metano.
Se han efectuado estudios en los que se analiza la producción de metano y su emisión a partir de los humedales, debido a que éste es un gas que contribuye aproximadamente con el 15% del efecto invernadero.
Estos estudios establecen que los humedales naturales y agrícolas, representan aproximadamente el 40% de las fuentes del metano atmosférico. Sin embargo, la mayor parte de los estudios relacionados con la producción, consumo y emisión del metano se han llevado a cabo en humedales boreales y templados, existiendo pocos estudios que evalúan estas actividades en humedales tropicales (Brasil y Panamá). En México no hay estudios al respecto.
Palabras clave: Humedales, metanogénesis, metano-oxidación, flujos de metano.
Introduction
Wetlands are ecosystems with intermediate characteristics between terrestrial and aquatic ecosystems that are usually flooded. They are covered by a great diversity of vegetation, from moss to herbaceous and vascular plants. Their hydrological properties and the abundance of vegetation make them sites with high rates of organic carbon deposition, which is mineralized mainly through anaerobic processes, being methanogenesis the dominant process in freshwater wetlands, whereas sulfate reduction predominates in brackish wetlands.
Because of the large extension of freshwater wetlands in the boreal and temperate regions, methanogenesis is the most important process, being accountable for the release of large amounts of methane (CH4) into the atmosphere from these ecosystems (Fung et al., 1991; Bartlett & Harris, 1993). However the thin oxic layer and the oxic plant rhizophere in wetlands promote activity of methane-oxidizing bacteria or methanotrophs. Thus, both CH4 formation and consumption are microbiological processes controlled by many factors, and its relationship influences on the magnitude of the flux from wetlands. CH4 release has important ecological implications because it is a gas involved in the greenhouse effect related with global climatic change.
Several studies have been made on methanogenesis, methane oxidation, and the emission rate of CH4 mainly in the boreal and temperate regions; whereas in the tropical regions there are few studies. In Mexico, where coastal wetlands are abundant, this type of studies are particularly scarce.
In this paper, we review the literature on the activity of microorganisms involved in the production and consumption of CH4, as well as the magnitude of CH4 emissions, and the variables influencing its control in natural wetlands. This review focused basically on peat-forming wetlands (bogs, fens, swamps) and some types of non-peat forming coastal wetlands (salt marshes, and mangroves); we also included rice paddy fields because these are responsible of the 20% methane global emissions. We didn't consider littoral sediments of lakes and reservoirs.
Definition and characteristics of wetlands
Wetlands are ecosystems with a scarce drainage area that, at least periodically, are saturated or covered by water and represent important constituents of the biosphere, as they perform an indispensable function in the biochemical cycles of carbon, nitrogen, and sulfur (Westermann, 1993b). In Mexico, wetlands are defined like transition areas between aquatic and terrestrial ecosystems, at least periodically, are saturated or covered by water (Carrera & de la Fuente León, 2003).
Wetlands are formed in zones where soil drainage is deficient due to the presence of a bed of impermeable rocks, permafrost, and/or where yearly precipitation exceeds evaporation; or they can be originated by the accumulation of organic matter in the shallow regions of lakes, giving rise to a biological succession phenomenon.
The hydrological characteristics, and the hydroperiod of wetlands produce unique physical-chemical conditions that make them very different ecosystems from the well-drained terrestrial systems, and the deep aquatic systems. The hydroperiod results from the balance between water supplies and their output, the soil and sub-surface conditions. The main water supplies are given by precipitation, superficial run-offs, underground water, tides, and river floods; which originate a transport of energy and nutrients into the wetlands. These supplies influence largely the biogeochemistry of the soils in these environments, and are the main selection force driving richness and composition of species. Hydrology affects primary production, organic matter accumulation and the nutrients cycle. In general, productivity is higher in those wetlands with elevated nutrient flows or in those presenting pulses in hydroperiods. Mineralization of organic matter is slower when there is a stagnant and anaerobic water column than under well drained conditions.
The soil or sediment of wetlands is the zone where many of the chemical transformation of the elements take place; it is often described as a hydric soil for it is poorly drained, it is water-saturated or flooded, and develops anaerobic conditions. Wetland soils are classified as mineral, and organic or peatlands (Table 1) (Mitsch & Gosselink, 1993). The peat covers the superficial strata of wetlands, its constituted by plant materials in diverse decomposition stages. The most important characteristics of peat are its botanical composition and decomposition state.
Botanical composition includes moss, herbaceous, and wood material, and leaves remnants. In most boreal wetlands, the most common moss is Sphagnum spp. Peatlands with herbaceous vegetation present freshwater grasslands (Phragmites spp., Carex spp., Cladium spp), wild rice (Zizania), brackish grasslands (Spartina spp.), and cattails (Typha spp). In the forestry wetlands, peat is formed from the detritus of trees, such as pines (Pinus spp.), cypresses (Taxodium spp.), or tupelo trees (Nyssa sp.).
Regarding the state of decomposition, as it advances the vegetal structure changes physically and chemically until the end product barely resembles the original material. Along the degradation of peat, the amount of large fibers (> 1.5 mm) decreases and the material becomes extremely fragmented. Chemically, the amount of lignin increases, while the cellulose and plant pigments decrease (Clymo, 1983).
Classification of wetlands. Classification of wetlands is mainly determined by the hydrological, ecological, and topographic properties, the nutrients regime, as well as the floristic composition and peat structure that constitute them. To establish a classification of the diverse types of wetlands is not easy because the used terminology is complex and often confusing between different countries. A large number of terms have been used depending on the region or continent. In this work, wetlands are grouped mainly based on their salinity characteristics (Table 2) (Clymo, 1983; Matthews & Fung, 1987; Zinder, 1993).
Natural wetlands are concentrated in the high latitudes of the northern hemisphere, where permafrost avoids soil drainage, dominating histosols (95%); as well as in the tropics, where precipitation rates are high, in this regions mangroves and flooding plains are the dominant wetlands.
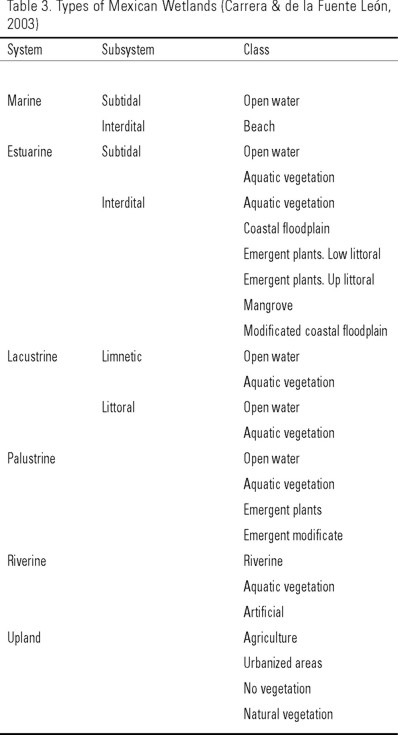
Mexico possesses barely 0.6% of the worldwide wetlands; most of them are coastal wetlands and these were recently classified according to the Cowardin et al. (1979) system, which is used by the National Program of Wetlands Inventory from the Department of Fishing and Wild Life, USA (Table 3) (Carrera & de la Fuente León, 2003).
Human activity has modified wetlands since the dawn of agriculture. Rubec et al. (1988) classified wetland land-use conversions into five categories: agricultural land reclamation, urban and industrial uses, energy development, peat harvesting and forest harvesting. Another wetland land use is the production of rice. Rice paddies account for approximately 20% of all methane emissions (Fung et al., 1991), for this reason they are included in this review.
Biogeochemical processes in the wetlands
Wetlands can be considered systems with three compartments: oxic surface, anoxic soil, and rhizosphere, each one with different physical-chemical conditions that influence the metabolic processes involved in the mineralization of organic matter.
Oxic zone
The oxic zone (aerobic) of the flooded soils is limited to the water column and the first milli- or centimeters of soil or sediment (King et al., 1990); within this zone, the regions close to the rhizosphere are also considered. The rhizosphere is the zone where the live roots of the plants constituting the wetland are located.
When the plant remnants from the wetlands are covered by water, their soluble substances are lost due to lixiviation. The lixiviate is constituted mainly by amino acids, sugars, volatile, and non-volatile fatty acids; these compounds are metabolized rapidly by the aerobic microflora of the system. The posterior degradation of this vegetal matter depends on the existing relationship among the diverse polymers (lignin, cellulose, and hemicellulose) in the deposits, their structure, and the physico-chemical characteristics of the ecosystem (Moran et al., 1989).
It has been established that fungi are the most important group of organisms involved in the aerobic decomposition of the vegetal matter in marshes, peatlands, and rice fields. Species of Penicillum, Fusarium, Alternaria, and Helicoon genera are commun (Williams & Crawford, 1983). Likewise, amylolytic, proteolytic, and chitinolytic bacteria are important agents in this process.
Anoxic zone
When the wetland soils are flooded, oxygen diffusion decreases drastically (oxygen diffuses 10,000 times slower in an aqueous solution than in the air). Because oxygen diffusion is slow and the organic carbon content is high, the oxide-reduction potential (Eh) decreases rapidly with depth, generating chemical gradients that influence the sequence of metabolic reactions occurring during organic matter degradation, as well as the spatial distribution of the microbiota that participates in that process (Stolzy et al., 1981; Sweerts et al., 1991). Once oxygen has been depleted, nitrate (NO3-) is the first electron acceptor that decreases, followed by manganese (Mn IV), iron (Fe III), sulfates (SO42-) and, finally, carbon dioxide (CO2), giving rise to the anaerobic processes (Fig. 1).
1. Denitrification, is a respiration process in which the electron acceptor is nitrate, it starts when oxygen concentration is < 10 µM. The resulting denitrification products are molecular nitrogen (N2) and nitrogen oxide (NOx). Anaerobic Gram-negative bacteria perform this process; among them, the genera Pseudomonas spp., Clostridium spp., Bacillus spp., and Alcaligenes spp. have been reported (Struwe & Kjoller, 1989). It has been estimated that denitrification is responsible for a 30-40% nitrogen loss from the soil in marshes and rice fields.
2. Manganese reduction, is the transformation of Mn3+ to Mn4+, it starts below +526 mV, and is carried out by a large diversity of facultative bacteria and microfungi.
3. Iron reduction, is a process carried out when the Eh descends to-47 mV. Several groups of facultative and anaerobic bacteria participate in it. Manganese-reduction and iron-reduction are relevant processes in those wetlands with high mineral supplies (Lovley, 1991).
4. Sulfate reduction. This metabolism is carried out by sulfate-reducing bacteria (SRB) when the Eh decrease to -120 mV. SRB are anaerobic bacteria and their cells depict a wide range of morphological shapes: bacilli, vibrio, cocci, sarcines, and filaments. SRB use mainly sulfate as their terminal electron acceptor in the anaerobic oxidation of organic substrates, and reduce it to hydrogen sulfide (H2S):

Based on their oxidative and metabolic capacities, SRB can be divided in two groups. The first includes those species that metabolize incompletely the long and short chain fatty acids, such as propionate, lactate, and pyruvate, to acetate. Genera Desulfovibrio spp. and Desulfomonas spp. belong to this group. The second group comprises those species that carry out a complete oxidation of organic acids, such as lactate, acetate, benzoate, succinate, or fumarate, to CO2. The genus Desulfotomaculum spp. belong to this group (Pfennig et al., 1981). SRB, are able to use other substrates as electron donors, such as phenolic and indolic compounds, and amino acids (Bak & Widdel, 1986; Gibson et al., 1988)
Many SRB can grow in a syntrophic relation with the methanogenic bacteria that use hydrogen when sulfates are absent (Widdel, 1988). Sulfate reduction is the most important mineralization mechanism in brackish wetlands, representing up to 67-80% of the total respiration in sediments; the main supplies of organic matter for this process are the products from the roots and rhizomes of grasses and mangroves, as well as the excretion of dissolved organic matter during fast-growth periods (Howarth, 1993).
In agricultural wetlands, sulfate reduction is important because the H2S released can influence the growth of rice plants. However, it has been observed that, in the oxidative layer near the roots the H2S oxidation is stimulated, providing protection to the plant. In most temporally flooded soils, H2S rarely accumulates until reaching toxic concentrations because it precipitates mainly as ferrous sulfide (Widdel, 1988).
5. Methanogenesis, is the last stage in the anaerobic degradation and is carried out when the concentration of sulfates decreases. The process requires redox potentials below -244 mV (Lovley & Phillips, 1987; Peters & Conrad, 1996). Methanogenic bacteria (MB) are a unique group of prokaryotes since they produce a hydrocarbon, methane (CH4), as the main product of their metabolism. The MB utilize simple organic substrates, such as formiate, methanol, methylamines, and acetate (Phelps & Zeikus, 1985).
The importance of metabolic processes in a given wetland depends on the concentration of the specific electron acceptors, and of the availability of organic matter. In coastal wetlands, such as salt marshes, all processes (except methanogenesis) depict high metabolic rates, although they are spatially separated in different strata or microniches (Table 4) (Howarth, 1993).

Because oxygen is present along the oxic-anoxic interface and in the rhizosphere, the electron acceptors regenerate from the re-oxidation of the reduced compounds, contributing to the maintenance of the anaerobic degradation chain.
Anaerobic decomposition in the wetlands is often incomplete, resulted in the accumulation of large amounts of organic carbon (Roulet, 2000). Therefore, the saturated soils of these ecosystems can contain approximately a third of all the organic matter deposited in the soils of the world (Eswaran et al., 1995).
Carbon cycle in the wetlands
The carbon cycle is controlled by the input of organic matter to the soil. The degradation of the organic matter by means of the aerobic respiration produce CO2, and the process is limited by the reducing conditions of the soils or sediments in the wetlands. In anaerobic conditions, the first step involved in the degradation of organic carbon is fermentation, in which the organic matter is the terminal electron acceptor, producing diverse low molecular weight organic acids and alcohols, such as lactic acid and ethanol, respectively. At the beginning, fermentative bacteria excrete enzymes that hydrolyze polysaccharides. The same group of bacteria converts the resulting monomer into alcohols, fatty acids, and hydrogen (H2). In the presence of other electron acceptors, these substrates are completely degraded to CO2. When there is a limitation of external electron acceptors, syntrophic bacteria degrade the alcohols and fatty acids to acetate, formiate, and CO2. An alternative pathway involves the direct conversion of monomers to acetate through the activity of homoacetogenic bacteria. Acetate and H2 are finally used as substrates by SRB and MB (Fig. 1). In these reactions, 4 mol of H2 plus 2 mol of acetate are produced for each mole of glucose. In most freshwater wetlands, the concentration of electron acceptors is low as compared to the availability of carbon, hence the predominating process is methanogenesis, which is of great relevance due to the release of CH4, a gas related to the greenhouse effect and global climatic change.
Methanogenesis. The analysis of the structure and activity of the methanogenic bacteria (MB) populations in the wetlands is important to understand the global emissions of CH4. MB are members of the Archaea domain. They comprise a morphologically diverse group of short and long bacilli, cocci, and several arrangements of the basic forms in large chains or aggregated clumps. All members possess two unique cofactors, factor 420 (F 420) and 2-mercaptoethanosulfonic acid (coenzyme M or CoM) (Mah & Smith, 1981). They are constituted by approximately 50 species grouped in the next genera: Methanobacterium, Methanothermobacter, Methanobrevibacter, Methanosphaera, Methanothermus, Methanococcus, Methanothermococcus, Methanocaldococcus, Methanoignis, Methanomicrobium, Methanogenium, Methanoplanus, Methanoculleus, Methanofollis, Methanocorpusculum, Methanospirillum, Methanolobus, Methanococcoides, Methanohalophilus, Methanohalobium, Methanosarcina, Methanosalsus and Methanosaeta (Boone et al., 1993).
The MB are metabolically classified in four physiological groups:
I. The genera that use exclusively acetate as substrate, such as Methanosaeta spp.:

II. The genera that use hydrogen and formiate, such as Methanobacterium spp., Methanobrevibacter spp., and Methanogenium spp.:

III. The genera that develop from methylated compounds (methanol and methylamines), among them are Methanolobus spp., and Methanococcus spp.

IV. The generalists that generate CH4 from acetate, H2, and methylated compounds, as occurs in the genus Methanosarcina spp. (Garcia, 1990).
In most natural wetlands with neutral pH, methanogenesis from acetate is the predominating pathway implicated in CH4 production, followed by reduction of CO2 with H2. CH4 production by the acetate fermentation pathway is favored in the shallow subsurface, while methanogenesis from the reduction of CO2 with H2 becomes predominant in less reactive peat (Hornibrook et al., 1997). Methanogenesis from acetate represents 69% in peatlands (0.69-8.54 µM/hr) and 51% in tidal estuaries (0.40-3.41 µM/h). However, in mid-latitude wetlands, apparently CH4 is not derived from acetate. These compound accumulate to high levels during anaerobic decomposition and is ultimately degraded aerobically to CO2 after diffusion into oxic regions of peat (Hines et al., 2001).
In acid wetlands, the reduction of CO2 with hydrogen is an important precursor of methane, having quantified a great abundance of hydrogenophilic methanogens in them, around 3x107 cel/g sediment (Horn et al., 2003). The hydrogenophilic methanogenic bacteria (HMB) are symbiotically associated to anaerobic organisms that produce hydrogen and contribute to maintain low levels of this gas during carbon flux. As a result, low H2 concentrations are emitted to the atmosphere. In natural wetlands, HMB belong to the families Methanobacteriaceae, Methanococcaceae, Methanosarcinaceae, and Methanomicrobiaceae.
In a tropical coastal wetland in Mexico (Chiapas State) we quantified a MB density higher that those reported in salt marshes from temperate regions (H2: 1.3x106-3.08x1010 cells MB/g sediment, and acetate: 1.53x105-1.41x1010 cells MB/g sediment) (Torres-Alvarado et al, 2005).
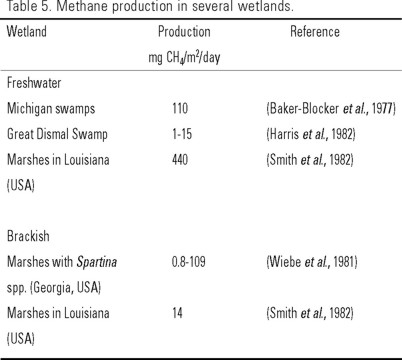
Table 5 depicts the amount of CH4 produced in several natural wetlands. Matthews and Fung (1987) determined that CH4 production rate is higher in tropical zones than in boreal zones, because increases in temperature and net primary production in tropical ecosystems favor the methanogenic process. Hence, wetlands from tropical latitudes dominate global methane production (Fung et al., 1991; Bartlett & Harris, 1993).
In agricultural wetlands, a difference must be made between the rice fields fertilized with straw and those not fertilized. In the first, methanogenesis from acetate represents 84-89% of the released CH4, whereas that from H2-CO2 represents 11-27% (Glissmann & Conrad, 1999). Production of CH4 in fertilized fields starts 8 days after incubation and reaches a stable state after 20 days (Weber et al., 2001). The methanogenic populations that colonize the rice straw belong to the families Methanosaetaceae, and Methanomicrobiaceae, being the genera Methanobacterium spp., and Methanosarcina spp. the most representative ones. However, the calculated diversity indexes (equitativity=0.29) are low as compared to the indexes reported for soils without fertilizers (0.54-0.85); this is due to the fact that the rice straw is predominantly an habitat for fermentative bacteria, whereas MB are more abundant in the soil around the fertilizer, where the products resulting from fermentation concentrate and serve as substrate.
In non-fertilized soils, it has been observed that acetoclastic methanogenesis represents 51-67% of the produced CH4, whereas H2-CO2 contributes with 17-31% of the total (Liesack et al., 2000). In rice fields, from 2.0x106 to 2.3x107 cell/g dry soil abundance of HMB has been quantified, and around 5.1x105 to 1.3x106 cell/g dry soil of acetoclastic methanogenic bacteria (AMB). The MB localized in the rice plants roots differs from that found in the anoxic soil, resulted in different pathways to CH4 formation between these two environments. In the roots of the rice plant (Oryza sativa, var. roma, type japonicus), the AMB belonged the species of Methanosarcina, and Methanosaeta genera. Because the Methanosarcina species have a greater affinity for acetate, these are more abundant at the roots with high acetate concentration; in contrast, in the anoxic soil, where acetate concentration decrease, the Methanosaeta species are more abundant because their affinity for acetate is lower. HMB are represented by the family Methanomicrobiaceae in the roots (Chin et al., 2004).
Global methanogenesis has increased due to the increment of the agricultural areas used for rice cultivation, these are, currently, responsible for half of the global production of CH4 and the microbiota located at the roots of the rice plants supplies 50% of the total production from the degradation of vegetal photosynthates excreted by the roots and for 3-6% of the CO2 photo-synthetically fixed (Anastasi et al., 1992).
Methane oxidation. Wetlands are the main ecosystems that emit CH4 to the atmosphere due to the large amount of organic matter (generated during primary production) degraded during methanogenesis. However, the amount of released CH4 depends on the existing radio between its production and consumption by diverse organisms. There are different microbial groups that carry out methane oxidation, in both aerobic and anaerobic conditions.
Aerobic conditions
Aerobic oxidation of CH4 is carried out by methane oxidizing bacteria (MOB), and nitrifying bacteria. These strict aerobic microorganisms oxidize CH4 to CO2 using oxygen as the electron acceptor, releasing methanol as intermediate product. Oxidation of CH4 demands about 14-29% of the total oxygen contained in the sediment.
MOB are non-mobile Gram-negative Proteobacteria that oxidize CH4 as the sole carbon and energy source generating bacterial biomass. The reaction is catalyzed by the mono-oxigenase enzyme (MMO). There are two MMO forms, a cytoplasmatic soluble form and a particulate form associated to the cellular membrane.
Phylogenetic studies indicate that there are two groups of MOB. Type I assimilates carbon through the ribulose monophosphate pathway, represented by genera Methylomonas, Methylobacter, Methylococcus, and Methylosinus, the latter two are the most active ones in histosols (Edwards et al., 1998). Type II use the serine route for carbon assimilation (Bowman et al., 1995). MOB inhabit peat and sediments, depict a notable capacity to survive in anoxic conditions and rapidly consume CH4 when oxygen is re-introduced into the ecosystem. MOB obtained from pure cultures are neutrophils, however, histosol communities are moderately acidophilic, with an optimal growth at pH between 4.5 and 5.5. Species Methylococcus capsulatus, and Methylosinus trichosporium, as well as the genus Methylocystis have been identified in these ecosystems (Dedysh et al., 1998).
In peatlands, 103-104 cell/ml of MOB have been quantified, whereas their abundance is higher in rice fields (2-18x106 cel/g dry soil) (DeBont et al., 1978). In peatlands Yavitt and Lang (1990) reported a CH4 oxidation rate of 80% of the total production, while King et al. (1990) demonstrated a consumption of 91% of the total CH4 produced in the Everglades (Florida, USA). It has been estimated that 80% of the produced methane is oxidized in rice fields.
Nitrifying bacteria also have a high affinity for CH4, oxidizing it under nitrogen limiting conditions of the soil (Chan & Parkin, 2001). In freshwater wetlands, this microbial group can compete for the available oxygen with the MOB (Megraw & Knowles, 1987).
The CH4 oxidation depends on the availability of oxygen, therefore this process occurs mainly in freshwater wetlands during the dry periods, when the level of the water table descends and the soil of the wetland is exposed to air (Harris et al., 1982; Yavitt & Lang, 1990). Likewise, CH4 oxidation is higher in the roots and rhyzome regions, where there is oxygen available coming from the atmosphere that is transported from the leaves to the roots (King, 1994). In peatlands, CH4 oxidation is accomplished in the first 7 mm as a function of oxygen penetration (Moore & Kowles, 1990; Moore & Roulet, 1993).
The aerobic oxidation of CH4 is influenced by changes in plant activity produced by their senescence in the autumn and new growth in the spring, which modify the superficial area available for colonization by MOB and by the organic matter supply for methanogenesis. Hydrogen concentration is also a factor related to CH4 oxidation; in alpine tundra soil, it has been observed that high H2 and CO2 concentrations stimulate CH4 oxidation up to 70 pmol/g/h, vs. 20 pmol/g/h, in soils not enriched with H2 and CO2 (West & Schmidt, 2002).
Anaerobic conditions
Anaerobic oxidation of methane (AOM) is carried out mainly in brackish water wetlands, participating in it at least two phylogenetically different groups of Archae, the ANME-1 and ANME-2. These bacteria generally form consortia with SRB, and the metabolism of its involves a syntrophic relationship based on inter-species electron transfer (Valentine, 1991; Orphan et al., 2002). Apparently, Archaea oxidize the CH4 and the resulting products are used by SRB (Table 6) (Blair & Aller, 1995; Valentine, 2002).
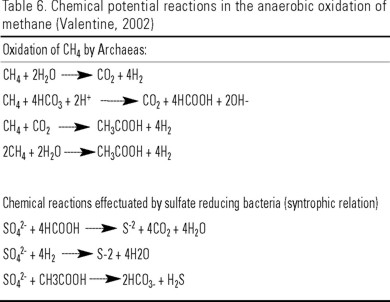
The ANME-1 Archae can oxidize methane without necessarily coupling oxidation with a syntrophic member (Orphan et al., 2002). The ANME-2 Archae, in contrast, couple to a syntrophic member. This group is related with Methanosarcina genus. The sulfate-reducing consortium comprises species of Desulfosarcina, and Desulfococcus genera, which are characterized by the capacity to accomplish complete oxidation of fatty acids to CO2.
The AOM is carried out in the transition zone between sulfate reduction and methanogenesis, since CH4 produced at deeper layer diffuses upwards where sulfate is available. The AOM inhibited gas transport to the atmosphere.
Among the factors related with AOM are organic matter content, supply rate of CH4, depth of sulfate penetration, temperature, pressure, mineralogy, sediment porosity, and seasonal changes, as well as anthropogenic activities that generally induce a decrease in CH4 consumption activities, as occurs with the change in use soil to agricultural purposes and the use of fertilizers (Ojima et al., 1993).
There is a relation between oxidation mechanisms and CH4 emission determined mainly by the speed of the internal gas fluxes within the ecosystem. This means that a fast displacement of CH4 from the soil avoids significant oxidation by oxidizing microbiota, inducing important gas emissions to the atmosphere.
Emission of methane
The loss of CH4 through the surface of soils or sediments is determined by the balance between its production in deep layers and its oxidation after it diffuses to zones with a positive redox potential. It has been estimated that global CH4 production rate from wetlands is 20% higher than the rate of its release (Reeburgh et al., 1993).
The deep layers of wetlands contain trapped CH4 due to the hydrostatic pressure of the overlaying water layer, and also because this gas is barely soluble in water (23-40 mg/l at 0-20°C). A large amount of CH4 trapped in sediments must be released periodically depending on the hydrostatic and atmospheric pressures, which means that CH4 can escape through the sediment to the atmosphere. There are three different routes involved in vertical transfer of CH4 to the atmosphere from the wetlands: diffusion, emergence in the form of gas bubbles, and transport controlled by vegetation (Fig. 2) (Cicerone & Oremland, 1988; Whiting & Chanton, 1992).
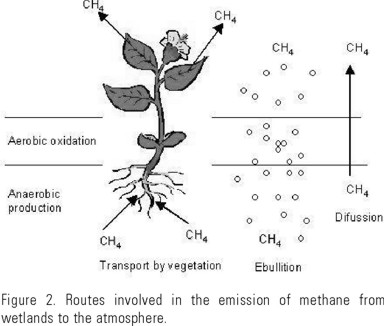
By diffusion CH4 molecules move from higher concentration regions (deep layers of the soil or sediment) to regions of lower concentrations. However the largest loss of CH4 towards the atmosphere is produced when the concentration in the interstitial water of the soil or sediment is higher than the hydrostatic pressure of the overlaying water layer forming gas bubbles than can escape to the surface by ebullition (Yavitt & Knapp, 1995).
Transport of CH4 in tidal wetlands and peatlands located in the state of Michigan (USA) is given by ebullition and diffusion. Chanton and Martens (1988) quantified an ebullition flow of 20.8 g CH4/m2/year and Kelley et al. (1990) determined a diffusion flow corresponding to 56% of the total emission.
Transport by vegetation is a response to the anoxic conditions of the soil, plants developed an adaptation strategy to aerate their submerged organs in the water by creating an internal ventilation system with gas localized in the stems, roots, and rhizomes, this space corresponds to the aerenchyma and acts as a gas channel, among these O2 and CH4.
Wetland plants present two diffusion gradients, one of them produced by the oxygen flux from the atmosphere to the roots and rhizomes located in the anoxic peat where CH4 is generated. The second one is produced by CH4 diffusion from peat to the atmosphere, which is facilitated by the introduction of CH4 to the aerenchyma of the roots. The transport of CH4 through plants includes its diffusion inside the root, conversion of the dissolved form to the gaseous form in the root cortex, diffusion through the cortex and aerenchyma, and, finally, release to the atmosphere through the micropores of the stems and the stomata of the leaves. It has been observed that CH4 flux reduces with closure of stomata because ventilation is stopped (Morrissey et al., 1993); however, emission can continue through the cuticle or the micropores present in the petiole and stems of plants, as occurs in Eriphorum angustifolium (Schimel, 1995).
Vascular plants exert a double effect on emission fluxes. On one side, penetration of roots into anoxic soils contributes to oxygen transport to these regions, creating oxic conditions near the rhizosphere that induce inhibition of methanogenesis and, hence, a decrease in CH4 production. The presence of aerobic sediments near the roots also stimulates CH4 oxidation by methanotrophic bacteria (King, 1994; 1996).
Aquatic vegetation is an important CH4 channel, in some cases, more than 80% of the total CH4 flux occurs through leaves and stems (Dacey, 1980; Whiting et al., 1991; Muller et al., 1994). In marsh peat with Typha latifolia and Sagittaria eurycarpum, a CH4 emission rate of 53-178 mg/m2/day and an oxidation rate of 9.6-97.6 mg/m2/day were calculated at the laboratory level, representing 8.8-43.2% of the produced CH4 (King, 1996). The importance of vegetation in CH4 transport has been demonstrated by Torn and Chapin (1993), who by removing vascular plants from a saturated tundra observed a reduction in CH4 emissions (Fig. 3).
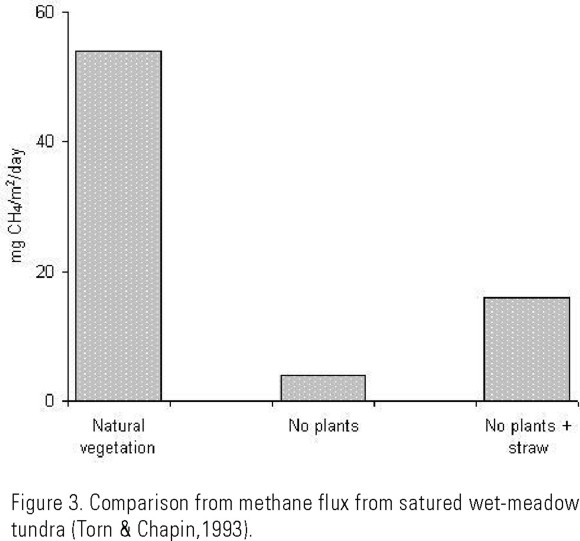
The physiological state of plants affects the emission flux, principally in those plants with an active transport of gases through the stomata than in those presenting diffusion transport through the micropores (Nouchi et al., 1990). Gaseous exchange is also related with environmental variables, such as temperature, solar radiation, and relative humidity.
Wetlands are the largest natural sources of CH4 to the atmosphere, accounting for about 20% of the current global annual emission. CH4 emissions have been quantified in different types of wetlands (Table 7). The fluxes among wetlands of the same latitude are very variable due to differences in organic matter supplies, depth of water column, exposition to air, type of vegetation, salinity, wetland area, and the method used to quantify these fluxes.
Out of the total amount of CH4 emitted, northern wetlands contribute with 34%, because of the large areas covered by these ecosystems (Wang et al., 1996). Histosols emit 3-7% of the total atmospheric CH4 and shows lower methane fluxes than those emitted by minerotrophic fen, this is usually due to a lower level of water table (Sebacher et al., 1986). In temperate regions, wetlands contribute with a minor proportion of the total and the salt marshes have very variable emission rates.
There is little information on the emissions from wetlands flooded during ephemeral periods, as those found in regions dominated by monsoons or in semi-arid zones, where precipitation is very low. From the information available on temporal wetlands, it has been determined that CH4 emissions are higher during summer floods than during winter because fermentative and methanogenic bacteria depend largely on temperature. In these wetlands, emissions become faster, once the wetland has been flooded as compared to rice fields. This behavior is caused by the fast decomposition rate of the organic matter coming from emerging macrophytes as compared to the rice straw. Ephemeral wetlands can consume atmospheric CH4 during the dry season, hence acting as CH4 sinks (Boon et al., 1997).
Bartlett & Harris (1993) performed an extensive review on the available information about CH4 fluxes from different natural wetlands and, by relating the emissions with the surface area occupied by these environments as established by Matthews and Fung (1987), obtained global CH4 emission values (Fig. 4). Based on their results, they determined a total emission of 109 T/year, where boreal and arctic wetlands contribute with 34% to the total, temperate wetlands with 5%, and tropical wetlands with 60%.

A significant proportion of the present increase of atmospheric CH4 concentration could be due to the increase in world-wide surface used to cultivate rice. Besides, most rice fields are located in tropical regions, where emissions are higher due to a higher methanogenic rate, considering also that the hollow stems of the rice plants favor CH4 transport to the atmosphere (Anastasi et al., 1992).
The contribution of the diverse transport processes of total emission of CH4 from the rice fields varies according to seasons. The ebullition process is the dominant mechanism during the first stages of the cultivation period. Along the growth period of the rice plants, ebullition rates decrease and transport by vegetation becomes the dominating process. It has been estimated that transport through the aerenchyma represents 70% of the annual CH4 flux; the percentages attributed to the ebullition and diffusion fluxes are 25%, and 5%, respectively (Schütz et al., 1989a).
Relation of environmental variables with methane emissions. The net CH4 flux from the wetlands results from diverse environmental variables that influence, in a complex manner, its production (methanogenesis), consumption (methanotrophy), and transport.
The relation between CH4 emission and environmental variables is difficult to understand for several reasons, for example, the episodic fluxes occurring in the subarctic wetlands (minerotrophic fens), mainly during spring, release the CH4 trapped in the ice during the winter. However, it is possible to establish some general aspects that influence CH4 flux to the atmosphere.
1. Temperature. From diverse studies it has been possible to establish that CH4 emissions are related to soil temperature, due to its influence on microbial metabolism. There is a direct relation between the increase in temperature and growth of MB (Westermann, 1993a). This relationship produces an exponential increase in CH4 flux while soil temperature increases (Hargreaves & Fowler, 1998). Hence, there are large emissions from the marshes and alluvial floodplains located in tropical regions. In this regions the optimal temperature of decomposition was 35ºC and methanogenesis did not proceed at 45ºC (Miyajima et al., 1997).
The temperature-emission relationship also explains the increase in methanogenic activity in wetlands from mid and high latitudes during high temperature seasons. Similar results have also been obtained experimentally, in which increases in temperature induce an exponential increase in the acetogenic, and hydrogenophilic methanogenic activity (Fig. 5) (Brooks Avery et al., 2002). Increases in temperature also produced an increase in CH4 consumption by methanotrophs (Krumholz et al., 1995).
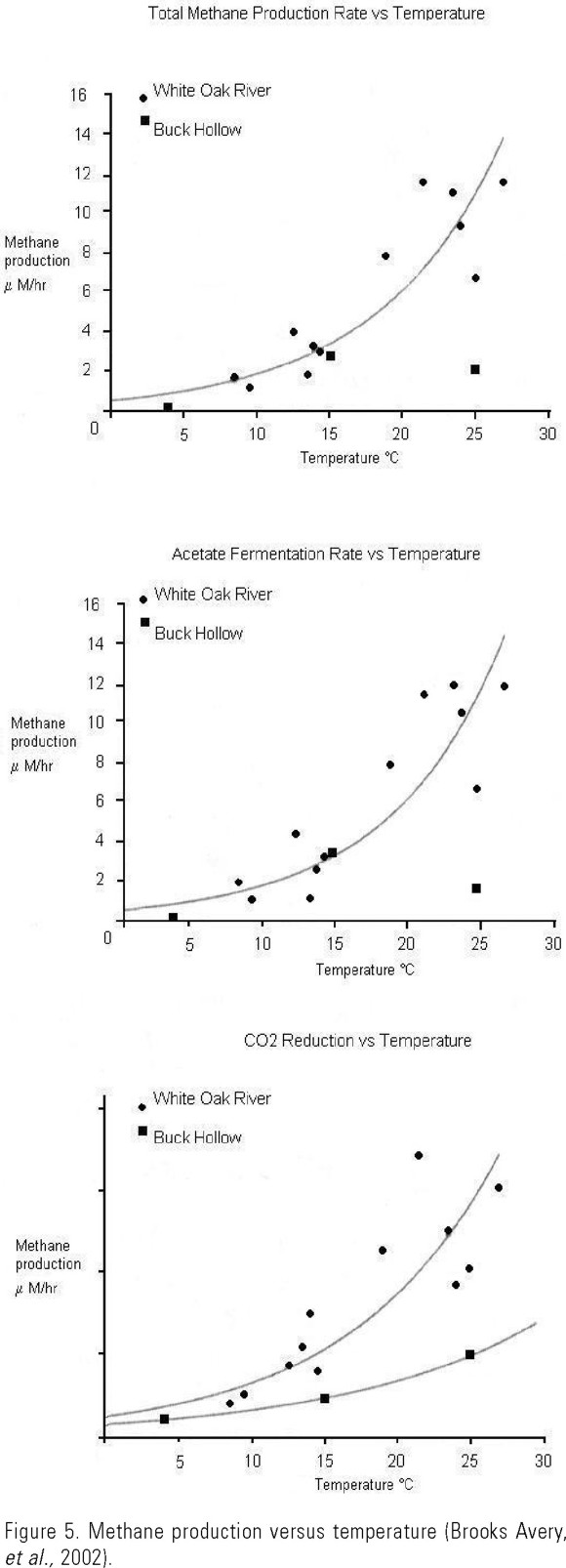
An important aspect related with temperature is the use of substrates by MB in the rice fields, as it has been observed that a decrease in temperature (from 30°C to 15°C) induces a decrease in CH4 production and a change in organic matter degradation pathways. When temperature diminished, a decrease in hydrogen partial pressure occurs with an accumulation of acetate, propionate, caproate, lactate, and isopropanol; hence, CH4 production from hydrogen decreases and the use of acetate increases (Conrad et al., 1987).
Besides, it has been observed that fermentation processes, related with the supply of organic substrates for methanogenesis, are more sensitive than even MB to temperature changes. Thus, temperature dependence of in situ CH4 production is also a reflection of the impact of this variable on the microbial processes involved in the production of substrates.
Soil temperature not always explains the differences in CH4 fluxes among the different wetlands, although some authors have reported an accurate statistical relation between emission rate and temperature (Harriss et al., 1985). According to Morrissey and Livingston (1992), temperature helps to explain 90% of the seasonal variation in CH4 production and emission.
2. Water table level. Its determines the depth at which aerobic and anaerobic conditions occur in wetlands, these conditions, in turn, control the methanogenic and metanotrophic processes (Kelley et al., 1995). The methanogenesis is a strict anaerobic process, and it will be stimulated during flooding periods, when the water table level increases (Reddy & Graetz, 1988). In contrast, with a decrease in flooding periods, CH4 production decreases. An inverse relation is observed with CH4 oxidation.
The mean position of the water table level is the best indicator of CH4 emissions; apparently a critical depth exists at which maximal emissions occur (Moore & Roulet, 1993; Moore & Dalva, 1993). It has been determined that depths of the water table greater than 18 cm do not produce high emissions, since CH4 production (methanogenesis) decreases and its consumption increases (methanotrophy). In contrast, when the water table level is 12 cm below the surface of peat, or exceeds it, emissions are high. Bubier et al. (1993) and Daulat and Clymo (1998) indicated that CH4 flux to the atmosphere from hollows is 5-60 times higher than that produced in hummocks, because the first have a lower water table depth than the hummocks (Fig. 6). The variables influencing directly or indirectly the water table level are very important to make regional extrapolations.

The water table level not only influences the amount of CH4 emitted to the atmosphere, but also its atmospheric removal. Harris et al. (1982) determined that peat from the Great Dismal Swamp contributes to remove the atmospheric CH4 when the water table level is below the surface of the peat during dry periods; in contrast when peat is well saturated with water it becomes an important CH4 source to the atmosphere.
3. Net production. The magnitude of emissions depends on the amount of organic compounds released by plants from the net primary production process, since this represents the substrate for MB (Chanton & Dacey, 1991). Most of the carbon assimilated by the plants from the wetlands during photosynthesis is deposited in the root tissues, so that in this zone a great potential for easily degradable carbon exists, which contributes to the development and proliferation of MB. Consequently, the photosynthesis rate exerts a great impact on methanogenesis because a high carbon assimilation implies necessarily a greater concentration of available labile carbon in the roots that will be used by MB when it enters to the rhizosphere (Whiting & Chanton, 1993). Whiting and Chanton (1992) determined a positive relation between CH4 emission and net primary production of a minerotrophic fen dominated by Carex spp, these authors attribute the high CH4 emission rates to methanogenesis stimulation by the supply of labile organic substrates derived from the photosynthetic process, as well as to the fact that plants act as gas conductors. It has been estimated that approximately 3% of the net production of wetland ecosystems escapes to the atmosphere in the form of CH4.
The relationship of the primary production process with CH4 emission also influences the diurnal variations of the emissions. Thomas et al., (1996) demonstrated an increment in CH4 flux from peat during the day and a decrease during the night. During the day, photosynthesis products contribute to the increase in root exudates that promote formation of CH4 by MB, which is transported through the vascular system of plants and released through the stomata. In addition, during the day, photosynthesis releases oxygen that displacement the oxic zone below the peat surface and around the roots, increasing CH4 oxidation. Therefore the emissions dynamics, during a short period, depends on the circadian physiology of the wetlands flora (Lloyd et al., 1998).
4. Supply of labile organic matter. In wetlands, availability of labile organic carbon susceptible to be used during the methanogenesis process depends, besides net production, from the amount of flooded vegetal matter. When the water column depth increases, decomposition of vegetal matter is greater under anaerobic conditions and, since these processes are slower, the generated organic substrates are of better quality (carbohydrates and proteins). In the other hand, with a lower water column depth, there is a smaller amount of flooded vegetal cover, favoring the aerobic mineralization processes, which are faster and, hence, generate substrates difficult to degrade as humic substances, biologically inert (Cicerone et al., 1992).
To this respect Miyajima et al. (1997) demonstrated a negative correlation between methanogenic rate with the lignin and fiber contents of the substrate plant materials. However in freshwater wetlands the presence of high concentrations of labile organic carbon increases the amount of total CH4 produced (Crozier & DeLaune, 1996). In marshes with Deyeuxia angustifolia, a CH4 content of 0.72 g/m2 with 0.68 mM of labile organic carbon was determined, whereas, in marshes with Carex lasiocarpa, a concentration of 1.61 g CH4/m2 and 1.86 mM of labile organic carbon was found (Ding et al., 2002).
5. Substrates concentration. A high acetate concentration promotes the growth of Methanosarcina spp., which requires 0.2-1.2 mM acetate, whereas Methanosaeta spp. needs < 10 µM. In fertilized rice fields where acetate concentrations up to 24 mM have been evaluated, Methanosarcina spp. dominates (Lovley & Klug, 1983; Lovley & Klug, 1986). It has been observed that MB can tolerate up to 100 mM of volatile fatty acids (acetate among them) at neutral pH; however at acidic pH bacteria become extremely sensitive to acetate concentrations. In acidic conditions, with an extracellular pH lower than 4.7, the non-dissociated acetic acid is abundant and can diffuse through the cellular membrane towards the interior of the cell, that remains at a neutral pH; once inside, the acid dissociates and induces uncoupling of the membrane protons producing cell death (Russell, 1991).
Finally, since at the beginning of the mineralization processes there is no available substrate for the MB, this bacterial population does not establish; therefore, fermentative activity limits methanogenesis (Shannon & White, 1996).
6. Vegetal cover and distribution. CH4 flux is not related with the total vegetal cover area but flux is related with the floristic composition. Bryophytes are more sensitive to changes in water table levels than vascular plants, hence they are better indicators of CH4 emission (Gignac & Vitt, 1990). In a smaller scale, the presence of certain species in the humid zones is a good indicator of the flux. Carex rostrata, C. limosa, and Cladopodiella fluitants are indicators of high CH4 emissions (higher than 100 mg/m2/day). The bryophytes Scorpidium scorpioides, and Limprichtia revolvens are associated to low emissions (9-14 mg/m2/day) (Bubier, 1995). Emissions magnitude is also related to the distribution of bryophytes in histosols, since in hollows when Sphagnum majus, S. cuspidatum, Cratoneuron filicinum, and Warnstorfia exannulatus dominate, CH4 flux is 156 mg/m2/day; in contrast in hummocks, with S. fuscum, emissions decrease to 5 mg/m2/day (Bubier et al., 1993).
In freshwater marshes, CH4 flux increases linearly with the increment in the density of stems from Deyeuxia angustifolia (25.7 mgCH4/m2/hr), Carex meyeriana (31.1 mgCH4/m2/hr), and Carex lasiocarpa (35.6 mgCH4/m2/hr). Emissions reached maximal levels in the area where root growth was more abundant (Ding et al., 2002). In nutrients-poor wetlands, characterized by the presence of Oxycoccus spp. and Andromeda spp., emissions increase (Sundh et al., 2000).
7. Sulfates concentration. In brackish wetlands characterized by constant supply of sulfates, was determinate a negative correlation between sulfate concentration and CH4 emission (Purvaja & Ramesh, 2001). In this environments the sulfate reduction process is favored in contrasting with the methanogenesis since SRB compete more efficiently for the available substrates, mainly acetate and hydrogen, as compared to MB (Lovley & Klug, 1986). In consequence, CH4 production decreases and methanogenesis is restricted to deep areas of sediments where sulfate supply decreases (Sinke et al., 1992). In a coastal wetland of Mexico (Chiapas) we observed than methanogenesis was higher in months associated to the rainy season when the sulfate concentration diminished (Torres-Alvarado et al., 2005).
Senior et al. (1982) demonstrated that CO2 reduction is the only mechanism for CH4 production in sediments from salt marshes where sulfate supply is high. The capacity of HMB to produce CH4 in the presence of competitive processes, such as sulfate reduction, allowed methanogenesis continue in this type of wetlands. In addition to the use of hydrogen, MB are able to use less competitive substrates, such as methanol and methylamines. Lovley and Klug (1983) determined that CH4 formation from methanol represents 2.8-4.0% of the total production and helps methanogenic bacteria to maintain their populations when sulfate is being supplied to the environment.
A complete inhibition of methanogenesis by addition of sulfate also been observed in rice fields (Achtnich et al., 1995). In this wetlands the activity of SRB is higher directly at the root of the rice plant (500 nmol/cm3/day) as compared to the rhizosphere (310 nmol/cm3/day) and the oxic-anoxic interface (100 nmol/cm3/day). The presence of two different physiological types of SRB has been suggested in these wetlands. The rhizosphere region is colonized by SRB that oxidize incompletely the substrates and have fast growth, such as species from the genus Desulfovibrio, whereas the soil is the habitat of those SRB that oxidize completely the substrate, belonging to the slow growth spore forming bacteria, such as species from the genus Desulfotomaculum (Liesack et al., 2000).
An important aspect related to the rate of CH4 emission is the effect of acid rain. In regions affected by this phenomenon, atmospheric deposition of sulfates occurs in the aquatic ecosystems and wetlands that might reduce the magnitude of CH4 flux (Nedwell & Watson, 1995). Watson and Nedwell (1998) demonstrated that in histosols of the Great Dun Fell, methanogenesis is inhibited by 250 µM sulfate concentrations supplied by the acid rain in England that favors the sulfate reduction process.
8. pH. In laboratory studies it has been demonstrated that CH4 production by MB depends on the pH, with optimal values between 5 and 7; however tolerant strains to the acid conditions of histosols have been reported (Williams & Crawford, 1984; Dunfield et al., 1993).
9. Phosphates. It has been observed that the acetoclastic methanogenic activity in the roots of rice plants is inhibited by phosphates concentration. In laboratory experiments with acetate, a CH4 production of 40% was obtained without phosphates and this production decreased to 20% with phosphates in the medium (Chin et al., 2004). In contrast, CH4 production increased with a carbonate buffer solution.
10. Soil texture. Soil and sediments texture is also a factor related to CH4 emissions. Soils with high clay content and high supply of organic matter have the highest CH4 production, whereas sandy soils are characterized by a low production potential (Neue et al., 1990). In clay soils average CH4 emission rates of 209 mg/m2/day were quantified, whereas in sandy slightly acid soils the average emission was 59.7 mg/m2/day.
11. Peat harvesting. The peat is an important CH4 source. Draining activities and peat harvesting to be used as oil, natural gas, and in medicine mainly in Canada, Russia, Ireland, and Nordic cities, alter CH4 flux. Before peat harvesting starts, the water table level where it is located, has decreased considerably due to draining processes. Because the superficial layer is more oxidized, a decrease in CH4 emission is produced; however, during the harvesting, peat becomes an important source of CH4 emission (15-93 mg/m2/hr) than drained peat (0.11 mg/m2/hr) (Roulet & Moore, 1995).
12. Alteration of river beds. River flows worldwide have been modified by human development, causing a decrease in fluvial volume and changes in its distribution. Fluctuations in water level affect flooding periods of wetlands, and this influences the production/consumption of CH4, changing the gas emission rate. In adjacent wetlands to Murria River (Australia), maximal CH4 emissions occurred in winter (rainy season) or spring (ice melting). However when the course of the river was changed, maximal emissions were quantified during the summer with the flooding period (Boon et al., 1997).
Methane and greenhouse effect
Global methane sources to the atmosphere range between 1012 to 1014 g CH4/year, producing a 1.75 ppm concentration of this gas in the atmosphere, with a mean life span of 7 to 11 years; however in the last decades this concentration has increased at a rate of 0.5-0.8% annually. Atmospheric CH4 is recognized as one of the most important gas involved in the greenhouse effect (Pearman & Fraser, 1988; Karl & Tilbrook, 1994).
Gases found in the atmosphere such as CO2, CH4, nitric oxide (N2O), and ozone (O3), entrap solar radiation when this is reflected by the earth surface (greenhouse effect), helping to maintain the earth warm with a global average temperature of 15°C, which induces development of biophysical processes. The warming effect produced by a gas is related to its concentration and permanence in the atmosphere, as well as with its radioactive force. In this sense, although CH4 has a considerably lower atmospheric concentration (1.7 ppm) than CO2 (345 ppm), it has a greater capacity to absorb infrared radiation, therefore addition of 1 kg CH4 to the atmosphere blocks better the heat emitted by the terrestrial surface than 1 kg CO2. Therefore, CH4 as a molecule is 30 times more effective to absorb heat during the greenhouse effect than CO2 (Tyler, 1991). For this reason, CH4 is the second gas of importance, after CO2, involved in the greenhouse effect; contributing approximately with 25% to atmospheric warming.
Methane emissions and global climatic change. The increase in concentrations of the main gases with greenhouse effect in the atmosphere in the last years has promoted an increase in the average temperature of the earth. This phenomenon is known as Global Climatic Change due to the effects produced on the earth's weather.
There are different factors affecting the climatic system, however many scientists claim that emission of gases CH4 and CO2, coming mainly from anthropogenic activities and wetlands, could be a key factor in the increase of temperature and the global climatic change. The increase in atmospheric temperature produces a greater activity in the hydrological cycle, changes in the distribution of rainfall and in climatic patterns, alterations of terrestrial ecosystems and of migration species patterns (EPOMEX, 1998). A loss of biodiversity is being fore-casted, particularly in the tropical regions, as well as a fusion of the polar caskets that will increase the level of water in oceans (Masera, 1992).
It has been anticipated that the effects of the Global Climatic Change will be more pronounced in the continental regions of the Northern Hemisphere because they contain most of the wetland ecosystems, where the flow of gases with green-house effects is relevant, producing between 22 to 70 Tn/year, (Anselmann & Crutzen, 1989). Likewise, tropical regions, due to their high biological activity, have the potential to emit large amounts of gases to the atmosphere. If an increase in Global Warming is produced, a change in peat saturation of wetlands could be induced, modifying CH4 fluxes (Conrad et al., 1987).
It is expected that Global Warming be more pronounced at high latitudes, calculating that, in the Arctic, the average temperature will increase approximately 3°C. As a consequence, in boreal wetlands, the rate of evapotranspiration will increase reducing the area of the saturated ecosystems, decreasing CH4 production and increasing its consumption through bacterial oxidation processes, hence, the increase in temperature would have a negative feedback control on atmospheric CH4 (Arah & Stephen, 1998). In contrast, in tropical regions, increase in temperature could would be accompanied by an increase in methanogenesis and CH4 emissions from wetlands (Cao et al., 1998).
Management and mitigation measures. Although recent environmental regulations recognize the fundamental relevance of wetlands as natural habitats and center of biogeochemical activity, not clearly mitigation measures have been established to attenuate the flux of CH4 emissions to the atmosphere. This is particularly difficult with natural wetlands where management measures require diverse studies before their implementation. Despite this, there have been some proposals to this respect.
Plants growth in wetlands produce a larger supply of organic matter for methanogenesis, incrementing the production and emission of CH4. In consequence, removal of vegetal residues could contribute to diminish methane fluxes. In Sweden, where harvest of peat is practiced, a regular cleaning of the dikes from which peat is extracted has been established as a mitigation measure to help maintain low emission levels (Sundh et al., 2000).
Several mitigation strategies have been established for rice paddy fields. One of the most drastic measures proposed has been the transition of the wetland to upland regions without flooding. This measure is not simple to implement in the agricultural practice mainly for production reasons. Most of the uplands used for rice cultivation produce 1-4 Ton/ha, whereas the flooded rice fields produce more than 7 Ton/ha (Institute, 1988).
A more promising measure involves a modification in the irrigation pattern to include short dry periods, since rice plants can recover after short dry periods once the flooding conditions are reestablished. An additional measure refers to the implementation of an internal drainage system in the cultivated fields that might facilitate oxygen introduction to increase CH4 oxidation. This measure would have a particular positive effect on saline and alkaline soils (De Datta, 1981).
The use of different types of fertilizers has been another of the analyzed measures; however, the results obtained until now have been contradictory. The use of ammonium sulfate in some regions in Italy has reduced CH4 emissions (Schütz et al., 1989b). However, its use in rice paddies from California did not attenuate methanogenesis (Cicerone & Shetter, 1981). Another proposal refers to the addition of enough amounts of iron (III) to the rice fields to promote development of iron- reducing microbiota, which competes more effectively for the available substrates, both for sulfate reduction and methanogenesis, which could decrease CH4 production in rice fields (Nouchi et al., 1990; Wassmann et al., 1993). This phenomenon also was observed in natural wetlands where incubation experiments demonstrated that microbial Fe(III) oxide reduction suppressed sulfate reduction and methanogenesis in surface sediments, whereas in rhizosphere sediment the Fe(III) oxide reduction accounted for 65% of total carbon metabolism, compared to 22% for methanogenesis (Roden & Wetzel, 1996).
Concluding remarks
This review has outlined some aspects of methanogenesis and CH4 oxidation in wetlands, and its relation with CH4 emissions from this environments. CH4 is considered one of the most important greenhouse gases in the atmosphere. Because of the strict anaerobic conditions required by CH4 generation by microorganisms, natural wetland ecosystems are one of the main sources of biogenic CH4 and tropical wetlands are one of the largest natural sources in the global methane budget due to high biological activities.
Increases in atmospheric CH4 and other greenhouse gas concentrations are predicted to rise global mean temperature with several implications. The only way by which the magnitude of climatic changes at a global scale can be assessed is by performing several quantifications of the production, oxidation, and emission of CH4 in a large variety of wetlands and characterize their response to environmental parameters, since the available database on CH4 emissions to the atmosphere is insufficient in regard to the large variety of climatological and edaphological factors that would allow to reliably extrapolate data at a global scale and to design more precise models on the impact of the Global Climatic Change leading to a better prediction of future scenarios. In Mexico is particularly important because there aren't studies to respect.
The increase in rice demands due to the growing human population could lead to further increases in the areas used for its cultivation and, therefore, would contribute to higher CH4 emissions. As a result, rice cultivation will have a large impact on future Global Warming. By these reason, the research on rice cultivation must be addressed to a better analysis of CH4 production, its oxidation and to the development of possible mitigation strategies to diminish and/or suppress emissions of this hydrocarbon.
References
ACHTNICH, C., A. SCHUHMANN, T. WIND & R. CONRAD. 1995. Role of interspecies H2 transfer to sulfate and ferric iron-reducing bacteria in acetate consumption in anoxic paddy soil. FEMS Microbiology Ecology. 16: 61-70 [ Links ]
ANASTASI, C., M. DOWDING & V. J. SIMPSON. 1992. Future CH4 emissions from rice production. Journal of Geophysical Research 97: 7521-7525. [ Links ]
ANSELMANN, I. & P. J. CRUTZEN. 1989. Freshwater wetlands: global distribution of natural wetlands and rice paddies, their net primary productivity, seasonality and possible methane emissions. Journal of Atmospheric Chemistry 8: 307-358. [ Links ]
ARAH, J. R. M. & K. D. STEPHEN. 1998. A model of the processes leading to methane emission from peatland. Atmospheric Environment 32: 3257-3264. [ Links ]
BAK, F. & F. WIDDEL. 1986. Anaerobic degradation of phenol and phenol derivates by Desulfobacterium phenolicum sp. nov. Archives of Microbiology 146: 177-180. [ Links ]
BAKER-BLOCKER, A., T. M. DONAHUE & K. H. MANCY. 1977. Methane flux from wetlands. Tellus 29: 245-250. [ Links ]
BARTLETT, K. B. & R. C. HARRIS. 1993. Review and assessment of methane emissions from wetlands. Chemosphere 26: 261-320. [ Links ]
BARTLETT, K. B., R. C. HARRIS & D. I. SEBACHER. 1985. Methane flux from coastal salt marshes. Journal Geophysical Research 90: 5710-5720. [ Links ]
BARTLETT, K. B., P. M. CRILL, D. I. SEBACHER, R. C. HARRIS, J. O. WILSON & J. M. MELACK. 1988. Methane flux from the central Amazonian flooodplain. Journal Geophysical Research 93: 1571-1582. [ Links ]
BLAIR, N. E. & R. C. ALLER. 1995. Anaerobic methane oxidation on the Amazon shelf. Geochimica et Cosmochimica Acta 59: 3707-3715. [ Links ]
BOON, P. I., A. MITCHELL & K. LEE. 1997. Effects of wetting and drying on methane emissions from ephemeral foodplain wetlands in south-eastern Australia. Hydrobiologia 357: 73-87. [ Links ]
BOONE, D. R., W. B. WHITMAN & P. ROUVIERE. 1993. Diversity and Taxonomy of Methanogens. In: Ferry, J. G. (Ed). Methanogenesis. Ecology, Physiology, Biochemistry and Genetics. Chapman and Hal, pp. 35-80. [ Links ]
Bowman, J. P., L. I. Sly & E. Stackebrandt. 1995. The phylogenetic position of the family Methylococceae. International Journal of Systematic Bacteriology 45: 182-185. [ Links ]
BROOKS AVERY, G. J., R. D. SHANNON, J. R. WHITE, C. S. MARTENS & M. J. ALPERIN. 2002. Controls on methane production in a tidal fresh-water estuary and a peatland: methane production via acetate fermentation and CO2 reduction. Biogeochemistry 00: 1-19. [ Links ]
BUBIER, J. L. 1995. The relationship of vegetation to methane emission and hydrochemical gradients in Northern Peatlands. Journal of Ecology 83: 403-420. [ Links ]
BUBIER, J. L., T. R. MOORE & N. T. ROULET. 1993. Methane emissions from wetlands in the midboreal region of Northern Ontario, Canada. Ecology 74: 2240-2254. [ Links ]
CAO, M., K. GREGSON & S. MARSHALL. 1998. Global methane emission fron wetlands and its sensitivity to Climate Change. Atmospheric Environment 32: 3293-3299. [ Links ]
CARRERA, E. G. & G. DE LA FUENTE LEÓN. 2003. Inventario y Clasificación de Humedales en México. In. Ducks Unlimited de México, Asociación Civil (DUMAC). México. p. 239. [ Links ]
CHAN, A. S. K. & T. B. PARKIN. 2001. Methane oxidation and production activity in soils from natural and agricultural ecosystems. Journal of Environmental Quality 30: 1896-1903. [ Links ]
CHANTON, J. P. & J. W. H. DACEY. 1991. Effects of vegetation on methane flux, reservoirs, and carbon isotopic composition. In: Sharkey, T. D., E. A. Holland & H. A. Mooney (Eds). Trace Gas Emissions by Plants. Academic Press, Great Britain. pp. 65-92. [ Links ]
CHANTON, J. P. & C. S. MARTENS. 1988. Seasonal variations in ebullitive flux and carbon isotopic composition of methane in a tidal freshwater estuary. Global Biogeochemical Cycles. 2: 289-298. [ Links ]
CHIN, K. J., T. LUEDERS, M. W. FRIEDRICH, M. KLOSE & R. CONRAD. 2004. Archaeal community structure and pathway of methane formation on rice roots. Microbial Ecology 47: 59-67. [ Links ]
CICERONE, R. J. & J. D. SHETTER. 1981. Sources of atmospheric methane: measurements in rice paddies and a discussion. Journal of Geophysical Research 86: 7203-7209. [ Links ]
CICERONE, R. J. & R. S. OREMLAND. 1988. Biochemical aspects of atmospheric methane. Global Biogeochemical Cycles 2: 229-327. [ Links ]
CICERONE, R. J., C. C. DELWICHE, S. C. TYLER & P. R. ZIMMERMAN. 1992. Methane emissions from California rice paddies with varied treatments. Global Biogeochemical Cycles 6: 233-248. [ Links ]
COWARDIN, L. M., CARTER, F.C. GOLET & E.T. LA ROE. 1979. Classification of wetlands and deepwater habitatas of the United States. USFWS/0BS-79/31. [ Links ]
CROZIER, C. R. & R. D. DELAUNE. 1996. Methane production by soils from different Louisiana marsh vegetation types. Wetlands. 16: 121-126 [ Links ]
CLYMO, R. S. 1983. Peat in mires:swamp, bog, fen and moor. In: Gore, A. J. P. (Ed). Ecosystems of the World. Elsevier, pp. 159-224. [ Links ]
CONRAD, R., H. SCHÜTZ & M. BABBEL. 1987. Temperature limitation of hydrogen turnover and methanogenesis in anoxic paddy soil. FEMS Microbiological Ecology 45: 281-289. [ Links ]
CRILL, P. M., K. B. BARTLETT, R. C. HARRIS, E. GORHAM, E. S. VERRY, D. I. SEBACHER, L. MADZAR & W. SANNER. 1988. Methane flux from Minnesota peatlands. Global Biogeochemical Cycles 2: 371-384. [ Links ]
DACEY, J. W. H. 1980. Internal winds in water lilies: an adaptation for life in anaerobic sediments. Science 210: 1017-1019. [ Links ]
DAULAT, W. E. & R. S. CLYMO. 1998. Effects of temperature and watertable on the efflux of methane from peatland surface cores. Atmospheric Environment. 32: 3207-3218. [ Links ]
DE DATTA, S. K. 1981. Principles and Practices of Rice Production. John Wiley and Sons. New York, USA. 250 p. [ Links ]
DEBONT, J. A. M., K. K. LEE & D. F. BOULDIN. 1978. Bacterial oxidation of methene in a rice paddy. Ecology Bulletin 26: 91-96. [ Links ]
DEDYSH, S. N., N. S. PANIKOV, W. LIESACK, R. GROBKOPF, J. ZHOU & J. M. TIEDJE. 1998. Isolation of acidophilic methane-oxiding bacteria from Northern peat wetlands. Science 282: 281-284. [ Links ]
DELAUNE, R. D., C. J. SMITH & W. H. PATICK. 1983. Methane release from Gulf Coast wetlands. Tellus 35B: 8-15. [ Links ]
DING, W., Z. CAI, H. TSURUTA & X. LI. 2002. Effect of standing water depth on methane emissions from freshwater marshes in Northeast China. Atmospheric Environment 36: 5149-5157. [ Links ]
DUNFIELD, P., R. KNOWLES, R. DUMONT & T. MOORE. 1993. Methane production and consumption in temperate and subartic peat soils: response to temperature and pH. Soil Biology and Biochemistry 23: 321-326. [ Links ]
EDWARDS, C., B. A. HALES, G. H. HALL, I. R. MCDONALDS, J. C. MURRELL, R. PICKUP, D. A. RITCHIE, J. R. SAUNDERS, B. M. SIMON & M. UPTON. 1998. Microbiological processes in the terrestrial carbon cycle: methane cycling in peat. Atmospheric Environment 32: 3247-3255. [ Links ]
EPOMEX. 1998. Las modificaciones antrópicas sobre el efecto de invernadero y sus consecuencias ambientales. Centro de Ecología, Pesquerías y Oceanografía del Golfo de México 9: 1-2. [ Links ]
ESWARAN, H., E. V. D. BERG, P. REICH & J. KIMBLE. 1995. Global soil carbon resources. In: Lal, J. K., E. Levine & B. A. Stewart (Eds). Soils and Global Change. CRC Lewis Publishers, pp. 27-43. [ Links ]
FUNG, I., J. JOHN, J. LERNER, E. MATTHEWS, M. PRATHER, L. STEELE & P. FRASER. 1991. Global budgets of atmospheric methane: results from a three-dimensional global model synthesis. Journal of Geophysical Research 6: 13033-13065. [ Links ]
GARCIA, J. L. 1990. Taxonomy and ecology of methanogens. FEMS Microbiological Reviews 87: 297-308. [ Links ]
GIBSON I, G. R., G. T. MACFARLANE & J. H. CUMMINGS. 1988. Occurrence of sulphate-reducing bacteria in human faeces and the relationship of dissimilatory sulphate reduction to methanogenesis in the large gut. Journal of Applied Bacteriology 65: 103-111. [ Links ]
GIGNAC, L. D. & D. H. VITT. 1990. Habitat limitations of Sphagnum along climatic, chemical, and physical gradients in mires of western Canada. Bryologist 93: 7-22. [ Links ]
GLISSMANN, K. & R. CONRAD. 1999. Fermentation pattern of methanogenic degradation of rice straw in anoxic paddy soil. FEMS Microbiological Ecology 31: 117-126. [ Links ]
HARGREAVES, K. J. & D. FOWLER. 1998. Quantifying the effects of water table and soil temperature on the emission of methane from peat wetland at the field scale. Atmospheric Environment 32: 3275-3282. [ Links ]
HARRIS, R. C. & D. I. SEBACHER. 1981. Methane flux in forested fresh-water swamps of the southeastern United States. Geophysical Research Letters 8: 1002-1004. [ Links ]
HARRIS, R. C., D. I. SEBACHER & F. P. D. DAY, JR. 1982. Methane flux in the Great Dismal Swamp. Nature 297: 673-674. [ Links ]
HARRIS, R. C., D. I. SEBACHER, K. B. BARTLETT, D. S. BARTLETT & P. M. CRILL. 1988. Sources of atmospheric methane in the south Florida environment. Global Biogeochemical Cycles 2: 231-243. [ Links ]
HARRISS, R. C., E. GORHAM, D. I. SEBACHER, K. B. BARTLETT & P. A. FLEBBE. 1985. Methane flux from northern peatlands. Nature 315: 652-653. [ Links ]
HINES, M. E., K. N. DUDDLESTON & R. P. KIENE. 2001. Carbon flow to acetate and C1 coumpounds in northern wetlands. Geophysical Research Letters. 28: 4251-4254 [ Links ]
HORN, M. A., C. MATTHIES, K. KÜSEL, A. SCHRAMM & H. L. DRAKE. 2003. Hydrogenotrophic methanogenesis by moderately acid-tolerant methanogens of a methane-emitting acid peat. Applied and Environmental Microbiology 69: 74-83. [ Links ]
HORNIBROOK, E. R. C., F. J. LONGSTAFFE & W. S. FYFE. 1997. Spatial distribution of microbial methane production pathways in temperate zone wetland soils: stable carbon and hydrogen isotope evidence. Geochimica et Cosmochimica Acta. 61: 745-753 [ Links ]
HOWARTH, R. W. 1993. Microbial processes in salt-marsh sediments. In: Ford, T. E. (Ed). Aquatic Microbiology. Blackwell Scientific Publications, Great Britain. pp. 239-260. [ Links ]
INSTITUTE, I. R. R. 1988. World Rice Statistics 1987. (IRRI, Manila Philippines) p. 257 [ Links ]
JOABSSON, A., T. R. CHRISTENSEN & B. WALLÉN. 1999. Vascular plant controls on methane emissions from northern peat forming wetlands. Trends in Ecology and Evolution 14: 385-388. [ Links ]
KARL, D. M. & B. D. TILBROOK. 1994. Production and transport of methane in oceanic particulate organic matter. Nature 368: 732-734. [ Links ]
KELLER, M., M. E. MITRE & R. F. STALLARD. 1990. Consumption of atmospheric methane in soils of central Panama: effects of agricultural development. Global Biogeochemical Cycles 4: 21-27. [ Links ]
KELLY, C. A., C. S. MARTENS & J. P. CHANTON. 1990. Variations in sedimentary carbon remineralization rates in the White Oak River Estuary, North Carolina. Limnology and Oceanography. 35: 372-383. [ Links ]
KELLEY, C. A., C. S. MARTENS & W. USSLER. 1995. Methane dynamics across a tidally flooded riverbank margin. Limnology and Oceanography 40: 1112-1129. [ Links ]
KING, G. M. 1994. Association of methanotrophs with the roots and rhizomes of aquatic vegetation. Applied and Environmental Microbiology 60: 3220-3227. [ Links ]
KING, G. M. 1996. In situ analyses of methane oxidation associated with the roots and rhizomes of a bur reed, Sparganium eurycarpum, in a Maine wetland. Applied and Environmental Microbiology 62: 4548-4555. [ Links ]
KING, G. M. & W. J. WIEBE. 1978. Methane release from soils of a Georgia salt marsh. Geochimica et Cosmochimica Acta 42: 343-348. [ Links ]
KING, G. M., P. ROSLEV & H. SKOVGAARD. 1990. Distribution and rate of methane oxidation in sediments of the Florida Everglades. Applied and Environmental Microbiology 56: 2902-2911. [ Links ]
KNOWLES, R. & T. R. MOORE. 1990. Methane emissions from fen, bog and swamp peatlands in Quebec. Biogeochemistry. 11: 45-61 [ Links ]
KRUMHOLZ, L. R., J. L. HOLLENBACK, S. J. ROSKES & D. B. RINGELBERG. 1995. Methanogenesis and methanotrophy within a Sphagnum peatland. FEMS Microbiology Ecology. 18: 215-224 [ Links ]
LIESACK, W., S. SCHNELL & N. P. REVSBECH. 2000. Microbiology of flooded rice paddies. FEMS Microbiological Reviews 24: 625-645. [ Links ]
LLOYD, D., K. L. THOMAS, J. BENSTEAD, K. L. DAVIES, S. H. LLOYD, J. R. M. ARAH & K. D. STEPHEN. 1998. Methanogenesis and CO2 exchange in an ombrotrophic peat bog. Atmospheric Environment 32: 3229-3238. [ Links ]
LOVLEY, D. R. 1991. Dissimilatory Fe(III) and Mn(IV) reduction. Microbiological Reviews 55: 259-287. [ Links ]
LOVLEY, D. R. & M. J. KLUG. 1983. Methanogenesis from methanol and methylamines and acetogenesis from hydrogen and carbon dioxide in the sediments of an eutrophic lake. Applied and Environmental Microbiology 45: 1310-1315. [ Links ]
LOVLEY, D. R. & M. J. KLUG. 1986. Model for distribution of sulfate reduction and methanogenesis in freshwater sediments. Geochimica et Cosmochimica Acta 50: 11-18. [ Links ]
LOVLEY, D. R. & E. J. P. PHILLIPS. 1987. Competitive mechanisms for inhibition of sulfate reduction and methane production in the zone of ferric iron reduction in sediments. Applied and Environmental Microbiology 53: 2636-2641. [ Links ]
MAH , R. A. & M. R. SMITH. 1981. The methanogenic bacteria. In: P.S. Mortimer, H. S., H.G. Trüper, A. Balows and H.G. Schlegel (Ed). The prokaryotes. A handbook on habitats isolation and identification of bacteria. Springer-Verlag, pp. 948-977. [ Links ]
MASERA, C. O. 1992. Emisiones de gases de invernadero en Latinoamérica. Situación actual y escenarios a largo plazo. Ciencia 43: 35-40. [ Links ]
MATTHEWS, E. & I. FUNG. 1987. Methane emission from natural wetlands: global distribution, area and environmental characteristics of sources. Global Biogeochemical Cycles 1: 61-86. [ Links ]
MEGRAW, S. R. & R. KNOWLES. 1987. Methane production and consumtion in a cultivated Humisol. Biology of Fertilizated Soils 5: 56-60. [ Links ]
MITSCH, W. J. & J. G. GOSSELINK. 1993. Wetlands. Van Nostrand Reinhold. New York. 539 [ Links ]
MIYAJIMA, T., E. WADA, Y. T. HANBA & P. VIJARNSORN. 1997. Anaerobic mineralization of indigenous organic matters and methanogenesis in tropical wetland soils. Geochimica et Cosmochimica Acta. 61: 3739-3751 [ Links ]
MOORE, T. & R. KOWLES. 1990. Methane emissions from fen, bog and swamp peatlands in Quebec. Biogeochemistry 11: 45-61. [ Links ]
MOORE, T. & N. ROULET. 1993. Methane flux: water table relations in northern wetlands. Geophysical Research Letters 20: 587-590. [ Links ]
MOORE, T. R. & M. DALVA. 1993. The influence of temperature and water table position on carbon dioxide and methane emissions from laboratory columns of peatland soils. Journal of Soil Science 44: 651-664. [ Links ]
MORAN, M. A., R. BENNER & R. E. HUDSON. 1989. Kinetics of microbial degradation of vascular plant material in two wetlands ecosystems. Oecologia 79: 158-167. [ Links ]
MORRISSEY, L. A., D. B. ZOBEL & G. P. LIVINGSTON. 1993. Significance of stomatal control on methane release from Carex-dominated wetlands. Chemosphere 26: 339-355. [ Links ]
MULLER, K. L. G. G. GANF & P. I. BOON. 1994. Methane flux from beds of Baumea arthrophylla (Nees) Boeckeler and Triglochin procerum R. Br. at Bool Lagoon, South Australia. Australian Journal of Marine and Freshwater Research. 45: 1543-1553 [ Links ]
NEDWELL, D. B. & A. WATSON. 1995. CH4 production, oxidation and emission in a U. K. ombrotrophic peat bog: influence of SO4 from acid rain. Soil Biology and Biochemistry 27: 893-903. [ Links ]
NEUE, H. U., P. BECKER-HEIDMANN & H. W. SCHARPENSEEL. 1990. Organic matter dynamics, soil properties, and cultural practices in rice lands and their relationship to methane production. In: Bouwman, A. F. (Ed). Soils and the Greenhouse Effect. John Wiley and Sons, USA. pp. 457-466. [ Links ]
NOUCHI, I., S. MARIKO & K. AOKI. 1990. Mechanism of methane transport from the rhizosphere to the atmosphere through rice plants. Plant Physiology 94: 59-66. [ Links ]
OJIMA, D. S., D. W. VALENTINE, A. R. MOSIER, W. J. PARTON & D. S. SCHIMEL. 1993. Effect of land use change on methane oxidation in temperate forest and grassland soils. Chemosphere 26: 675-685. [ Links ]
ORPHAN, V. J., C. H. HOUSE, K.-U. HINRICHS, K. D. MCKEEGAN & E. F. DELONG. 2002. Multiple microbial groups mediate methane oxidation in anoxic marine sediments. Proccedings Natural Academic Science.
PEARMAN, G. I. & P. J. FRASER. 1988. Sources of incresed methane. Nature 332: 489-490. [ Links ]
PETERS, V. & R. CONRAD. 1996. Sequential reduction processes and initiation of CH4 production upon flooding of oxic upland soils. Soil Biology and Biochemistry 28: 371-382. [ Links ]
PFENNIG, N., F. WIDDEL & H. G. TRUPER. 1981. The dissimilatory sulfate-reducing bacteria. In: Starr, M. P., H. Stolp, H. G. Truper, A. Ballows & H. G. Schlegel (Eds). The Procaryotes. A handbook on habitats isolation and identification of bacteria. Springer-Verlag, USA. pp. 926-940. [ Links ]
PHELPS, T. J. & J. G. ZEIKUS. 1985. Effect of fall turnover on terminal carbon metabolism in Lake Mendota sediments. Applied and Environmental Microbiology 50: 1285-1291. [ Links ]
PURVAJA, R., & R. RAMESH. 2001. Natural and anthropogenic methane emission from coastal wetlands of South India. Environmental Management. 27: 547-557 [ Links ]
REDDY, K. R. & D. A. GRAETZ. 1988. Carbon and nitrogen dynamics in wetland soils. In: Hook, D. D. (Ed). The Ecology and Management of Wetlands. Croom Helm, pp. 307-318. [ Links ]
REEBURGH, W. S., S. C. WHALEN & M. J. ALPERIN. 1993. The role of methylotrophy in the global methane budget. In: Murrell, J. C. & D. P. Kelly (Eds). Microbial Growth on C-1 Compounds. Kluwer Academic Publishers, Great Britain. pp. 1-14. [ Links ]
RODEN, E. E. & R. G. WETZEL. 1996. Organic carbon oxidation and suppression of methane production by microbial Fe(III) oxide reduction in vegetated and unvegetated freshwater wetland sediments. Limnology and Oceanography. 41: 1733-1748. [ Links ]
ROULET, N. T. & T. R. MOORE. 1995. The effect of foresty drainage practices on the emission of methane from northern peatlands. Canadian Journal of Forest Research. 25: 491-499. [ Links ]
ROULET, N. T., R. ASH & M. T. R. 1992. Low boreal wetlands as a source of atmospheric methane. Journal Geophysical Research. 97: 3739-3749. [ Links ]
ROULET, N. T. 2000. Peatlands, carbon storage, greenhouse gases, and the Kyoto Protocol: prospects and significance for Canada. Wetlands. 20: 605-615 [ Links ]
RUBEC, C. A., P. LYNCH-STEWART, G. M. WICKWARE & I. KESSEL-TAYLOR. 1998. Wetland utilization in Canada. In: National Wetlands Working Group. Wetlands of Canada. Polyscience Publications and Environment Canada. Montreal, Canada. pp. 318-412. [ Links ]
RUSSELL, J. B. 1991. Intracellular pH of acid-tolerant ruminal bacteria. Applied and Environmental Microbiology 60: 1332-1240. [ Links ]
SCHIMEL, J. P. 1995. Plant transport and methane production as controls on methane flux from artic wet meadow tundra. Biogeochemistry 28: 183-200. [ Links ]
SCHÜTZ, H., W. SEILER & R. CONRAD. 1989A. Processes involved in formation and emission of methane in rice paddies. Biogeochemistry 7: 33-53. [ Links ]
SCHÜTZ, H., A. HOLZAPFEL-PSCHORN, R. CONRAD, H. RENNENBERG & W. SEILER. 1989B. A three years continuous record on the influence of daytime, season, and fertilizer treatment on methane emission rates from an Italian paddy field. Journal of Geophysical Research 94: 16405-16416. [ Links ]
SEBACHER, D. I., R. C. HARRIS, K. B. BARTLETT, S. M. SEBACHER & S. S. GRICE. 1986. Atmospheric methane sources: Alaska tundra bogs, an alpine fen and a subartic boreal marsh. Tellus 38B: 1-10. [ Links ]
SENIOR, E., E. B. LINDSTROM, M. B. IBRAHIM & D. B. NEDWELL. 1982. Sulfate reduction and methanogenesis in the sediment of a salt-marsh on the east coast of the United Kingdom. Applied and Environmental Microbiology. 43: 987-996. [ Links ]
SHANNON, R. D. & J. R. WHITE. 1996. The effects of spatial and temporal variations in acetate and sulfate on methane cycling in two Michigan peatlands. Limnology and Oceanography 41: 435-443. [ Links ]
SINKE, A. J. C., C. A. A., T. E. CAPPENBERG & A. J. B. ZEHNDER. 1992. Seasonal variation in sulfate reduction and methanogenesis in peaty sediments of eutrophic Lake Loosdrecht, The Netherlands. Biogeochemistry 16: 43-61. [ Links ]
SMITH, C. J., R. D. DELAUNE & J. W. H. PATRICK 1982. Carbon and nitrogen cycling in a Spartina alterniflora salt marsh. In: Freney, J. R. & I. E. Galvally (Eds). The cycling of Carbon, Nitrogen, Sulfur, and Phosphorus in Terrestrial and Aquatic Ecosystems. Springer-Verlag, USA. pp. 97-104. [ Links ]
SMITH, L. K. & W. M. LEWIS. 1992. Methane emissions from the Orinoco River floodplain, Venezuela. In: Aquatic Science Meeting. ASLO (Ed). Santa Fe, New Mexico [ Links ]
STOLZY, L. H., D. D. FOCHT & H. FLÜHLER. 1981. Indicators of soil aeration status. Flora 171: 236-265. [ Links ]
STRUWE, S. & A. KJOLLER. 1989. Field determination of denitrification in water-logged forest soils. FEMS Microbiology Ecology 62: 71-78. [ Links ]
SUNDH, I., M. NILSSON, C. MIKKELÄ, G. GRANBERG & B. H. SVENSSON. 2000. Fluxes of methane and carbon dioxide on peat-mining areas in Sweden. Ambio 29: 499-503. [ Links ]
SWEERTS, J.-P. R. A., M.-J. BÄR-GILISSEN, A. A. CORNELESE & T. E. CAPPENBERG. 1991. Oxygen-consuming processes at the profundal and littoral sediment-water interface of a small meso-eutrophic lake (Lake Vechten, The Netherlands). Limnology and Oceanography 36: 1124-1133. [ Links ]
TATHY, J. P., R. A. DELMAS, A. MARENCO, B. CROS, M. LABAT & J. SERVANT. 1992. Methane emission from flooded forest in Central Africa. Journal Geophysical Research 97: 6159-6168. [ Links ]
THOMAS, K. L. J. BENSTEAD, K. L. DAVIES & D. LLOYD. 1996. Role of wetland plants in the diurnal control of CH4 and CO2 fluxes in peat. Soil Biology and Biogeochemistry. 28: 17-23. [ Links ]
TORN, M. S. & F. S. CHAPIN. 1993. Environmental and biotic controls over methane flux from Artic Tundra. Chemosphere. 26: 357-368. [ Links ]
TORRES-ALVARADO, M. R., F. J. FERNÁNDEZ, I. D. L. A. BARRIGA-SOSA, V. H. RIVERA-MONROY & F. RAMÍREZ-VIVES. 2005. Seasonal shifts in the abundance and spatial distribution of methanogenic and sulfate-reducing bacteria in sediments of a tropical coastal lagoon system (Chantuto-Panzacola, Chiapas, Mexico). Microbial Ecology. Submitted. [ Links ]
TYLER, S. C. 1991. The global methane budget. In: Rogers, J. E. & W. B. Whitman (Eds). Microbial production and consumption of greehouse gases: methane, nitrogen oxides and halomethanes. American Society for Microbiology, USA. pp. 7-38. [ Links ]
VALENTINE, D. L. 1991. Thermodynamic ecology of hydrogen-based syntrophy. In: J., S. (Ed). Symbiosis: Mechanisms and Model Systems. Kluwer Academic Publishers, Great Britain. pp. 147-161. [ Links ]
VALENTINE, D. L. 2002. Biogeochemistry and microbial ecology of methane oxidation in anoxic environments: a review. Antonie van Leeuwenhoek 81: 271-282. [ Links ]
WANG, Z., D. ZENG & W. H. JR. PATRICK. 1996. Methane emissions from natural wetlands. Environmental Monitoring and Assessment. 42: 143-161 [ Links ]
WASSMANN, R., H. PAPEN & H. RENNENBERG. 1993. Methane emission from rice paddies and possible mitigation strategies. Chemosphere 26: 201-217. [ Links ]
WATSON, A. & D. B. NEDWELL. 1998. Methane production and emission from peat: the influence of anions (sulphate, nitrate) from acid rain. Atmospheric Environment. 32: 3239-3245. [ Links ]
WEBER, S., T. LAUDERS, M. W. FRIEDRICH & R. CONRAD. 2001. Methanogenic populations involved in the degradation of rice straw in anoxic paddy soil. FEMS Microbiology Ecology 38: 11-20. [ Links ]
WEST, A. E. & S. K. SCHMIDT. 2002. Endogenous methanogenesis stimulates oxidation of atmospheric CH4 in Alpine Tundra soil. Microbial Ecology 43: 408-415. [ Links ]
WESTERMANN, P. 1993A. Temperature regulation of methanogenesis in wetlands. Chemosphere 26: 321-328. [ Links ]
WESTERMANN, P. 1993B. Wetland and swamp microbiology. In: Ford, T. E. (Ed). Aquatic Microbiology. Blackwell Scientific Publications, Boston, USA. pp. 215-238. [ Links ]
WHALEN, S. C. & W. S. REEBURGH. 1990. Consumption of atmospheric methane by tundra soils. Nature 346: 160-162. [ Links ]
WHITING, G. J. & J. P. CHANTON. 1992. Plant-dependent CH4 emission in a subartic Canadian fen. Global Biogeochemical Cycles 6: 225-231. [ Links ]
WHITING, G. J. & J. P. CHANTON. 1993. Primary production control of methane emission from wetlands. Nature 364: 794-795. [ Links ]
WHITING, G. J., J. P. CHANTON, D. S. BARTLETT & J. D. HAPPELL. 1991. Relationships between CH4 emission, biomas, and CO2 exchange in a subtropical grassland. Journal of Geophysical Research 96: 13067-13071. [ Links ]
WIDDEL, F. 1988. Microbiology and ecology of sulfate and sulfur reducing bacteria. In: Zehnder, A. J. B. (Ed). Biology of Anaerobic Microorganisms. John Wiley and Sons., pp. 469-586. [ Links ]
WIEBE, W. J., R. R. CHRISTIAN, J. A. HANSEN, G. KING, B. SHERR & G. SKIRING. 1981. Anaerobic respiration and fermentation. In: Pomeroy, L. R. & R. G. Wiegert (Eds). The Ecology of a Salt Marsh. Springer-Verlag, USA. pp. 137-159. [ Links ]
WILLIAMS, R. T. & R. L. CRAWFORD. 1983. Effects of various physico-chemical factors on microbial activity in peatlands: aerobic degradative processes. Canadian Journal of Microbiology 29: 1430-1437. [ Links ]
WILLIAMS, R. T. & R. L. CRAWFORD. 1984. Methane production in Minnesota peatlands. Applied and Environmental Microbiology 47: 1266-1271. [ Links ]
YAVITT, J. B. & G. E. LANG. 1990. Methane production in contrasting wetland sites: response to organic chemical components of peat and to sulfate reduction. Geomicrobiology Journal 8: 27-46. [ Links ]
YAVITT, J. B. & A. K. KNAPP. 1995. Methane emission to the atmosphere through emergent cattail (Typha latifolia L.) plants. Tellus 47B: 521-534. [ Links ]
YAVITT, J. B., G. E. LANG & A. J. SEXSTONE. 1990. Methane fluxes in wetland and forest soils, beaver ponds, and low-order streams of a temperate forest ecosystem. Journal Geophysical Research 95: 463-474. [ Links ]
ZINDER, S. H. 1993. Physiological ecology of methanogens. In: Ferry, J. G. (Ed). Methanogenesis: Ecology, Physiology, Biochemistry and Genetics. Chapman and Hall, New York, USA. pp. 128-206. [ Links ]














