Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Hidrobiológica
versión impresa ISSN 0188-8897
Hidrobiológica vol.14 no.1 Ciudad de México jun. 2004
Artículo
First basis for a sustained juvenile production technology of fat snook Centropomus parallelus Poey
Primeras bases para una tecnología sostenible de producción de juveniles de robalo chucumite Centropomus parallelus Poey
Luis Alvarez-Lajonchère, Vinicius Ronzani Cerqueira, Israel Diniz Silva, Jaqueline Araujo and Marcos dos Reis
Universidade Federal de Santa Catarina, Centro de Ciencias Agrarias - Departamento de Aqüicultura, Caixa Postal 476, Florianópolis 88040.900, Santa Catarina, Brasil.
Recibido: 28 de junio de 2003
Aceptado: 22 de febrero de 2004
Abstract
A larval, weaning and nursery trial with fat snook Centropomus parallelus Poey was conducted in Santa Catarina, Brazil. Spawning of captive broodstock was induced with 50 µg/kg LHRHa and fertilized eggs (82.7%) were stocked in two 1,800 L tanks at a mean density of 41.3 eggs/L. Larval hatching averaged 92%. Nannochloropsis oculata (Droop) Hibberd was introduced daily from day 1 until day 20 post-hatch (d20ph). Rotifers Brachionus rotundiformis Muller were supplied during the first month and Artemia meta-nauplii from d19ph to d66ph. Weaning was carried out from day 51 to day 66 with a 43.6% protein dry diet, supplied together with Artemia meta-nauplii. An abrupt temperature drop of 5ºC during the night of d26ph began the first mass mortality period, which lasted for 3 weeks. Mortality during weaning and nursery was less than 4%. A total of 10,200 juveniles (52.4 ± 1.4 mm and 1.25 ± 0.06 g) were harvested with a survival of 6.5%, mean final density of 2.8 fish/L and biomass of 3.5 kg/m3. Food conversion rate during nursery was 1.283 and the increase in variance of the size distribution in total length was 7.5%.
Key words: Centropomus parallelus, larviculture, weaning, nursery, juvenile, snook.
Resumen
Se realizó un ensayo de cría larval, destete y alevinaje con el robalo gordo Centropomus parallelus Poey, en Santa Catarina, Brasil. Se indujo el desove de reproductores cautivos con 50 µg/kg de LHRHa y los huevos fertilizados (82.7%) se introdujeron en dos tanques de 1,800 l a una densidad media de 41.3 huevos/L. La eclosión promedió 92%. Diariamente se introdujo Nannochloropsis oculata (Droop) Hibberd desde el día 1 hasta el día 20 después de la eclosión, rotíferos Brachionus rotundiformis Miller durante el primer mes y meta-nauplios de Artemia desde el día 19 al 66. El destete se realizó del día 51 al 66 con una dieta seca con 43.6% la proteína, suministrada con los meta-nauplios de Artemia. Una disminución brusca de temperatura de 5ºC durante la noche del día 26 inició el primer periodo de mortalidad masiva, que duró tres semanas. La mortalidad en el destete y el alevinaje fue de menos del 4%. Se cosechó un total de 10,200 juveniles (52.4 ± 1.4 mm y 1.25 ± 0.06 g) con una supervivencia de 6.5%, una densidad final media de 2.8 juveniles/L y una biomasa de 3.5 kg/m3. La tasa de conversión de alimento durante el alevinaje fue de 1.283 y el aumento en la variación de la distribución de la longitud total fue de 7.5%.
Palabras clave: Centropomus parallelus, larvicultura, destete, alevinaje, juvenil, robalo.
Introduction
The marine members of the family Centropomidae are catadromous fish with a wide tropical distribution. Their commercial and recreational fisheries are important and several species have aquaculture potential (Tucker & Jory 1991; Barlow et al., 1996). Most centropomid aquaculture production involves the Asian seabass Lates calcarifer (Bloch). In America there are several species of the genus Centropomus among which, the fat snook Centropomus parallelus Poey is one of the middle size, which is the most abundant snook in Santa Catarina, Brazil, and has been proposed for cultivation (Patrona, 1984).
Induced spawning and larviculture are well-established commercial practices with L. calcarifer (National Institute of Coastal Aquaculture, 1986; Dhert et al., 1992). In contrast, juvenile production is still at experimental scale with common snook Centropomus undecimalis (Bloch) (Edwards & Henderson, 1987; Tucker, 1987) and C. parallelus (Honczaryk & Cerqueira, 1994; Cerqueira, 1995; Cerqueira et al., 1995; Cerqueira & Bernardini, 1995).
The present study was carried out during the natural spawning season to improve the rearing methodology already developed with C. parallelus and to adapt some standard production practices to promote sustained juvenile mass production techniques for supply commercial operations.
Materials and methods
Fertilized eggs of the fat snook C. parallelus were obtained from a broodstock at the Laboratorio de Piscicultura Marinha, Universidade Federal de Santa Catarina (Brazil) in January 1998. As part of a maturation research in progress, the broodstock was maintained in concrete-8,000-L tanks with sea water (34-35 ups) and a biological filter in the spawner's tank bottom.
Induced spawning treatment was based on a single intramuscular injection of synthetic analog of luteinizing hormone - releasing hormone (LHRHa), des Gly10 [D-Ala6] LHRH ethylamide purchased from SIGMA, 50 µg/kg for the female (1.1 kg) with postvitellogenic oocytes 0.4 mm in diameter examined by ovarian biopsy, and the same for the two males (0.470 and 0.515 kg) with running milt, which were re-introduced in the same broodstock tank. Eggs were collected from an overflow tank on the following afternoon, 14-15 h after fertilization after blastopore closure, and introduced into the larval rearing tanks
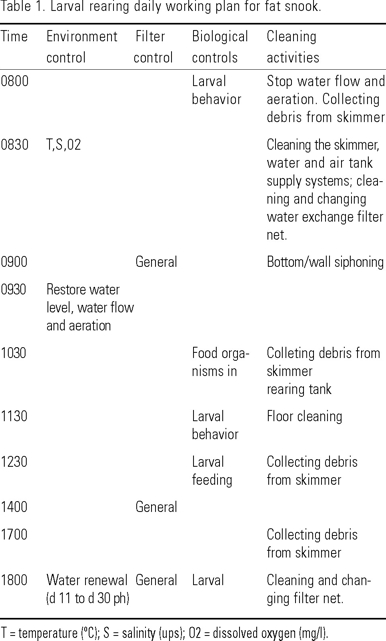
Larvae were reared in two blue rectangular 1,800-L fiberglass tanks, with U-shape bottom and a maximum water depth of 0.70 m in the middle. Spawned eggs were stocked at a density of 41.3 eggs/L, with the same spawning conditions of temperature (26º C) and salinity (35 ups). An environment management protocol during egg incubation, larval rearing, weaning, and nursery was established (Fig. 1) as well as daily working plan (Table 1). Eggs were allowed to hatch in the rearing tanks with mild aeration from three diffusers (0.2 L/min each). Indoors tanks were under a partially translucent fiberglass roof, illuminated with four 40 W fluorescent bulb. Water level was maintained at one end of the tank by an exterior overflow stand pipe fitted to an interior screened vertical pipe.
Two surface cleaners, similar to the one described by Sweetman (1992), were used all through the rearing cycle, starting from the first day. The microalgae Nannochloropsis oculata (Droop) Hibberd was introduced into the rearing tanks with fertilized eggs, and maintained at a density of 500,000 cells/ml until day 20 post-hatch (d20ph).
A feeding regime and daily feeding program (Table 2) were established. On the second day after hatching, the S-type rotifer Brachionus rotundiformis Tschugunoff (mean length 180 µm and mean width 118 µm) was supplied at a density of 10/mL and from d3ph to d24ph, and were added to the tanks twice each day to establish densities of 20-30 rotifers/mL and lowered to 10-15 rotifers/mL until d29ph (Table 2). Enrichment during the first 10 days was carried out with a secondary 3-6 h culture of a dense culture of N. oculata, and from d11ph onwards was enriched by the direct method for 3-6 h with a cod liver oil emulsion (Watanabe et al., 1982).
Artemia nauplii were obtained by decapsulation of Great Salt Lake (GSL) cysts and incubated for 24 h with a mean hatching efficiency of 100,000 nauplii/g. Nauplii were enriched by the direct method with the cod liver oil emulsion (Watanabe et al., 1982) for 18 - 24 h before being introduced to the rearing tanks. Meta-nauplii were supplied from d19ph to d66ph, starting with a density of 0.5/mL, which was gradually increased up to 7/mL on d35ph and then gradually decreased. In the rearing tanks, the desired Artemia meta-nauplii density was supplied in the morning, which was considered as 50% of the daily ration; the other 50% was supplied in two equal rations at midday and afternoon (Table 2).
Weaning started on d51ph and required 16 days with an experimental dry diet prepared at the laboratory with a dietary protein, lipid and carbohydrate levels of 43.6%, 15% and 23% respectively, having an estimated metabolizable energy (ME) of 402.6 kcal 100/g, and a protein:energy ratio (P:E) of 108.3 mg of protein per kcal. At the beginning, larvae were fed with the dry diet at a rate of 10% of body weight with particle size of 0.5-0.9 mm, subsequently on d75ph the particle size was 1.0-1.2 mm and the rate was gradually lowered to 5% on d85ph.
During the first 9 days of weaning, dry diet was regularly given together with 1L of concentrated and enriched Artemia meta-nauplii, supplied 4 times between 0800 h and 1800 h, and alternating two times with Artemia meta-nauplii alone, each with 50% of the daily ration (Table 2). Between d60ph and d66ph the dry diet was supplied 8 times alternating again with two times of Artemia metanauplii (Table 2). Feed amounts were recorded and used to calculate a feed conversion ratio (FCR = total dry matter feed fed (g) / wet weight gain of fish (g).
The experiment was completed on the morning of d90ph, and all the juveniles of both tanks were harvested after a light sedation level with 17 ppm of benzocaine. Total number was estimated by visual appreciation in polypropylene trays (0.1 m2) filled with 10 L of water from the rearing tanks. The density in each tray was compared with that of a control in which the exact number of juveniles (250) was previously determined.
Wet weight (g ± 0.001) and total length (nearest mm) measurements were taken from 120 juveniles collected on the harvest day, rinsed with a 0.5 mm net and then excess water was blotted with an absorbent paper. Growth rates were calculated as specific growth rates (SGR) according to the equation SGR = 100 (ln Wt - ln Wo)/t, where Wo is weight at the beginning of weaning, Wt is weight at harvest, and t is the number of days during weaning and nursery. The increase in variance of the size distribution with time, was calculated by the increase in the coefficient of variation of mean length ( CV) from the beginning of weaning (CV1) to harvest day (CV2). Protein efficiency ratio (PER) during nursery was calculated as weight gain (g) / protein fed (g) during nursery.=
Results
Spawning took place during the second night, 38-h after injection with 82.7% fertilization. No abnormal yolk-sac larvae were observed. Hatching rates were 89% in tank 1 and 95% in tank 2, averaging 92%, and initial larval densities were 36.8 larvae/L and 39.2/L, respectively. At 26-27ºC the mouth was open on d3ph, oil droplet and yolk-sac were consumed on d4ph. On d9ph more than 80% of the larvae exhibited normal gas bladder inflation.
Salinity was maintained between 34 and 36 ppt and dissolved oxygen between 5.2 and 5.8 mg/L, during the whole rearing cycle. Average daily water temperature during the experimental trial was 25.8 ± 1.2ºC.
On d19ph microalgae culture collapsed, their supply to the larval rearing tanks was stopped on d21ph, hence rotifers fed with baker's yeast were given to the larvae.
On d25ph, early in the morning, a few larvae died suddenly early in the morning when the siphon was introduced in the tank, but this incident was not repeated. During the night of d26ph, the temperature was dropped to 21ºC due to a strong cold front, causing a mass mortality estimated to be roughly around 10,000 larvae in each tank. Temperature was restored with a 2 kW immersion heater at a rate of 1ºC/h and maintained within the desired range (25-26ºC) together with a water flow of 4L/min during night hours in the following 23 days, and with a thermostat thereafter.
Following the mass mortality, around 50 to 100 larvae were found dead every morning. On d33ph another mass mortality occurred early in the morning when a sudden shadow cover the tank water surface, resulting immediately in mass mortality. Dead larvae had their whole body with excess mucus and their mouth completely open. The dead larvae were estimated by weight to be around 10,000 individuals. During the following 6 days, 50-200 larvae were found dead each morning.
Larvae were stimulated to eat the dry diet by simultaneously supplying concentrated Artemia nauplii. After the first week, all them were eating the artificial diet quite well without Artemia. During the trial, 13.6 kg of Artemia cysts were used, supplying a mean daily ration of 15 nauplii/mL, 8,866 nauplii per stocked larvae, and a general cyst consumption index of 1.335 kg per 1,000 juveniles produced.
During weaning and nursery the total mortality was estimated as less than 4%. Cannibalism was observed frequently, and the smaller larvae were eaten completely by a mouthful with the head first by larger fish. After d45ph, a few predators were found dead with digestive tract obstructions from their preys. The total length difference between predators and preys was 30 to 100%.
On d90ph 4,300 juveniles were harvested from tank 1 and 5,800 from tank 2, which represented final survival rates of 6.2 and 6.6%, respectively (mean 6.5%). Final densities were 2.4 and 3.2 juveniles/L respectively (mean 2.8/L), and final biomass was 3.0 and 4.0 kg/m 3, respectively (mean 3.5 kg/m3).
Total length (TL) of juveniles was 52.4 ± 1.4 mm (range: 31 - 80 mm) with a CV= 14.28%. Wet weight (W) was 1.25 ± 0.06 g (range: 0.50 - 1.88 g). Specific growth rate was 8.5%/day, and daily growth in TL was 0.57 mm/day. Growth can be expressed by the equation: TL = e (1.15244 + 0.0330103 t) (r = 0.9758) where TL is total length in mm and t is the age in days. The increase in the size distribution variance from d51ph to d90ph was 7.5% considering that on d51ph when weaning started. Estimated W was 75 ± 1.5 mg and TL was 19.2 ± 0.4 mm with a CV = 6.8%.
Final weaning TL and W were 27 ± 0.7 mm and 198 ± 5.3 mg respectively. Daily body weight gain during weaning and nursery were 7.7 ± 0.3 mg and 43.8 ± 2.5 mg, respectively, with an overall daily weight gain of 29.4 ± 1.2 mg during formulated feed supply. PER during nursery was 2.19. Juvenile length-weight relationship was W = 1.1170 x10-5 TL 2.921172763 (r = 0.9876) at harvest. Condition factor of the pooled sample at harvest was 0.869 ± 0.112. Mean feed conversion rate during nursery was 1.083.
Discussion
Egg quality was high, with no abnormal larvae and a satisfactory viable hatching rate was noticed higher than the previous results with this species (Cerqueira, 1995), with common snook (Roberts, 1987), and Asian seabass (Maneewongsa & Tattanon, 1982; National Institute of Coastal Aquaculture, 1986).
One of the changes introduced in the rearing protocol that could have improved survival during the first month was the increased light intensity to a level similar to that reported by Edwards & Henderson (1987) and the 24-h photoperiod. The effects of the new light regime should have positively influenced the successful establishment of external larval feeding (Rosenthal & Hempel, 1970).
Maintenance of stable water temperature is a key factor for some marine fish larvae, especially during the night when drastic changes can result in immediate larval mortality (Lim, 1993), as occurred in the present study, and could have been avoided with reliable immersion heaters with thermostat control.
Surface cleaners, although commonly employed only during the swim bladder inflation period were used throughout the whole rearing trial as applied by Lim (1993). This enabled the improvement of water quality by removing Artemia cysts, uneaten dry feed, and other floating debris, as well as acting partially as a foam fractionator by collecting dissolved organics. Cleaning and disinfecting routines were followed systematically and should have improved disease prevention. One of the three areas where larval rearing research and development efforts have been concentrated in Europe is hygiene (Sweetman, 1992) and routine procedures established in the present trial must be improved to enable a healthy and stable production as needed for commercial scale.
Exogenous nutrition was established on rotifers as first food without difficulties. Rotifer densities used in the present study, higher than in previous trials (non-published data) and in other centropomid larval rearing (National Institute of Coastal Aquaculture, 1986; Tucker, 1987; Dhert et al., 1992), could also have positively influenced early survival.
Mortality showed a constant mortality curve during the first 4 weeks, which characterizes marine fish larval rearing without critical periods, where a certain percentage of established larvae die each day, as pointed out by Kraul (1983).
As metamorphosis approached, stress symptoms preceded mass mortalities, which started with the abrupt temperature drop, characterizing the first critical period faced on this study. A critical period at the end of the first month has been reported on earlier studies with this species (Cerqueira et al., 1995), with common snook (Shafland & Koehl, 1979; Edwards & Henderson, 1987), Asian seabass (Dhert et al., 1992) and other marine fishes (Lim, 1993; Alvarez-Lajonchère et al., 1996).
Blaxter (1988) considered metamorphosis as one of the potentially critical periods of fish larval rearing. In the present study, mortalities related to this period were probably due to nutritional deficiencies, especially highly unsaturated fatty acids (HUFA), as reported previously for fat snook (Cerqueira et al., 1995), common snook (Tucker & Jory, 1991) and Asian seabass (Rimmer & Reed, 1990; Dhert et al., 1992; Barlow et al., 1996).
In previous trials with fat snook, mass mortalities during this period were minimized by the direct enrichment of Artemia nauplii with fish oil emulsion technique of Watanabe et al. (1982). In the present study, the above mentioned method alone could not avoid stress shock mass mortalities possibly due to the synergetic influence of several stress factors, such as: i) high density due to higher survival during the first 4 weeks, ii) 24 h photoperiod during the first 20 days, iii) the abrupt temperature drop, iv) the absence of microalgae in the rearing tanks, v) the use of rotifers cultured with baker's yeast instead of Nannachloropsis oculata during the preceding week.
To avoid stress shock mass mortalities related with metamorphosis and to increase resistance to physical sorting required for snook species to lower cannibalism, larval nutrition must be improved in several ways. Better rotifer and Artemia nauplii enrichment methods should be applied to reach higher levels of highly unsaturated fatty acids (HUFA), as already achieved with Asia seabass (Sorgeloos et al., 1988; Dhert et al., 1992).
The quantity of Artemia cysts used was much higher than the general Artemia cyst consumption index in marine fish larviculture of 200-500 g per 1,000 fry produced (Lavens & Sorgeloos, 1996), although the index of nauplii per stocked larvae of the present trial were similar to that practiced commercially in countries like Italy (Dr. Maximo Caggiano, personal communication to the senior author). To lower cyst consumption index and juvenile production costs, future studies should consider increasing cyst hatching efficiency, and also larval rearing efficiency could be improved by lowering larval mortality, as well as reducing absolute Artemia cysts consumption, by reducing the density and supply period by means of introducing other live prey, such as large rotifers Brachionus plicatilis Muller, copepods and cladocerans, as well as progressively bigger Artemia juveniles at the end of the live food schedule.
In the present study, weaning started at an age which has been reported to give the best results for fat snook (Borba, 1997), much later than in common snook (Edwards & Henderson, 1987; Tucker, 1989; Serfling, 1998) and Asian seabass (National Institute of Coastal Aquaculture, 1986; Tucker et al., 1988; Dhert et al., 1992). Considering the recommendations of Hecht & Pienaar (1993), a delayed weaning would not have favored the fast growers and together with an extended live feed/dry feed co-feeding period could reduce cannibalism.
Synthetic attractants were used in earlier trials with fat snook (Honczaryk & Cerqueira, 1994; Borba et al., 1994; Cerqueira & Bernardini, 1995; Borba, 1997). Although Cerqueira & Bernardini (1995) recommended the addition of natural attractants in the diet, in the present study the strategy was not to include in the artificial diet the attractants identified as responsible for the chemical stimuli of Artemia (Kolkovski et al., 1995) but to add nauplii together with the dry feed, mixed just before supplying it to the larvae. This combination improved acceptance of the pelleted feed, which did not have problems with palatability as noticed in other trials (Cerqueira & Bernardini, 1995). The acceptance of the formulated feed together with an extended supply of live food as a supplement has been shown to be important for cannibalism control (Hecht & Pienaar, 1993).
The supply of Artemia increased the rate of assimilation of the microdiet-fed larvae and their growth, regardless of the age of the fish, enhancing the efficiency of microdiets in other species, possibly by the influence of microdiet ingestion by visual and chemical stimuli and/or the direct influence of nauplii biochemical composition on larval digestion and assimilation (Kolkovski et al., 1997a, b).
The common phenomenon of size hierarchy with an increasing size range, as the larvae grow, was present in this study as a result of competition for food and social dominance in high density (Blaxter, 1988). This is one of the key factors for cannibalism on smaller individuals in snooks (Edwards & Henderson, 1987; Parazo et al., 1991; Dhert et al., 1992) and other fishes (Dowd & Clarke, 1989; Hecht & Pienaar, 1993).
Due to the stress condition of the larvae after mass mortalities started, and considering that the dead larvae belonged to the biggest size group, no size selection was carried out because larvae could present handling sensitivity. This was reflected in the broad size range of the harvested juveniles. The increase in the variance of the size distribution was intermediate in this study compared with reports for other species (Goldan et al., 1997).
Parazo et al. (1991) reported cannibalism in Asian seabass, with a maximum prey size of 61-67% of predator total length whereas for common snook is the prey:predator size ratio was found to be 38% at d44ph by Edwards & Henderson (1987). In mangrove habitats, Luczkovich et al. (1995) found a prey:predator ratio of less than 30% for common snook juveniles consuming mainly small fish species. A ratio of 33% greatly increased cannibalism in common snook with a five-fold increase in consumption rate (Dowd & Clarke, 1989). Parazo et al. (1991) recommended no more than a 33% difference in total length, which could also be adequate for fat snook according to results obtained in the present study, although cannibalism could not be quantified as in other studies (Edwards & Henderson, 1987).
High survival during weaning and nursery were among the best results in centropomids (Dhert et al., 1992; Borba, 1997). Wide variations in larval survival have been reported for Centropomus species in laboratory and pond systems. Most studies with Asian seabass obtained survival around 20-40% at d30ph, the end of larval rearing period, and higher than 80% during nursery (Tattanon & Maneewongsa, 1982; National Institute of Coastal Aquaculture, 1986; Parazo et al., 1991; Dhert et al., 1992).
Stocking density in the present study was higher than those used for common snook (Edwards & Henderson, 1987) and similar to those used for Asian seabass larviculture (Tattanon & Maneewongsa, 1982; National Institute of Coastal Aquaculture, 1986; Dhert et al., 1992). The density at harvest found in this study was similar to that recommended for Asian seabass at the end of the larval rearing around Day 30 (National Institute of Coastal Aquaculture, 1986) after several reductions by size-grading the juveniles, and fall within the range obtained by Serfling (1998) with common snook. Final biomass in the present study was higher than in other species (Kraul, 1983; Person Le Ruyet et al., 1991; Kraul, 1993) and similar to the highest obtained during nursery with Pacific threadfin Polydactylus sexfilis (Cuvier & Valenciennes) by Ostrowski et al. (1996).
Daily weight gain was intermediate in comparison with reports of other finfish species (Ostrowski et al., 1996). Mean growth was similar to that reported by Cerqueira et al. (1995) and within the mean range of reported data for the species. The SGR was lower than the previously reported for fat snook by Borba (1997), but much lower than reports for common snook (Tucker, 1989).
The condition factor at harvest was slightly smaller than those reported by Borba (1997) for 85 day old fat snook, but much smaller than in her 75 day old juveniles and those reported by Honczaryk & Cerqueira (1994) and Cerqueira & Bernardini (1995).
Crude protein and fat in the experimental diet of the present study were within the range of formulated diets for other carnivorous fishes for best performance, as with Asian seabass juveniles (Boonyaratpalin, 1988; Catacutan & Coloso, 1995, 1997) and fat snook juveniles (Cerqueira et al., 1995; Honczaryk & Cerqueira, 1994), although Tucker et al. (1988) and Borba (1997) supplied higher crude protein diets with better results.
Carbohydrates in the present diet were high, like in the diet supplied by Cerqueira et al. (1995), higher than those reported by Catacutan & Coloso (1997) and Tucker et al. (1988) with Asian seabass and Honczaryk & Cerqueira (1994) and Borba (1997) with fat snook. The carbohydrates level should be lowered and the protein content increased proportionally to increase the P:E ration to a level close to that reported as optimum by Boonyaratpalin (1988) and Catacutan & Coloso (1995, 1997). This will also improve PER which was slightly lower than those reported by Catacutan & Coloso (1997).
FCR was lower than that of the reports for the same species and only higher than those reported by Borba (1997). Best performances of other finfish species usually approximate unity as Tucker (1989) reported for common snook and Asian seabass, while Catacutan & Coloso, (1997) obtained ratios of 1.21 and 1.65 for Asian seabass.
Changes introduced in the rearing protocol of fat snook larviculture were successful, reducing mortality during the first three weeks, as well as during weaning and nursery. The still critical period is now limited to one month, from around d21ph to d50ph. The present results could be taken as a benchmark for future studies to improve seed production technology, and further changes should be made to increase survival, growth and stress resistance:
a) Rearing tanks should be bigger, i.e. 5-10 m3 cylindrical fiberglass tanks with black walls.
b) Stocking density could be lowered to 25-30 eggs/L.
c) Water temperature should be thermostatically maintained between 26 and 28ºC.
d) The 24-h photoperiod should be only during the first 15 days of larval rearing.
e) Improve rotifer algae enrichment with higher n-3 HUFA levels by increasing N. oculata density (20-40 x 106 cells/mL) and adding Isochrysis T-ISO (1 x 106 cells/mL).
f) Improve the emulsion enrichment procedures for rotifers and Artemia with products that increase n-3 HUFA too much higher levels, and other nutrients.
g) Live food regime could be improved to reduce costs as well as cannibalism by delaying the supply of enriched Artemia meta-nauplii in favor of the smallest larvae by including the supply of other organisms as well as progressively bigger Artemia.
h) Frequent size-grading with free rotating rod graders, starting as early as the larvae could resist manipulation and the total length of prey:predator size ratio not higher than 33%.
i) Artificial feeds should be further improved, following guidelines of the present study.
Acknowledgements
Funding was partially provided by the Canadian International Development Agency (Brazilian Mariculture Linkage Project), and a grant from the Conselho Nacional de Desenvolvimento Cientifico e Tecnologico (No. 300801/97-1 NV) to the senior author. The authors wish to express their gratitude to the staff and students of the Marine Fish Culture Laboratory of the University for their valuable technical assistance, and to Luciano Gonzaga from the Universidade Federal de Santa Catarina for the feed proximate analysis. Thanks are also given to Dr. S. Kraul for his valuable comments on the manuscript.
References
ALVAREZ-LAJONCHÈRE, L., L. PÉREZ SÁNCHEZ, O. G. HERNÁNDEZ MOLEJÓN & E. TORRES GÓMEZ. 1996. Mass production of striped patao Eugerres brasilianus juveniles in Cuba. Journal of the World Aquaculture Society 27: 347-352. [ Links ]
BARLOW, C., K. WILLIAMS & M. RIMMER. 1996. Sea bass culture in Australia. INFOFISH International 2: 26-33. [ Links ]
BLAXTER, J. H. S. 1988. Pattern and variety in development. In: Hoar, W. S. & D. J. Randall (Eds.). Fish Physiology vol. XI, The Physiology of developing fish, Part A Eggs and larvae. Academic Press, London, pp. 1-58. [ Links ]
BOONHYARATPALIN, M. 1988. Asian seabass, Lates calcarifer. In: Wilson, R. P. (Ed.). Handbook of Nutrient Requirements of finfish, CRC Press Inc., Boca Raton, Florida, pp. 5-11. [ Links ]
BORBA, M. R. 1997. Efeito da idade e da utilizacao de compostos sinterticos como atrativos na adaptacao da larva do robalo (Centropomus parallelus Poey, 1860) ao alimneto formulado. Tesis de Maestría en Ciencias, Universidad Federal de Santa Catarina, Brasil, 58 pp. [ Links ]
BORBA, M. R., V. R. CERQUEIRA, N. L. NELLI & J. A. G. MACCHIAVELLO. 1994. Utilizacao de diferentes fontes proteicas na dieta artificial de juvenis de robalo (Centropomus parallelus) em cultivo experimental. In: Resumenes VIII Simpósio Brasileiro de Aquicultura, Piracicaba, Sao Paulo, 11 a 14 de outubro 1994, 43 pp. [ Links ]
CATACUTAN, M. & R. COLOSO. 1995. Effect of dietary protein to energy ratios on growth, survival and body composition of juvenile Asian seabass, Lates calcarifer. Aquaculture 131: 125-133. [ Links ]
CATACUTAN, M. R.& R. M. COLOSO. 1997. Growth of juvenile Asian seabass, Lates calcarifer, fed varying carbohydrate and lipid levels. Aquaculture 149: 137-144. [ Links ]
CERQUEIRA, V. R. 1995. Testes de indução de desova do robalo, Centropomus parallelus, do litoral da Ilha de Santa Catarina com gonadotrofina coriônica humana. In: Anais Congresso Brasileiro de Engenharia de Pesca, 7, 1991, Recife, Associação dos Engenheiros de Pesca de Pernambuco - SUDENE, pp. 95-102. [ Links ]
CERQUEIRA, V. R. & M. E. BERNARDINI. 1995. The weaning of the fat snook Centropomus parallelus larvae with experimental and commercial artificial diets. In: Lavens, P., E. Jaspers & I. Roelants (Eds.). Larvi'95- Fish & Shellfish Larviculture Symposium, Ghent, Belgium, 3-7 September, 1995. European Aquaculture Society Special Publication 24: 272-275. [ Links ]
CERQUEIRA, V. R., J. A. G. MACCHIAVELLO & A. M. BRÜGGER. 1995. Produção de alevinos de robalo, Centropomus parallelus Poey, 1860, através de larvicultura intensiva em laboratório. In: Anais, Simpósio Brasileiro de Aquicultura, 7, 1992, Peruíbe, ACIESP, pp. 191-197. [ Links ]
DHERT, P., P. LAVENS & P. SORGELOOS. 1992. State of the art of Asian seabass Lates calcarifer larviculture. Journal of the World Aquaculture Society 23 (4): 317-329. [ Links ]
DOWD, C. E. & M. E. CLARKE. 1989. An experimental investigation of cannibalism in hatchery-reared juvenile redfish (Sciaenops ocellatus) and snook (Centropomus undecimalis). In: Blaxter, J. H. S., J. C. Gamble & H. von Westernhagen (Eds.). The early life history of fish. The third ICES Symposium, Bergen, 3-5 October 1988. Vol. 191, 486 p. [ Links ]
EDWARDS, R. E. & B. D. HENDERSON. 1987. An experimental hatchery project: studies of propagation, culture and biology of snook (Centropomus undecimalis). Proceedings of the Gulf and Caribbean Fisheries Institute 38: 211-221. [ Links ]
GOLDAN, O., D. POPPER, U. & I. KARPLUS. 1997. Management of size variation in juvenile gilthead sea bream (Sparus aurata). I: Particle size and frequency of feeding dry and live food. Aquaculture 152: 181-190. [ Links ]
HECHT., T. & A. G. PIENAAR 1993. A review of cannibalism and its implications in fish larviculture. Journal of the World Aquaculture Society 24: 246-261. [ Links ]
HONCZARYK, A. & V. R. CERQUEIRA. 1994. Adaptation of fat snook Centropomus parallelus (Poey) larvae to artificial diets. In: MacKinlay, D. D. (Ed.). High Performance Fish, Proceedings of an International Fish Physiology Symposium, 16-21 July, 1994, University of British Columbia, Vancouver, Canada, pp. 383-388. [ Links ]
KOLKOVSKI, S., A. TANDLER & M. S. IZQUIERDO. 1997a. Effects of live food and dietary digestive enzymes on the efficiency of microdiets for seabass (Dicentrarchus labrax) larvae. Aquaculture 148: 313-322. [ Links ]
KOLKOVSKI, S., A. ARIELI & A. TANDLER. 1995. Visual and olfactory stimuli are determining factors in the stimulation of microdiet ingestion in gilthead seabream Sparus auratus larvae. In: Lavens, P., E. Jaspers & I. Roelants (Eds.). Larvi'95 - Fish & Crustacean Larviculture Symposium, European Aquaculture Society Special Publication 24: 289-292. [ Links ]
KOLKOVSKI, S., W. KOVEN & A. TANDLER. 1997b. The mode of action of Artemia in enhancing utilization of microdiet by gilthead seabream Sparus auratus larvae. Aquaculture 155: 193-205. [ Links ]
KRAUL, S. 1983. Results and hypotheses for the propagation of the grey mullet, Mugil cephalus L. Aquaculture 30: 273-284. [ Links ]
KRAUL, S. 1993. Larviculture of the mahimahi Coryphaena hippurus in Hawaii, USA. Journal of the World Aquaculture Society 24: 410-421. [ Links ]
LAVENS, P. & P. SORGELOOS. 1996. Manual on the production and use of live food for aquaculture. FAO FisheriesTechnical Papers 361: 1-295. [ Links ]
LIM, L. C. 1993. Larviculture of the greasy grouper Epinephelus tauvina F. and the brown-marbled grouper E. fuscoguttatus F. in Singapore. Journal of the World Aquaculture Society 242: 262-374. [ Links ]
LUCZKOVICH, J. J., S. F. NORTON & R. G. GILMORE, JR. 1995. The influence of oral anatomy on prey selection during the ontogeny of two percoid fishes, Lagodon rhomboides and Centropomus undecimalis. Environmental Biology of Fishes 44: 79-95. [ Links ]
MANEEWONGSA, S. & T. TATTANON. 1982. Nature of eggs, larvae and juveniles of seabass. In: Report of Training Course on seabass spawning and larval rearing, Songkhla, Thailand, 1-20 June 1982, SCS/GEN/82/39, pp. 22-24. [ Links ]
NATIONAL INSTITUTE OF COASTAL AQUACULTURE. 1986. Technical manual for seed production of seabass. Songkhla (Thailand), National Institute of Coastal Aquaculture, 49 pp. [ Links ]
OSTROWSKI, A. C., T. IWAI, S. MONAHAN, S. UNGER, D. DAGDAGAN, P. MURAKAWA, A. SCHIVELL & C. PIGAO. 1996. Nursery production technology for Pacific threadfin (Polydactylus sexfilis). Aquaculture 139: 19-29. [ Links ]
PARAZO, M. M., E. M. AVILA & D. M. REYES, JR. 1991. Size and weight dependent cannibalisms in hatchery bred sea basss (Lates calcarifer Bloch) Journal of Applied Ichthyology 7: 1-7. [ Links ]
PATRONA, L. D. 1984. Contribution a la biologie du robalo Centropomus parallelus (Pisces, Centropomidae) du Sudest de Bresil: possibilités aquacoles. Institut National Polythecnique de Toulouse, France, Doctoral Thesis, 175 pp. [ Links ]
PERSON LE-RUYET, J., F. BAUDIN-LAURENCIN, N. DEVAUCHELLE, R. METAILLER, J.-L. NICOLAS, J. ROBIN & J. GUILLAUME. 1991. Culture of turbot (Scophthalmus maximus). In: McVey, J. P. (Ed.). CRC Handbook of Mariculture, Vol. II, Finfish Aquaculture, CRC Press, Boca Raton, Florida, pp. 21-41. [ Links ]
RIMMER, M. A. & A. REED. 1990. Effects of nutritional requirement of live food organisms on growth and survival of barramundi/seabass Lates calcarifer (Bloch) larvae. In: Barret, J. (Ed.). Advances in tropical aquaculture. Tahiti, French Polynesia, February 20 - March 4 1990, pp. 611-623. [ Links ]
ROBERTS, D. E., JR. 1987. Induced maturation and spawning of common snook, Centropomus undecimalis. Proceedings of the Gulf and Caribbean Fisheries Institute 38: 222-230. [ Links ]
ROSENTHAL, H. & T. HEMPEL 1970. Experimental studies in feeding and food requirements of herring larvae (Clupea harengus L.). In: Steele, J. H. (Ed.), Marine food chains. Oliver & Boyd, Edinburgh, pp. 344-364. [ Links ]
SERFLING, S. A. 1998. Breeding and culture of snook, Centropomus undecimalis, in a closed-cycle, controlled environment culture system. In: Book of Abstracts, Aquaculture'98, February 15-19, 1998. Las Vegas (USA), World Aquaculture Society, 482. [ Links ]
SHAFLAND, P. L. & D. H. KOEHL. 1979. Laboratory rearing of the common snook. Proceedings Annual Conference Southeasten Association of Fish and Wildlife Agencies 33: 425-431. [ Links ]
SORGELOOS, P., P. LÉGER & P. LAVENS. 1988. Improved larval rearing of European and Asian seabass, seabream, mahi-mahi, siganid and milkfish using enrichment diets for Brachionus and Artemia. World Aquaculture 19: 78-79. [ Links ]
SWEETMAN, J. W. 1992 . Larviculture of Mediterranean marine fish species: current status and future trends. Journal of the World Aquaculture Society 23: 330-337. [ Links ]
TATTANON, T. & S. MANEEWONGSA. 1982. Larval rearing of seabass. In: Report of Training Course on seabass spawning and larval rearing, Songkhla, Thailand, 1-20 June 1982, SCS/GEN/82/39, pp. 29-30. [ Links ]
TUCKER, J. W. JR. 1987. Snook and tarpon culture and preliminary evaluation for commercial farming. Progressive Fish-Culturist 49: 49-57. [ Links ]
TUCKER, J. W., JR. 1989. Research on coastal finfish aquaculture in Florida and Australia. Proceedings of the Gulf and Caribbean Fisheries Institute 39: 415-419. [ Links ]
TUCKER, J. W., JR. & D. E. JORY. 1991. Marine fish culture in the Caribbean region. World Aquaculture 22: 10-27. [ Links ]
TUCKER, J. W., JR., M. R. MACKINNON, D. J. RUSSELL, J. J. O'BRIEN & E. CAZZOLA. 1988. Growth of juvenile barramundi (Lates calcarifer) on dry feeds. Progressive Fish-Culturist 50: 81-85. [ Links ]
WATANABE, T., M. OHTA, M. KITAJIMA & S. FUJITA. 1982 . Improvement of dietary value of brine shrimp Artemia salina for fish larvae by feeding them on w3 highly unsaturated fatty acids. Bulletin of the Japanese Society of Scientific Fisheries 48: 1775-1782. [ Links ]














