Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Hidrobiológica
versión impresa ISSN 0188-8897
Hidrobiológica vol.12 no.2 Ciudad de México dic. 2002
Article
Temporal variability in the abundance of the bay anchovy Anchoa mitchilli (Valenciennes, 1848) eggs and spawing biomass in Pueblo Viejo Lagoon, Veracruz, Mexico
Variabilidad temporal en la abundancia de huevos y biomasa de puestas de la anchoveta Anchoa mitchilli (Valenciennes, 1848) en la laguna de Pueblo Viejo, México
Marina Sánchez-Ramírez and Alberto Ocaña-Luna
Lab. de Ecología. Dpto. de Zoología. Escuela Nacional de Ciencias Biológicas, Instituto Politécnico Nacional. Prolongación de Carpio y Plan de Ayala s/n Col. Sto. Tomás. C. P. 11340. México, D. F. E-mail: msanchez@encb.ipn.mx
Recibido: 30 de mayo de 2002
Aceptado: 28 de octubre de 2002
Abstract
The spawning of Anchoa mitchilli is analyzed and its adult biomass is estimated in Pueblo Viejo Lagoon, Veracruz, Mexico. Samples were collected at 19 stations in October and December of 1992, in March, April, June and August of 1993, using a zooplankton net with a 505 µm mesh size and a 50 cm mouth to which a flowmeter was fixed. The spawning of A. mitchilli was analized with respect to the abundance of eggs in the lagoon (number of eggs/m3) and the adult biomass was calculated through the annual production of eggs. A total of 51,348 eggs was obtained of which 50,205 (97.7%) corresponded to A. mitchilli. This is a species that spawns inside the lagoon throughout the year, except during the rains (July-October), with a peak (96.8%) in March-April in temperatures of 19-20°C and salinities of 14-21 psu. Temperature (15-32 °C) was observed to be one of the main factors that determine the spawning period, whereas salinity inhibits spawning below 10 psu. A. mitchilli biomass in Pueblo Viejo Lagoon was 793 metric tons and 5.7 g/m3. A latitudinal gradient from north to south is evident for the biomass of this species, and this is determined by an increase in the diversity towards the tropical regions and the resulting decrease in the biomass of the species, down to the southern limit of its distribution in the Peninsula of Yucatan, Mexico.
Key words: Adult biomass, bay anchovy, ichthyoplankton, latitudinal changes, spawning season.
Resumen
En esta investigación se analiza el desove y se estima la biomasa adulta de Anchoa mitchilli, en la Laguna de Pueblo Viejo, Veracruz, México. Las muestras fueron recolectadas en 19 estaciones en octubre y diciembre de 1992; marzo, abril, junio y agosto de 1993, con una red de zooplancton de abertura de malla de 505 µm y 50 cm de boca con un flujómetro adaptado a la misma. Se analizó el desove de A. mitchilli en base a la abundancia de huevos en la laguna (número de huevos/m3) y se calculó la biomasa adulta a través de la producción anual de huevos. Se extrajeron un total de 51,348 huevos de los cuales 50,205 (97.7%) correspondieron a A. mitchilli. Es una especies que desova dentro de la laguna casi todo el año, excepto en la época de "lluvias" (julio-octubre), con un pico (96.8%) en marzo-abril en temperaturas de 19-20°C y salinidades de 14-21 psu. Se observó que la temperatura (15-32 °C) es uno de los principales factores que determinan el período de desove, mientras que la salinidad inhibe el desove por abajo de 10 psu. La biomasa de A. mitchilli en la Laguna de Pueblo Viejo fue de 793 toneladas métricas y de 5.7 g/m3. Es evidente un gradiente latitudinal de la biomasa de esta especies, decreciendo de norte a sur, determinado por un incremento en la diversidad específica hacia las regiones tropicales, que provoca un decremento de la biomasa de la especie hacia el límite sur de su distribución en la Península de Yucatán, México.
Palabras clave: Biomasa de adultos, bay anchovy, ictioplancton, cambios latitudinales, época de desove.
Introduction
The bay anchovy Anchoa mitchilli (Valenciennes, 1848) is an engraulid fish distributed from Maine to Florida, U.S.A. and throughout the Gulf of Mexico (Hoese and Moore, 1998) down to Yucatan, Mexico (Hildebrand, 1963). This species is found along the coast down to a depth of 36 m, but is more common in areas with muddy bottoms and brackish waters where it tolerates a range of salinity of 0.5 to 80 psu (Whitehead 1978). The early juvenile stages also enter freshwater areas (Tucker, 1988).
The bay anchovy is the main source of food for predatory fish along its distribution range (Baird and Ulanowicz 1989; Juanes et al., 1993). This species is of enormous trophic importance as a primary consumer (Olney 1983). Its production links secondary plankton production to fisheries output and is important to the energy processes of the ecosystem (Luo and Musick, 1991). It is possible that this abundant planktivore species may exercise significant top-down control over productivity in estuaries, and that its production level may limit piscivore production (Wang and Houde, 1995).
A. mitchilli is a dominant species throughout its life cycle and the adults have been reported as abundant community components by Reis and Dean (1981), Voutlitois et al. (1987), Castillo-Rivera et al. (1994) and Griffith and Bechler (1995).
Because eggs can be sampled more reliably than trawled adults, the egg production method may provide a more accurate estimate of biomass (Rilling and Houde, 1995). Estimations of the spawning biomass are important to the management of marine resources, not only of those under exploitation but also of those that are considered a potential resource. These are also useful to evaluate the part that abundant species such as A. mitchilli play in trophic chains and its ultimate effect on populations for which it constitutes an important food item.
A. mitchilli shows its greatest numerical abundance in Pueblo Viejo Lagoon and occupies the second place with respect to weight among the 75 species captured here (Castillo-Rivera et al., 1994), in spite of being a small species with a maximum total length of 102 mm (Jones et al., 1978). The dominance of this species in the ichthyoplankton of several coastal lagoons of the Gulf of Mexico has been reported by Flores-Coto (1987), Dokken et al., (1984), Leak and Houde (1987), Vouglitois et al., (1987) and Monteleone (1992).
Given the importance of A. mitchilli due to its abundance, short life cycle, massive spawning and trophic role within estuarine-lagoon systems, the objectives of this study were to determine the abundance of this species' eggs and to estimate its spawning biomass in Pueblo Viejo Lagoon.
Materials and methods
Pueblo Viejo Lagoon is located in the northern area of the state of Veracruz, between 22° 05' and 22° 13' N, and 97° 50' and 98° 00' W. It communicates with the Panuco river to the north through a channel, approximately 10 km away from its mouth into the Gulf of Mexico, and with the Tamacuil river to the south (Fig. 1).

Three climatic seasons are characteristic throughout the year the dry season (March to June), the rainy season (July to October) and the northers season (November to February) (Castillo-Rivera and Kobelkowsky, 1993). Six sampling dates were selected, October and December of 1992 and March, April, June and August of 1993, and 19 sampling stations were located throughut the lagoon (Fig. 1).
Samples were collected using a conic zooplankton net with a 505 µm mesh size and a 50 cm mouth to which a flowmeter was fixed, and trawling for 5 minutes at each station. Samples were preserved with 4% formalin that was neutralized with sodium borate.
The eggs were extracted from the 114 samples and those corresponding to A. mitchilli were used to calculate the density per sampling station (Cij). This was standardized as number of eggs/m3 as follows:
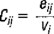
where:
Cij = number of eggs/m3 at station i during cruise j.
eij = number of eggs collected at station i during cruise j.
vi = filtered volume at sampling station i.
The total abundance of eggs for each cruise (Aj) was calculated by multiplying the egg density by the volume filtered at each station, and then adding all the products:

where:
Aj = total abundance of eggs estimated for the cruise j.
Cij = as defined above.
n = number of sampling stations.
vi = volume at station i.
The volume at each station was estimated using the Sette and Ahlstrom's polygons method (1948), considering the modification for coastal lagoons proposed by Chavance et al. (1984) who used the average depth value for these systems. An average depth of 1.5 m was used for Pueblo Viejo Lagoon as was reported by Iniestra-Gómez and Moreno-Arcuri (1991).
The daily egg production (Pd) was obtained according to the method established by Houde (1977). For this the residence time was estimated as the time (days) elapsed between spawning and hatching, which for this species is of one day according to Jones et al. (1978). It is:

where:
Pd = daily egg production: total number of eggs spawned in one day.
Aj = as defined above.
Tr = residence time of the eggs in the plankton.
The production per cruise (Pc) was calculated as the product of the daily production multiplied by the number of days of each cruise (adding to the sampling date half of the days since the previous cruise and half of the days to the next cruise) (Sette and Ahlstrom, 1948):
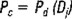
where:
Pc = egg production during the cruise j.
Pd = as defined above.
Dj = number of days represented by cruise j.
The annual production of eggs is an estimation of the abundance of eggs produced over the entire spawning season:

where:
Pa = annual production of eggs.
Pc = as defined above.
N = number of cruises during the spawning season.
Finally, the adult biomass was estimated according to Houde (1977), using the relative fecundity data (704.4 ovocytes/per gram of eviscerated female) and the sex ratio (0.56) of A. mitchilli, as recorded by Iniestra-Gomez and Moreno-Arcurri (1991):

where:
B = adult biomass.
Pa = as defined above
F = relative fecundity.
k = sex ratio.
Results and discussion
A total of 50,205 eggs of A. mitchilli were analyzed, and this represented 97.7% of all eggs collected. This species spawns in the lagoon almost throughout the year in salinities of 1 to 21 psu and temperatures of 19 to 30.5°C, with the exception of August to October (the rainy season). There is a spawning peak in March-April (the dry season) in temperatures of 19 to 20°C and salinities of 14 and 21 psu. The greater abundance of eggs was recorded to the south of Mata Grande Island and in the northeastern and central areas of the lagoon (Table 1; Fig. 1).

The months when no eggs were observed coincide closely with the recess period of the females (September-December) as Iniestra-Gómez and Moreno-Arcurri (1991) recorded. These authors observed that maturity is reached from February to March with a reproductive peak in this period that corresponds cosely with the greatest abundance of eggs in the lagoon, during March-April (Table 1).
These findings apparently differ from other authors' reports for other localities along the United States' Atlantic coast with respect to the spawning of A. mitchilli and to the temperature and salinity ranges in which it takes place. For example, Monteleone (1992) mentioned that eggs of this species are abundant in Great South Bay, New York, from the end of May to August in temperatures of 15.1 to 27.5°C and salinities above 20.0 psu, while at Barnegat Bay, New Jersey spawning begins in April with a peak during June-July (Vouglitois et al., 1987). Similarly, at Chesapeake Bay, the spawning peak begins in May (15.0-28.0°C) and ends after August according to Olney (1983).
Furthermore, spawning was recorded to take place in the York river from May to mid-September by Luo and Musick (1991). At Biscayne Bay, Florida, it takes place throughout the year, with a peak during spring and summer at temperatures between 28.5 and 32°C and salinities of 30 to 34.5 psu (Houde and Lovdal, 1984).
Apparently the spawning peak occurs earlier at Pueblo Viejo Lagoon. This is a consequence of the different latitudes at which these systems are located and it points to changes in the environmental conditions that do not strictly coincide with the time of year. Thus, while at locations along the North Atlantic the spawning peak occurs from May to August at temperatures above 15°C, at Pueblo Viejo Lagoon the peak occurs during March-April at temperatures of 19 to 20°C. These lie within the interval reported for this species' spawning in North America of 15 to 32°C, where the maximum occurs at temperatures above 15°C as was recorded by Monteleone (1992). For this reason, temperature is one of the main factors that determine the spawning period, as has been observed for marine fish by Brett (1970), who also pointed out that temperature has a marked effect on the reproduction period. On the other hand, the lower salinity that the anchovy eggs have being collected is of 10 psu at Biscayne Bay by Houde and Lovdal (1984). The low salinity that prevails during the rainy season (0-3 psu) in the lagoon as a result of input from the Panuco River, particularly at this time of the year, hinders the occurrence of spawning.
The adult biomass calculated for A. mitchilli was 793 metric tons (Table 2), with a biomass corresponding to this species in Pueblo Viejo Lagoon of 5.7 g/m3. At other localities such as Chesapeake Bay, calculated biomass reaches up to 9.3 g/m3 according to Wang and Houde (1995). At Tamiahua Lagoon, Veracruz, located to the south of Pueblo Viejo Lagoon, the biomass calculated is 3.1 g/m3 according to Gaspar-Dillanes and Sánchez-Iturbe (1985), whereas at Términos Lagoon Campeche, it varied from 1.7 to 1.9 g/m3 according to Flores-Coto et al. (1988). Considering that the water volume in Pueblo Viejo represents only 0.56% of the total in Chesapeake Bay, 3.4% of the total in Terminos Lagoon and 7.1% of the total in Tamiahua Lagoon, it is interesting to point out these differences as it is in this smaller lagoon where one of the highest biomass values for A. mitchilli have been recorded (Table 3).

A decrease in the biomass of A. mitchilli is evident along a latitudinal gradient (Table 3). This is probably due to an increase in species diversity towards tropical regions, with a subsequent decrease in the biomass of individual species, as Cushing (1975) has mentioned. He also pointed out that this has resulted in the main fisheries being located at higher latitudes. It is also important to consider that the southern limit of distribution of this species lies in the Peninsula of Yucatan as another cause of this gradient.
Acknowledgements
The authors wish to thank the General Coordination of Post-graduate Studies and Research of the Instituto Politécnico Nacional for funding the last phase of this study (Project CGPI-990311). We also thank Ma. Guadalupe Ramírez-Orta for her participation in the field work and separation of samples, Ma. Elena Sánchez and Andrea Raz-Guzmán Macbeth, for helping with the translation of the original manuscript into English, and anonymous reviewers that considerably improved the manuscript.
References
Baird, D. and R. E. Ulanowicz, 1989. The seasonal dynamics of the Chesapeake Bay Ecosystem. Ecological Monographs 59: 329-364. [ Links ]
Brett, J. R., 1970. Temperature. Animals. Fishes. Functional Responses. pp. 515-560. In: O. Kinee (Ed). Marine Ecology. A Comprehensive, Integrated Treatise on Life in Oceans and Coastal Waters, Volume I Environmental Factors. Part I. Wiley-Interscience. Chichester. [ Links ]
Castillo-Rivera, M. and A. Kobelkowsky, 1993. Comportamiento ambiental de la Laguna de Pueblo Viejo, Veracruz, México. Biotam 5: 1-12. [ Links ]
Castillo-Rivera, M., G. Moreno and R. Iniestra, 1994. Spatial, seasonal, and diel variation in abundance of the bay anchovy, Anchoa mitchilli (Teleostei: Engraulidae), in a tropical coastal lagoon of Mexico. The Southwestern Naturalist 39: 263-268. [ Links ]
Cushing, D. H., 1975. Ecología Marina y Pesquerías. Acribia. Zaragoza: 57-77. [ Links ]
Chavance, P., C. Flores-Coto and A. Sánchez-Iturbe, 1984. Early life history and adult biomass of sea bream in the Terminos Lagoon, Campeche. Transactions of the American Fisheries Society 113: 166-177. [ Links ]
Dokken, Q. R., G. C. Matlock and S. Cornelius, 1984. Distribution and composition of larval fish populations within Alazan Bay, Texas. Contributions in Marine Science 27: 205-222. [ Links ]
Flores-Coto, C., 1987. Estudio comparativo de la estructura de la comunidad ictioplanctónica de tres lagunas costeras del sur del Golfo de México. Anales del Instituto de Biología. Universidad Nacional Autónoma de México. Serie Zoología 58(2): 707-726. [ Links ]
Flores-Coto, C., F. Barba-Torres and J. Sánchez-Robles, 1983. Seasonal, diversity, abundance and distribution of ichthyoplankton in Tamiahua Lagoon, Western Gulf of México. Transactions of the American Fisheries Society 122: 247-256. [ Links ]
Flores-Coto, C., A. Ocaña-Luna, A. Luna-Calvo y F. Zalava-García, 1988. Abundancia de algunas especies de anchoas en la Laguna de Términos (México), estimada a través de la captura de huevos. Anales del Instituto de Ciencias del Mar y Limnología. Universidad Nacional Autónoma de México 15: 125-134. [ Links ]
Gaspar-Dillanes, M. T. y A. Sánchez-Iturbe, 1985. Estimación de la biomasa desovante de Anchoa mitchilli Cuvier y Valenciennes, 1848 (Pises: Engraulidae) y determinación de algunos parámetros ecológicos y poblacionales a partir de estudios ictioplanctónicos, en la Laguna de Tamiahua, Veracruz, México (1984/1985). Biología de campo, Area. Ciencias del Mar (Ecología). Fac. Ciencias, Dep. Biología, Univ. Nal. Autón. Mexico. [ Links ]
Griffith, S. A. and D. L. Bechler, 1995. The distribution and abundance of the bay anchovy, Anchoa mitchilli, in a southeast Texas marsh lake system. Gulf Research Reports 9:117-122. [ Links ]
Hildebrand, S. F., 1963. Family Engraulidae. pp. 152-249. In: Y. H. Olsen (Ed). Fishes of the Western North Atlantic. Part Three: soft-rayed bony fishes. Sears Foundation for Marine Research Bingham Oceanographic Laboratory Yale University. Copenhagen. [ Links ]
Hoese H. D. and R. H. Moore, 1998. Fishes of the Gulf of Mexico. Texas, Louisiana, and Adjacent Waters. Texas A & M. Texas. 422 p. [ Links ]
Houde, E. D., 1977. Abundance and potential yield in the round herring Etrumeus teres, and aspects of its early life history in the eastern Gulf of Mexico. Fishery Bulletin 75:61-89. [ Links ]
Houde, E. D. and J. A. Lovdal, 1984. Seasonality of occurrence, foods an food preferences of ichthyoplankton in Biscayne Bay, Florida. Estuarine, Coastal and Shelf Science 18:403-419. [ Links ]
Iniestra-Gómez, R. y G. Moreno-Arcuri, 1991. Contribución al conocimiento de aspectos biológicos y ecológicos de Anchoa mitchilli (Osteichthyes: Engraulidae) en la Laguna de Pueblo Viejo, Veracruz, México. Tesis Profesional. Escuela Nacional de Estudios Profesionales Zaragoza, Universidad Nacional Autónoma de México, 100 p. [ Links ]
Jones, P. W., F. D. Martin and J. D. Hardy Jr., 1978. Development of fish of the Mid-Atlantic Bight. An Atlas of egg, larval and juvenile stages vol. I. Acipenseridae through Ictaluridae. Power Plant Project. Office of biological Services. Fish and Wildlife Service, U. S. Department of the interior. Washington D. C. 153-168. [ Links ]
Juanes, F. R., E. Marks, K. A. McKown and D. O. Conover, 1993. Predation by Age-O blue fish on age-O anadromous fishes in the Hudson River Estuary. Transactions of the American Fisheries Society 122:348-356. [ Links ]
Leak, J. C. and E. D. Houde, 1987. Cohort growth and survival of bay anchovy Anchoa mitchilli larvae in Biscayne Bay, Florida. Marine Ecology Progress Series 37:109-122. [ Links ]
Luo, J. and J. A. Musick, 1991. Reproductive biology of the bay anchovy in Chesapeake Bay. Transactions of the American Fisheries Society 120:701-710. [ Links ]
Monteleone, D. M., 1992. Seasonality and abundance of ichthyoplankton in Great South Bay New York. Estuaries 15:230-238. [ Links ]
Olney, J. E., 1983. Eggs and early larvae of the bay anchovy, Anchoa mitchilli, and the weakfish, Cynoscion regalis, in lower Chesapeake Bay with notes on associated ichthyoplankton. Estuaries 6:20-35. [ Links ]
Reis, R. R. and J. M. Dean, 1981. Temporal variation in the utilization of an intertidal Creek by the Bay Anchovy (Anchoa mitchilli). Estuaries 4:16-23. [ Links ]
Rilling, G. C. and E. D. Houde, 1995. Regional and temporal variability in distribution and abundance of Bay Anchovy (Anchoa mitchilli) eggs, larvae, and adult biomass in the Chesapeake Bay. Estuaries 22 (4): 1096-1109. [ Links ]
Sette, O. E. and E. H. Ahlstrom, 1948, Estimations of abundance of eggs of the Pacific pilchard (Sardinops caerulea) off southern California during 1940 and 1941. Sears Found Journal Marine Research 7:511-542. [ Links ]
Tucker Jr. J. W., 1998. Energy utilization in bay anchovy Anchoa mitchilli, and black sea bass, Centropristis striata striata, eggs and larvae. Fishery Bulletin 78:279-293. [ Links ]
Vouglitois, J. J., K. W. Able, R. J. Kurtz and K. A. Tighe, 1987. Life history and population dynamics of the bay anchovy in New Jersey. Transactions of the American Fisheries Society 166:141-153. [ Links ]
Wang, S. B. and E. D. Houde, 1995. Distribution, relative abundance, biomass and production of bay anchovy Anchoa mitchilli in the Chesapaeake Bay. Marine Ecology Progress Series 121: 27-38. [ Links ]
Whitehead, P. J. P., 1978. Engraulidae. pp. 1-26. In: W. Fischer (Ed). FAO species identification sheets for fishery purposes. Western Central Atlantic. (Fishing area). Vol. II. Food and Agriculture Organization of the United Nations. Rome. [ Links ]














