Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Hidrobiológica
versión impresa ISSN 0188-8897
Hidrobiológica vol.15 no.2 Ciudad de México 2005
Artículo
Wild-caught Hybrids Between Sailfin and Shortfin Mollies (Poeciliidae, Poecilia): Morphological and Molecular Verification
Híbridos silvestres entre molis de vela y de dorsal corta (Poeciliidae: Poecilia): verificación morfológica y molecular
Michele M. Kittell1, Megan N. Harvey1, Salvador Contreras-Balderas2 and Margaret B. Ptacek1
1 Department of Biological Sciences, Clemson University, Clemson, South Carolina, U.S.A.
2 Laboratorio de Ictiología, Facultad de Ciencias Biológicas, Universidad Autónoma de Nuevo León, Monterrey, Nuevo León, México. E-mail: mptacek@clemson.edu
Recibido: 4 de octubre de 2004.
Aceptado: 16 de abril de 2005.
Abstract
This study documents four wild-caught, interspecific hybrids between sailfin mollies (Poecilia velifera or P. petenensis) and shortfin mollies (P. mexicana or P. orri) from the Yucatán peninsula and Isthmus of Tehuantepec regions of México. In canonical discriminant analysis of morphological data all four putative hybrid males were intermediate in shape between shortfin and sailfin molly species, falling well outside 95% confidence ellipses for those putative parental species. For two of the four hybrid individuals, we used allele size differences at the nuclear Xsrc gene between sailfin and shortfin species to determine that one was a first (F1) or early generation (F2, BC1) hybrid and the other was a later generation (>F1) hybrid. Sequences of the mtDNA control region (483 bp) and Xsrc nuclear gene (636 bp) indicated that the female parent of the early generation hybrid individual was P. mexicana and the male parent was P. velifera. Thus, while rare in the wild, interspecific hybridization and introgression between sailfin and shortfin mollies does occasionally occur despite the existence of behavioral pre-mating isolating mechanisms.
Key words: Premating reproductive isolation, naturally-occurring hybrids, Poecilia velifera, Poecilia petenensis, Poecilia mexicana, Poecilia orri.
Resumen
El presente estudio documenta registros de cuatro híbridos interespecíficos espontáneos entre molis de vela (Poecilia velifera o P. petenensis) y de dorsal corta (P. mexicana o P. orri) de las regiones de la Península de Yucatán y del Istmo de Tehuantepec en México. En el análisis discriminante de datos morfológicos los cuatro híbridos putativos entre molis de dorsal de vela y dorsal corta tuvieron morfología intermedia, cayendo bastante fuera de las elipses a 95% de confianza de las especies progenitoras putativas. En dos de los cuatro individuos híbridos, usamos diferencias en el tamaño de los alelos en el gene nuclear Xsrc entre los molis de vela y de dorsal corta para determinar que uno de estos dos individuos híbridos resultó de primera generación (F1) o híbridos de generación temprana (F2, BC1); el otro resultó ser híbrido de una generación posterior (>F1). Las secuencias de la región de control de mtDNA (483 bp) y el gene nuclear Xsrc (636 bp) indicaron que la madre progenitora del individuo de generación híbrida temprana era P. mexicana y el macho progenitor fue P. velifera. Por lo tanto, aunque rara en molys la hibridación e introgresión interespecífica entre molys de dorsal de vela y dorsal corta ocasionalmente se presenta, a pesar de la existencia de mecanismos de aislamiento precopulatorios en el comportamiento.
Palabras clave: Aislamiento reproductivo precopulatorio, híbridos silvestres, Poecilia velifera, Poecilia petenensis, Poecilia mexicana, Poecilia orri.
Introduction
Information on the frequency and direction of interspecific hybridization in natural populations is critical to our understanding of the evolution of pre-mating reproductive isolating mechanisms and the role of such mating signal divergence in speciation. Documenting mating mistakes in natural sympatric populations confirms a role for selection in the evolution of courtship signals and mating preferences for those signals (Gerhardt et al., 1994). In this paper, we confirm the existence of interspecific hybrids between sailfin and shortfin species of mollies (Poeciliidae, Poecilia), a group in which such hybrids are thought to be extremely rare as a result of pre-mating behavioral isolation (Woodhead & Armstrong, 1985; Farr, 1989; Ptacek, 1998). Interspecific crosses readily produce fertile and viable offspring of both genders in the laboratory, suggesting that post-mating isolation is weak in mollies (Hubbs & Hubbs, 1946; Parzefall, 1989; Ptacek, 2002). However, a paucity of reports of wild-caught hybrids (e.g., Contreras-Balderas, 1990) persists despite numerous instances of sympatry between sailfin and shortfin species documented by intensive collecting efforts throughout the distribution of mollies in the Yucatán region of México (Miller 1975, 1983; Schmitter-Soto, 1998; Cashner et al., 2003).
The one exception to the extreme rarity of naturally occurring molly hybrids is the Amazon molly (P. formosa (Girard, 1859)), an all-female biotype found in northeastern México. This gynogenetic form is descended from a hybridization event between the sailfin molly P. latipinna (Lesueur, 1821) and the shortfin molly P. mexicana (Steindachner, 1863) (Hubbs & Hubbs, 1932; Hubbs, 1933; Avise et al., 1991) that occurred at least 100,000 years ago (Schartl et al., 1995; Dries, 2003).
Here, we document four male interspecific hybrids between sailfin and shortfin mollies found in the Yucátan peninsula and Isthmus of Tehuantepec regions of México. These specimens, from three different localities, were all sexually mature with fully formed gonopodia, the specialized anal fin used for sperm transfer in poeciliids. They were first identified as putative hybrids on the basis of intermediate dorsal fin morphology (Fig. 1), which is characteristic of first generation and backcross hybrids from lab-crossed sailfin and shortfin species (Parzefall, 1989; Ptacek, 2002). All four putative hybrid males were collected in areas of interspecific sympatry. Two came from an unusual site where three species of mollies co-occur, P. petenensis (Günther, 1866), P. velifera (Regan, 1914) and P. mexicana, one in a site where P. petenensis and P. mexicana co-occur, and the fourth individual, from a site where P. velifera and P. orri (Fowler, 1943) co-occur.
We used morphometric shape analyses to compare the four wild-caught putative hybrids to males of all four potential parental species and to laboratory-reared F1 hybrids of P. velifera and P. mexicana. For two of the putative hybrid individuals (V/M225 and V/M226) we used the Xsrc nuclear gene region and the mitochondrial DNA control region to genetically determine whether they were first- or later-generation hybrid offspring and to determine which parental species produced them. The other two putative hybrids could not be verified using molecular analyses; one was preserved in formalin (P/M02), the other (V/O548) lacks species-specific differences in length of the Xsrc nuclear gene region for its parental species, P. velifera and P. orri (Schartl et al., 1995).
Materials and methods
Collection of Fishes. Four species of mollies are common in the Yucatán peninsula, two sailfin species (P. petenensis and P. velifera) and two shortfin species (P. mexicana and P. orri), and there are numerous regions of sympatry where each sailfin species and one or the other shortfin species co-occur including the three sites where the four putative hybrid males were collected (Fig. 2). The Tabasco site was a creek (Río Samaria) in the Río Grijalva drainage, located at 18∞ N and 94∞ W, and was sympatric between P. petenensis and P. mexicana. This site was highly disturbed, and the density of fishes was low (52 P. mexicana , 21 P. petenensis and 1 hybrid were collected) compared with collections at nearby sites. The single hybrid from this site (P/M02) was collected with a seine (3.1 m x 1.8 m, .0.95 cm mesh) by one of us (SCB) in August 1967 and deposited as voucher specimen (UANL 2402) in the fish collection at UANL. Specimens of males and females of both parental species were also deposited in the collection at UANL. The Campeche site was a small natural impoundment, enlarged by dredging, located at 19∞ 14.23' N and 90∞ 50.11' W (near Playa Guadalupe). This is the only reported site where both P. petenensis and P. velifera co-occur along with P. mexicana. The two hybrids from this site (V/M225 and V/M226) were collected with a cast net (1.83 m diameter) by one of us (MBP) in May 2001 and deposited as voucher specimens (V/M225: CUSC 7462 and V/M226: CUSC 7463) in the Campbell Museum of Natural History, Clemson University. The Quintana Roo site was a small cenote, located at 20∞ 17.420' N and 87∞ 22.666' W (near Punta Soliman), where P. velifera and P. orri were sympatric. The single hybrid male (V/O548) was collected with a cast net (1.83 m diameter) by one of us (MBP) in June 2003 and deposited as a voucher specimen (CUSC 7464) in the Campbell Museum of Natural History, Clemson University. Both males and females of all potential parental species from both the Campeche and Quintana Roo sites were collected at the same time as the putative hybrid males and have been deposited with the Campbell Museum of Natural History. One of the putative hybrids (P/M02) was preserved in 10% formalin and later stored in 50% isopropanol; the other putative hybrid individuals were preserved in 95% ethanol and later stored in 70% ethanol.

Morphological measurements. Anesthetized (MS222, Sigma) males of P. velifera (N = 24), P. petenensis (N = 27), P. orri (N = 21) and P. mexicana (N = 21), as well as the four wild-caught putative hybrid males, were photographed with a Sony DSC F707 Cybershot digital still camera. Males of P. velifera, P. petenensis and P. mexicana came from the Campeche site where V/M225 and V/M226 were collected. Males of P. orri came from the Quintana Roo site where V/O548 was collected. We also photographed known F1 hybrid males (N=21) from three different families (P. velifera sire X P. mexicana dam) that had been produced and reared in our laboratory for studies on inheritance patterns of dorsal fin morphology and courtship behavior (Ptacek and Kittell, unpubl. data). For each individual, we used image analysis software (NIH Image 1.62, http://rs-b.info.nih.gov//nih-image/, and tpsDig, Version 1.28, http://life-.bio.sunysb.edu/morph/index.html; F. J. Rohlf) to measure 14 morphological characters from the digital images: length of the dorsal fin at the base, length of the first dorsal fin ray, length of the last dorsal fin ray, area of the dorsal fin, length of the gonopodium, body depth at the caudal peduncle, body depth at mid-body, length of the caudal fin, height of the caudal fin, caudal fin area, predorsal fin distance, preanal fin distance, standard length and body area (see Ptacek, 1998 for specific locations of linear measures). All linear measures were natural log (ln) transformed and size-adjusted (ln trait - ln standard length). Fin areas (dorsal and caudal) were ln-transformed and size-adjusted with body area (ln fin area - ln body area). Transformations and size adjustments resulted in normally distributed trait values for the pooled data set that included the four parental species and the F1 hybrids.
We classified individual specimens to one of six categories: P. velifera, P. petenensis, P. orri, P. mexicana, laboratory-reared F1 hybrids, and unknowns (P/M02, V/M225, V/M226, and V/O548). Multivariate analysis of variance (MANOVA) was performed on the 12 morphological shape variables (excluding standard length and body area) to determine whether species and hybrids showed significant shape differences. Canonical discriminant analysis was used on the 12 ln-transformed morphological shape variables to determine which best separated the six categories in multivariate morphological space.
Molecular analyses. We used the G'NOME DNA extraction kit (BIO 101 Lab, Carlsbad, CA) to obtain genomic DNA from the muscle tissue of a single individual each of P. velifera, P. petenensis, P. mexicana, P. formosa, and from the two putative hybrid individuals (V/M225, and V/M226). A single individual of P. formosa from a site in the Río Tigre, Tamaulipas, México was included as a hybrid control (see below). We PCR-amplified a nuclear DNA region (Xsrc proto-oncogene) using the Xiphophorus primers srcE and srcF (Schartl et al., 1995) under standard PCR conditions (Palumbi, 1996 and as modified in Schartl et al., 1995). This gene region is known to differ in base pair (bp) length between sailfin and some shortfin species of mollies (P. mexicana but not P. orri); the Xsrc allele found in the shortfin molly P. mexicana is 50 bp shorter (650 bp) than the allele (700 bp) found in the sailfin mollies P. latipinna, P. velifera and P. petenensis (Schartl et al., 1995). Both alleles are present in the hybrid clonal species P. formosa, in which all individuals are the products of a first generation (F1) hybridization event (Schartl et al., 1995). Similarly, F1 or early generation (F2 or BC1: 50% of progeny are heterozygous) hybrid status could be tested in our putative hybrid individuals (V/M225 and V/M226). To compare the putative hybrid genotypes to those of the potential parental species we amplified one individual each of P. velifera (P. petenensis has the same-sized allele and thus, was not included) and P. mexicana and electrophoresed the products in 2% agarose gels, stained with 10% ethidium bromide and visualized with UV light. Gel images were captured and saved to computer file using a FujiFilm LAS1000 gel imager system.
Once early generation hybrid status was genetically verified for one of the hybrid males (V/M225), we amplified and sequenced an approximately 500-bp segment of the mitochondrial DNA control region using primers L15926 (Kocher et al., 1989) and H16498 (Shields & Kocher, 1991). This area was also sequenced for potential parental species of this individual: P. mexicana, P. velifera, and P. petenensis from the same locality as V/M225. Because in animals mtDNA is generally inherited maternally (Wilson et al., 1985), it provides an opportunity to determine direction in hybrid crosses (Avise & Saunders, 1984). By comparing the sequence of V/M225 to that of the potential parental species we could determine the species identity of its maternal parent. Once the maternal species was identified as the shortfin species, P. mexicana, we sequenced the 700 bp "sailfin" allele of the Xsrc nuclear region for P. velifera, P. petenensis, and V/M225 to determine which sailfin species was the paternal parent. We compared sequences from V/M225 and the two, sailfin species, P. velifera and P. petenensis to the sequence of P. mexicana mexicana (GenBank Accession No. X79659; Schartl et al., 1995) to insure proper alignment of homologous base pair positions.
To sequence the "sailfin" band from V/M225, we excised it from a 2% agarose gel using a razor blade, then purified this PCR product using the QIAquick Gel Extraction Kit (Qiagen Inc., Valencia, CA). The other PCR products for the three parental species and V/M225 from the mtDNA control region and for the three parental species from the nuclear Xsrc region were purified using Qiaquick PCR purification columns (Qiagen, Inc., Valencia, CA). All purified products were used in sequencing reactions using the BigDyeTM Terminator Cycle Sequencing Kit (Version 3.1, Applied Biosystems, Inc.) and sequences were generated on an Applied Biosystems Model 377 Automated DNA sequencer at Arizona State University. Sequences were aligned initially using Sequencher (Version KS 5.0.1) and ambiguities were corrected manually. All sequences generated in this study are deposited in GenBank (mtDNA control region sequences, Accession No. AY599415 -AY599417, AY660025; nuclear Xsrc sequences, Accession No. AY599412 - AY599414).
Results
Morphological verification of hybrids. The overall MANOVA revealed that parental species and hybrids differed significantly (Wilks' _ = 0.0005, F60,476 = 32.60, P < 0.0001) in morphological shape. Discriminant axis 1 of the discriminant analysis explained 89.9% of the variance and was most highly correlated with length of the dorsal fin at the base (R = 0.994, P < 0.001), pre-dorsal fin distance (R = −0.981, P < 0.001), and dorsal fin area (R = 0.901, P < 0.001). This axis distinguished shortfin from sailfin mollies, and all hybrids were morphologically intermediate (Fig. 3). Discriminant axis 2 of the discriminant analysis explained an additional 4.7% of the variance and was most highly correlated with caudal fin area (R = −0.504, P < 0.001), caudal fin height (R = −0.438, P < 0.001), and length of the first dorsal fin ray (R = −0.406, P < 0.001). This axis showed considerable overlap between parental species with most of the wild-caught and laboratory-reared hybrids having somewhat smaller caudal fins than parental species (Fig. 3). All four putative wild-caught hybrids fell outside the 95% confidence intervals for each of the four potential parental species, and were intermediate in morphological shape between sailfin and shortfin species, as were the laboratory-reared F1 hybrids (Fig. 3).
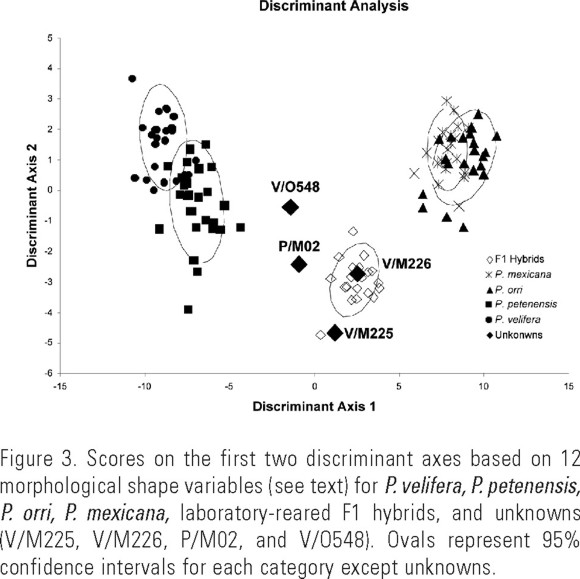
The discriminant analysis used two canonical discriminant factors to correctly classify all 21 males (100%) of the laboratory-reared F1 hybrids to their hybrid category. For males of the two sailfin species, 26 of 27 (96%) P. petenenesis were correctly classified (one was misclassified as P. velifera), and 23 of 24 (96%) P. velifera were correctly classified (one was misclassified as P. petenensis). There was more overlap between shapes of the two shortfin species. Nineteen of 21 (90%) male P. mexicana were correctly classified (two were misclassified as P. orri). For P. orri, 19 of 21 males (90%) were correctly classified (two were misclassified as P. mexicana). Of the four unknowns, V/M225, V/O548, and P/M02 were correctly classified (75%), but V/M226 was misclassified as an F1 hybrid.
Molecular verification of hybrids. Fragment profiles for the Xsrc region verified an early generation hybrid status (F1, F2 or BC1) for V/M225. The control, P. formosa and V/M225 both showed the sailfin allele (from either P. velifera or P. petenensis for V/M225) and the P. mexicana allele (Fig. 4). V/M226 showed only the P. mexicana allele, suggesting that it arose from a backcross to P. mexicana (e.g., BC1: 50% of progeny are homozygous for backcross parental allele) based on its morphological intermediacy (Fig. 3). The DNA sequence of the Xsrc region of V/M225 was identical to that of P. velifera, demonstrating that species to be its male parent (based upon mtDNA identification of P. mexicana as the female parent; see below). The two sailfin species, P. velifera and P. petenensis differed in DNA sequence from one another at 11 nucleotide positions. In addition, a 4-bp deletion was found in P. petenensis but not in any of the other species or V/M225, further excluding it as the sailfin male parent.

The mtDNA control region sequence of V/M225 was identical to that of P. mexicana and differed from those of P. petenensis and P. velifera at 55 and 50 nucleotide positions, respectively. This confimed P. mexicana as the female parent of V/M225.
Discussion
Our discovery of interspecific hybridization between sailfin and shortfin mollies in the Yucatán region of México is important for two reasons. First, despite intensive collecting efforts by us, and others (Miller 1975, 1983; Lozano-Vilano & Contreras-Balderas, 1987; Obregón-Barbosa et al., 1994; Schmitter-Soto, 1998) for over 30 years, the four wild-caught hybrids reported here are the first to be documented from this part of the geographic distribution of mollies in México. It is possible that female hybrids, which are harder to detect morphologically, have gone unnoticed. However, the paucity of wild-caught hybrids identified in collections from sympatric populations of sailfin and shortfin mollies does support earlier suggestions that interspecific hybridization in mollies is rare, most likely due to strong pre-mating reproductive isolation between sympatric species (Hubbs, 1955; Farr, 1989; Ptacek, 1998).
Second, though laboratory crosses between the sailfin species P. latipinna or P. velifera and the shortfin species P. mexicana have long been known (Hubbs & Hubbs, 1946; Parzefall, 1989; Dries, 2000; Ptacek, 2002), our genetic verification of a wild backcross or >F1 generation hybrid individual (V/M226) confirms that interspecific hybrids in nature are viable and fertile, and that introgression with P. mexicana is not only possible, but has occurred, at least at one site (Campeche).
Possible explanations for breakdown of pre-mating behavioral isolation in certain sympatric situations include the observation that females of shortfin mollies showed weaker conspecific mating preferences in dichotomous laboratory choice tests (Ptacek, 1998) and, again in the lab, females of both P. mexicana and P. orri preferred males of the sailfin species P. latipinna to males of heterospecific, shortfin species. Thus, shortfin molly females may have a pre-existing mating bias towards the overall larger size of sailfin males (conferred by the presence of the enlarged dorsal fin), which could potentially promote mating mistakes where P. mexicana or P. orri occur sympatrically with sailfin species.
Additionally, differences in the frequency of sailfins and shortfins in sympatric sites could favor interspecific hybridization. The so-called Hubbs principle (Hubbs, 1955) suggests that when one species is rare at a site compared to another, the rare species is more likely to mate with more abundant heterospecifics (Mayr, 1963; Gerhardt et al., 1994; Grant & Grant, 1997). Males of P. velifera at the Campeche site were outnumbered almost 3:1 by P. petenensis and P. mexicana males and at the Quintana Roo site, P. orri was considerably less common than P. velifera (Ptacek, unpubl. data).
While we cannot definitively conclude the cause of hybridization in these three sympatric populations of mollies, our results provide both morphological and genetic verification that interspecific hybridization does occasionally occur in the wild between sailfin and shortfin molly species. To our knowledge, the only other report of a wild-caught hybrid molly (excluding P. formosa) was that of a single hybrid (P. latipunctata x P. mexicana) in north-central México (collected in the Río Tamesí near Llera de Canales, Tamaulipas, México (Contreras-Balderas, 1990)). Future studies should focus on determining the frequency of hybridization and backcrossing in sympatric populations of mollies and identifying the factors that promote such interbreeding. Regardless of the cause of hybridization, our discovery of interspecific hybrids at three different sympatric sites suggests that while selection does operate to promote interspecific divergence in mating signals in mollies, in some situations, such pre-mating isolation can break down.
Acknowledgments
We gratefully acknowledge the Mexican government for granting collecting permission to M.B.P. (Permit No. 10.04.01.613.03, 2001, No. 240103.613-03, 2003) and S.C.B. (1967). We thank A. Aranguren, J. Barger, E. Harrison, J. Ramirez, C. Roelke, J. Schmitter-Soto, M. Valtierra Vega, and El Colegio de la Frontera Sur (ECOSUR), Chetumal, Quintana Roo, Mexico for assistance and logistical support during field collections, M. Childress and S. Hankison for assistance with morphometric analyses, A. Rivera for help preparing Figure 1, C. Gabor for providing the specimen of P. formosa, and A. Kodric-Brown, J. Schmitter-Soto, G. Smith, J. Travis, and an anonymous reviewer for helpful comments on a previous draft of this paper. Animal care of stock populations of parental species of mollies followed a protocol approved by the Clemson University Animal Research Committee (Protocol # 20044). Financial support was provided by the Howard Hughes Medical Institute through an Undergraduate Student Research Award (to MNH), by the National Science Foundation, Animal Behavior Program (to MBP; IBN 0296173), and by "Acuario Municipal Felipe de J. Benavides" at Monterrey, Mexico (to SCB).
References
AVISE, J.C. & N.C. SAUNDERS. 1984. Hybridization and introgression among species of sunfish Lepomis: analysis by mitochondrial DNA and allozyme markers. Genetics 108 (1): 237-256. [ Links ]
AVISE, J.C., J.C. TREXLER, J. TRAVIS & W.S. NELSON. 1991. Poecilia mexicana is the recent female parent of the unisexual fish P. formosa. Evolution 45(6): 1530-1533. [ Links ]
CASHNER, F.M., G.R. SMITH & R.C. CASHNER. 2003. Robert Rush Miller and Francis Hubbs Miller. Copeia 2003 (4): 910-916. [ Links ]
CONTRERAS-BALDERAS, S. 1990. Nuevo híbrido natural, Poecilia mexicana X P. latipunctata, del río Tamesí, Tamaulipas, Mexico (Pisces: Poeciliidae). Universidad y Ciencia 7 (14): 61-64. [ Links ]
DRIES, L.A. 2000. The evolutionary persistence of the gynogenetic Amazon molly, Poecilia formosa. Doctoral Dissertation, University of Texas. Austin. [ Links ]
DRIES, L.A. 2003. Peering through the looking glass at a sexual parasite: Are Amazon mollies red queens? Evolution 57 (6): 1387-1396. [ Links ]
FARR, J.A. 1989. Sexual selection and secondary sexual differentiation in poeciliids: Determinants of male mating success and the evolution of female choice. In: Meffe, G.K. & F.F. Snelson, Jr. (Eds.). Ecology and Evolution of Livebearing Fishes (Poeciliidae). Prentice Hall. Englewood Cliffs, pp. 91-123. [ Links ]
GERHARDT, H.C., M.B. PTACEK, L. BARNETT & K.G. TORKE. 1994. Hybridization in the diploid-tetraploid treefrogs Hyla chrysoscelis and H. versicolor. Copeia 1994 (1): 51-59. [ Links ]
GRANT, P.R. & B.R. GRANT. 1997. Hybridization, sexual imprinting and mate choice. American Naturalist 149 (1): 1-28. [ Links ]
HUBBS, C.L. 1933. Species and hybrids of Mollienesia. The Aquarium 1 (1): 263-268, 277. [ Links ]
Hubbs, C.L. 1955. Hybridization between fish species in nature. Systematic Zoology 4 (1): 1-20. [ Links ]
HUBBS, C.L. & L.C. HUBBS. 1932. Apparent parthenogenesis in nature in a form of fish of hybrid origin. Science 76 (1983): 628-630. [ Links ]
HUBBS, C.L. & L.C. HUBBS. 1946. Experimental breeding of the Amazon molly. Aquarium Journal 17 (1): 4-6. [ Links ]
KOCHER, T.D., W.K. THOMAS, A. MEYER, S.V. EDWARDS, S. PÄÄBO, F.X. VILLABLANCA & A.C. WILSON. 1989. Dynamics of mitochondrial DNA evolution in animals: amplification and sequencing with conserved primers. Proceedings of the National Academy of Sciences 86 (16): 6196-6200. [ Links ]
LOZANO-VILANO, M.L. & S. CONTRERAS-BALDERAS. 1987. Lista zoogeográfica y ecológica de la ictiofauna continental de Chiapas, México. The Southwestern Naturalist 32 (2): 223-236. [ Links ]
MAYR, E. 1963. Animal Species and Evolution. Belknap. Cambridge, 811 p. [ Links ]
MILLER, R.R. 1975. Four new species of Mexican poeciliid fishes of the genera Poecilia, Gambusia, and Poeciliopsis. Occasional Papers of the Museum of Zoology University of Michigan 672: 1-44. [ Links ]
MILLER, R.R. 1983. Checklist and key to the mollies of Mexico (Pisces: Poeciliidae: Poecilia, Subgenus Mollienesia). Copeia 1983 (3): 817-822. [ Links ]
OBREGÓN-BARBOSA, H., S. CONTRERAS-BALDERAS & M.L. LOZANO-VILANO. 1994. The fishes of Northern and Central Veracruz, Mexico. Hydrobiologia 286 (1): 79-95. [ Links ]
PALUMBI, S.R. 1996. PCR and molecular systematics. In: Hillis, D., C. Moritz, & B. Mable (Eds.). Molecular Systematics. Sinauer. Sunderland, pp. 205-247. [ Links ]
PARZEFALL, J. 1989. Sexual and aggressive behaviour in species hybrids of Poecilia mexicana and Poecilia velifera: (Pisces, Poeciliidae). Ethology 82 (2): 101-115. [ Links ]
PTACEK, M.B. 1998. Interspecific mate choice in sailfin and shortfin species of mollies. Animal Behaviour 56 (5): 1145-1154. [ Links ]
PTACEK, M.B. 2002. Patterns of inheritance of mating signals in interspecific hybrids between sailfin and shortfin mollies (Poeciliidae: Poecilia: Mollienesia). Genetica 116 (2-3): 329-342. [ Links ]
SCHARTL, M., B. WILDE, I. SCHLUPP & J. PARZEFALL. 1995. Evolutionary origin of a parthenoform, the Amazon Molly Poecilia formosa, on the basis of a molecular genealogy. Evolution 49 (5): 827-835. [ Links ]
SCHMITTER-SOTO, J.J. 1998. Catálogo de los peces continentales de Quintana Roo. ECOSUR. San Cristóbal de Las Casas, 239 p. [ Links ]
SHIELDS, G.L. & T.D. KOCHER. 1991. Phylogenetic relationships of North American Ursids based on analysis of mitochondrial DNA. Evolution 45 (1): 218-221. [ Links ]
WILSON, A.C., R.L. CANN, S. CARR, M. GEORGE, U. GYLLENSTEN, R.H.K. HELM-BYCHOWSKI, S.R. PALUMBI, E. PRAGER, R.D. SAGE & M. STONEKING. 1985. Mitochondrial DNA and two perspectives on evolutionary genetics. Biological Journal of the Linnean Society 26 (1985): 375-400. [ Links ]
WOODHEAD, A. D. & N. ARMSTRONG. 1985. Aspects of mating behaviour of male mollies (Poecilia spp.). Journal of Fish Biology 27 (1985): 593-601. [ Links ]














