Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Hidrobiológica
versão impressa ISSN 0188-8897
Hidrobiológica vol.11 no.2 Ciudad de México Dez. 2001
Artículos
Effect of the culture conditions on the growth and lipid contents of two strains of Nannochloris sp. to be used aquaculture
Dora E. Hernández-Ceballos and Sergio F. Martinez-Díaz
Centro Interdisciplinario de Ciencias Marinas (CICIMAR), Departamento de desarrollo de tecnologias. Playa el Conchalito s/n. La Paz B.C.S., México. C. P. 23000. E-mail sdiaz@redipn.ipn.mx
Recibido: 21 de febrero de 2001
Aceptado: 27 de septiembre de 2001
Abstract
Two strains of microalgae were obtained from the Texcoco lake,Mexico and San Pedrito Oasis Baja California Sur Mexico, and named C1 and C2 respectively, previously were selected as good candidates to improve the mass culture of a native strain of Brachionus plicatilis. In the laboratory, the isolates were purified and acclimated to growth under marine conditions at 35 ppt salinity and at 27-30" C. Both strains were identified as Nannochloris sp. based primarily on their a, b chlorophyll and carotene contents and on light and transmission electron microscopy of the cell. Different culture conditions were tested in order to evaluate their effect on growth and lipid content. Differences in the response of both strains were found. Nannochloris sp strain C2 was better adapted to different culture conditions (media, nitrogen concentration and culture vessel); this strain achieved higher growth and major lipid contents than strain C1. The conditions which improved the yield of both strains, are described. Also it was found that Nannochloris sp. strain C2 (isolated from a coastal oasis) has apparent advantages in yield over Nannochloris sp. strain C1 for use in aquaculture.
Key words: Nannochloris, culture systems, tropical aquaculture.
Resumen
Dos cepas de microalgas fueron obtenidas del lago de Texcoco México y del Oasis de San Pedrito Baja California Sur México y fueron denominadas C1 y C2 respectivamente, previamente estas cepas fueron seleccionadas como buenas candidatas para mejorar el cultivo masivo del rotífero Brachionus plicatilis. En el laboratorio, las cepas aisladas fueron purificadas y aclimatadas a condiciones marinas, de 35 ppm y a temperatura de 27-30" C. Ambas cepas fueron identificadas como Nannochloris sp con base en su contenido de clorofilas a y b, carotenos y con base en microscopia óptica y electrónica de las células. Se probaron diferentes condiciones de cultivo con el propósito de evaluar su efecto sobre el crecimiento y el contenido de lípidos. Se encontraron diferencias en la respuesta de las cepas. La cepa Nannochloris sp C2 se adaptó mejor a las condiciones de cultivo (medios de cultivo, concentración de nitrógeno y recipiente de cultivo); esta cepa mostró un mayor crecimiento y un mayor contenido de lípidos que la cepa C1. Se describen las condiciones que mejoraron el rendimiento de ambas cepas. Además se encontró que la cepa C2 de Nannochloris sp. (aislada de un oasis costero) posee claras ventajas en rendimiento sobre la cepa C1 de Nannochloris sp. para uso en acuacultura.
Palabras clave: Nannochloris, sistemas de cultivo, acuacultura tropical.
Introduction
Marine fish culture is an expanding industry, the number of marine and estuarine species being reared commercially for human consumption and other purposes increasing each year. Microalgae have an important role in mariculture as food for larval stages of crustaceans and fish, for all stages of bivalves, and as food for zooplankton (rotifers, copepods, and brine shrimp) that are fed to late larval stages and juvenile fish and crustaceans (Volkman et al., 1989). According to Borowitzka (1997), the number of algae used in aquaculture is quite small when compared with the diversity of phytoplankton. Continued research will provide a wider range of species with improved nutritional properties and possibly better suited for large-scale culture in some parts of the world. The demand for tropical microalgae is increasing, so growth rates and nutritional value are important criteria in their evaluation as food source for aquaculture (Renaud et al., 1999).
Northwest Mexico is an arid region with extensive coastal areas and great potential for fish culture. However, the extreme environmental conditions limit the introduction of temperate and cold-water species. The use of native strains naturally adapted to extreme conditions could help to the success in the regional aquaculture. During the last decade, a native strain of Brachionus plicatilis has been produced to feed fish larvae (Ramirez-Sevilla et al., 1991). Improvements in rotifer fecundity and yields were obtained using two native microalgae (Rueda-Jasso, 1996). Initially those strains were considered Nannochloropsis sp. and Nannochloris sp., however their identity was not confirmed and studies to improve their culture conditions were not done. In this study it was analysed the taxonomic identity of those microalgae and evaluated the effect of different culture conditions on their growth and lipid content.
Materials and methods.
The strain C1 used in this study was originally isolated from the Texcoco lake Mexico. In the laboratory, it was acclimated to marine conditions in M1 medium, composed of sea water at 35 salinity, enriched with 1.1 M NaNO3, 0.1 M Na2HPO4, 0.15 M Na2SiO3·9H2O, 0.02 M FeSO4·7H2O, 0.01 M MnSO4·H2O, 0.025 M ZnCl2, 0.00015 M CuCl2·6H2O, 0.00015 M CuCl2·2H2O, 0.005 M Na2MoO4·H2O plus 1 mg cyanocobalamin, 1 mg biotin, and 100 mg thiamine per litre.
The strain C2 was isolated from Oasis San Pedrito, Baja California Sur, Mexico (23°23'24"N, 110°12'32"W). During summer 1985, samples of water were collected and 2 mL samples were inoculated in tubes with 20 mL of M1 media. The tubes were incubated for 72 h at 27-30°C, under continuous light (Fluorescent lamps at ca.5,000 lux). Then each tube was transferred to 250 mL of M1 media and incubated with continuous aeration. At 15 days, the biomass was harvested by centrifugation and the microalgae were purified by successive subculturing on fresh solid media (M1 plus 2% agar) using a mixture of antibiotics (100 mg penicillin G, 50 mg streptomycin, and 10 mg chloramphenicol) as described by Stein (1973). The isolated microalgae were cloned and stored in agar tubes.
For characterisation and identification, the isolated strains were cultured at 27 "C under both continuous light (Fluorescent lamps at ca.5,000 lux) and natural daylight. The gross morphology was determined using living specimens under phase contrast at 1,000x. Samples for transmission electronic microscopy (TEM) were fixed in glutaraldehyde 3% in phosphate buffer (PB) (0.1 M, pH 7.2) at room temperature. Specimens were washed two times in PB (overnight) and post-fixed in 2% osmium tetroxide in PB, for two hours at 4" C. Next, they were dehydrated and embedded in epoxy plastic. Sections produced were stained with uranyl acetate and examined in a Zeiss TEM. Pigment content was analysed using thin-layer chromatography, according to Lewin (1989). The identification of isolates was done using the descriptions of Butcher (1952); Wilhelm et al. (1982), Brown and Elfman (1982), Sarokin and Carpenter (1982), and Turner and Gowen (1984).
The growth characteristics were evaluated under different culture conditions. Four different media of common use in aquaculture were tested; M1-medium (as previously described), Beta-medium (Guillard, 1975). Fa-medium (Fabregas et al., 1985). and FI-medium (Nellys et al., 1988) in 2-L Erlenmeyer flasks and 19-L carboys. The microalgae were cultured in triplicate at an initial density of 4.5 × 106 cells/mL. The cultures were incubated at 27°C under continuous aeration and illumination (ca. 5,000 lux). Every 7 days, the cell concentration was directly evaluated using a Neubauer chamber. Two millilitre samples were fixed in 4% formalin and stained with lugol solution. Counts were made in triplicate under 400x magnification. In addition, 20 mL of each culture was centrifuged at 2,000 g for 10 min and dried under vacuum. The total content of lipids was measured after methanol-chloroform (2:1) extractions (Bligh and Dyer, 1959, modified by Kates, 1972). The extracts were evaporated to dryness under nitrogen atmosphere and weighed. Every sample was assayed in triplicate.
The growth in each of the experimental conditions was evaluated using the logistic model of population growth, Nt=K(1 + ea+rt)-1, where K (the capacity of the system) was estimated for each combination of recipient and culture media using the Simplex and quasi-Newton algorithm of Statistica® 5.0 A (StatSoft inc.Tulsa, USA). The growth rates (r in the model) were compared by the Tukey test for multiple comparisons among slopes (Zar, 1995) using the log-linear model Ln(KNt - 1) = a + rt.
The lipid content (mg/cell) during the growth of each strain under the different experimental conditions was compared using a MANOVA analysis. Where differences were found, a Dunnet test was performed.
The effect of different nitrogen concentrations was evaluated. Growth at 0.5, 1.5, 5, and 10 mM of NaNO3 was undertaken in suitably modified M1 media. In each case, the nitrogen:phosphorus relation was maintained constant at 15:1. The cultures were made in triplicate in 2-L flasks under continuous illumination and aeration. The number of cells and the lipid content were evaluated during 21 days as previously described.
The effect of the nitrogen content in the media was evaluated comparing the growth rates (r) as previously described and the biomass produced at the end of the experiment by ANOVA test.
Results
Using morphological and ultrastructural characteristics, the two strains were identified as Nannochloris sp., Chlorophyceae: Chlorococcales. (Fig. 1).

The cells are small (2 to 5 mm), reproducing by binary fission and by autospore formation. They possess a parietal chloroplast without a pyrenoid, which occupies most of the cell, and the thylakoids arrayed in long lamellae surrounded by a double membrane. There is one prominent nucleus and a mitochondrion with a double membrane. In both strains, the formation of 8-µm autospores was observed up to 3 and 6 cells, and the next steps in autospore formation were difficult to follow by either light microscopy or in the serial sections of electron microscopy preparations. In mature stages, the cell has reddish granules. Chlorophyll a, b, and carotenes were found in both strains.
Although both strains grew in the three media tested, under our experimental conditions differences their behaviour were observed. In both strains the growth rate was higher in flasks than carboys (F = 81.5, P<0.0001) (Fig.2). In flasks C1 showed different growth rates in the three media used (P < 0.005), the higher yield was found using M1 medium (P < 0.001) and the lowest yield in FA medium (Fig 2a). In carboys, no significant differences in the final yield were observed between the three media used (F (2,18) = 2.46, P = 0.1134) (Fig.2b). For Strain C2, no significant differences in growth were observed between flasks and carboys (F (1,30) = 1.95, P > 0.15) (Fig. 2c,d) however a better yield was obtained using the FA medium in flasks (F (2,27) = 7.36, P < 0.03) (Fig. 2c) and lower growth rate was found within the M1 media. In carboys the growth of C2 showed a similar pattern in the three media used, growth rates and final yield were similar (F = 2.247, P > 0.15) (Fig. 2d).
The total content of lipids in C2 was larger than found in C1 (F (1,35) = 170.78, P > 0.0001). Also the content of lipids in C1 was not affected by the culture vessel (F(1,18) = 2.27, P < 0.15), and no significant changes were observed during growth (F (2,18) = 0.96, P > 0.4) (Fig. 3a,b). In contrast, during the growth of C2 we found an increase in the lipid content (Fig. 3), but, this increase was not statistically significant (P > 0.05). In carboys the total content of lipids of C2 reach the maximum value at day 21 (9.5 pg/cell), decreasing during consecutive days (Fig. 3d).

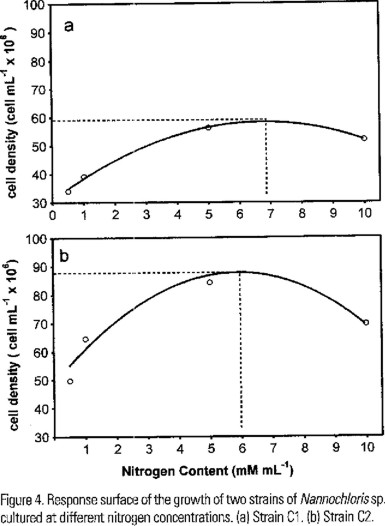
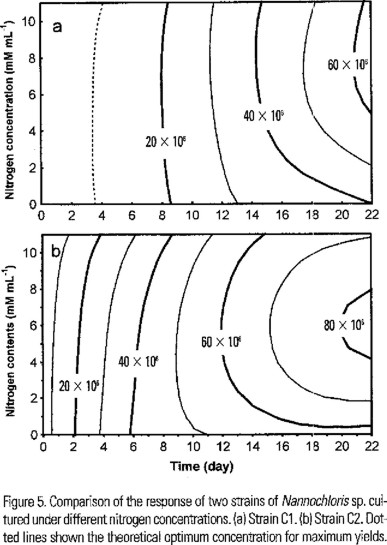
Under different nitrogen concentrations a typical pattern was recorded in the growth of both strains, but, differences in the growth rates were observed (Fig. 4a,b ). Also, we found significant differences in the total biomass produced at the end of the experiment (F (1,64) = 187.14, P < 0.0001). The yield of the C2 was almost twice that of C1 at all nitrogen concentrations tested (Fig. 4a,b). The best yield of C1 was obtained at 5 mM of N, but the theoretical optimum concentration is near 7 mM (Fig. 5a). C2 shown the best growth at 5 mM and the theoretical optimum is near 6 mM (Fig. 5b). Also significant differences in the content of lipids were no found by effect of nitrogen concentration (F (3,58) = 2.3655, P < 0.0802).
Discussion
According to our taxonomic analysis, both strains were identified as Nannochloris sp. Both have a very small cell size, spherical to avoid shape, divide into two daughter cells, and the cells, are not bound by a mother cell wall during cell division. In a previous analysis, strain C2 was identified as Nannochloropsis sp (Rueda-Jasso and Ortíz-Galindo, 1995), but, we identified chlorophyll b which distinguishes Nannochloris from Nannochloropsis (South and Wittick, 1987).
Although both strains were identified as Nannochloris, we find differences in their kinetic behaviour and nutritional requirements. Those differences and their origin suggest that they could be different species, but we did not find structural differences to support this hypothesis. Nannochloris has been very important in aquaculture with good yields obtained in outdoor cultures (Fulks and Main, 1991). Several strains of Nannochloris have been isolated and are used for feeding rotifers. The most common species used in aquaculture are Nannochloris atomus, Nannochloris oculata, and Nannochloris sp. (Brown, 1991). However, there is confusion between Nannochloris oculata and Nannochloropsis oculata. Sarokin and Carpenter (1982) suggested a mistake in the classification of Nannochloris oculata as a member of the Chlorophyceae, and should be included in Eustigmatophyceae. As in the present study, several strains of Nannochloris used in aquaculture have been identified only at genus level. However, they have been isolated from different areas, it is possible that several distinct species are in use worldwide. Further research (eg. genetic analysis) is necessary to clarify the actual status of the genus Nannochloris and to corroborate the adequate inclusion of those strains within the genus Nannochloris.
In our experiments, C2 showed a greater adaptability to different culture conditions. The growth of this strain was not affected by the culture vessels, grew appropriately in M1 and FA media and produced a biomass almost double that of C1 at any nitrogen concentration. Also, we found greater lipid contents in C2 than in C1, and apparently the lipid content in C2 increases during growth (Fig. 3). Hodgson et al. (1991) and Sukenik and Carmeli (1990) found a similar behaviour during the growth of Nannochloropsis oculata and Nannochloropsis sp. This behaviour was correlated with the cell division rateat any particular point on the batch-culturegrowth curve, where in periods of low growth rate, such as the lag and stationary phases, the lipid contents in the cell became elevated.
During the last decade, several studies have shown the importance of lipids, particularly the fatty acids, during larval development (Ostrowki and Divakaran, 1990). Mostly these lipids are produced by the microalgae and are transported through the trophic chain when ingested by zooplankton (Frolov et al., 1991). Greater lipid content in microalgae is considered advantageous in aquaculture. Although we found a greater lipid content in C2, further research is necessary to evaluate the fatty acids proportions in order to assure advantages in fish larviculture.
The biochemical composition of algae is generally affected by cultivation conditions, and factors such as temperature, light, and nutrient composition of the culture medium are important. Recent studies have shown that deficiencies in nitrogen concentration induce an increase in the lipid concentration of members of the Chlorophyceae, but this was not observed in Nannochloris atomus. Hodgson et al. (1991) and Dunstan et al. (1993) found that during the growth of Nannochloropsis oculata the concentration of lipids stays constant, but they found changes in the proportion of fatty acids. In this study, the total concentration of lipids was not significantly affected by the nitrogen concentration, but we do not know where the proportions of fatty acids remained without changes.
We concluded that C2 has avdantages over C1 as a candidate for aquaculture. The C2 strain was isolated from a coastal oasis in a region with extreme changes in salinity and temperature and it has shown good ability to adapt to diverse culture conditions. Those strains has been donated by our lab to several research institutions and marine aquariums of Mexico and proved to have advantages over other microalgae commonly used in rotifer production (Rueda-Jasso, 1996).
Acknowledgements.
We thank the asistance received from Heydi Ruth Fuentes Solis during sample analysis. We also thank Silvie Dumas for helpful discussion. This research was supported by DEPI IPN and Grant of COTEPABE.
References
BLIGH, E. G. and W. J. DYER 1959. A rapid method of total lipid extraction and purification. Canadian Journal of Biochemistry and Physiology 31: 911-917. [ Links ]
BOROWITZKA, M. A., 1997. Microalgae for aquaculture: Opportunities and constraints. Journal Applied Phycology 9: 393-401. [ Links ]
BROWN, R. M., 1991. The amino-acid and sugar composition of 16 species of microalgae used in mariculture. Journal of Experimental Marine Biology and Ecology 145: 79-99. [ Links ]
BROWN, L. M. and B. ELFMAN.,1982. Is autosporulation a feature of Nannochloris?. Canadian Journal of Botanic 61: 2647-2656. [ Links ]
BUTCHER R. W., 1952. Contributions to our knowledge of the smaller marine algae. Journal of Marine Biology Association, U.K. 31: 175-191. [ Links ]
DUNSTAN, G. A., J. K.VOLKMAN, S. M. BARRETT and C. D. GARLAND, 1993. Changes in the lipid composition and maximisation of the polyunsaturated fatty acid contentof three microalgae grown in mass culture. Journal of Applied Phycology 5: 71-83. [ Links ]
FABREGAS, J., C. HERRERO, B. CABEZAS and J. ABALDE, 1985. Mass culture and biochemical variability of the marine microalga Tetraselmis suecica Kylin (Butch) with high nutrient concentrions. Aquaculture 49: 231-244 [ Links ]
FROLOV, A. V., S. L. PANKOV, K. N. GERADZE, S. A. PANKOVA and L. V. SPEKTOROVA, 1991. Influence of the biochemical composition of food on the biochemical composition of the rotifer Brachionus plicatilis. Aquaculture 97: 181-202. [ Links ]
FULKS, W. and K. L. MAIN, 1991. Rotifer and microalgae culture systems. Proceedings of a U.S. Asia Woskshop, 28-31 January 1991, Honolulu, Hawaii. The Ocean Institute. 355 p. [ Links ]
GUILLARD, R. R. L., 1975. Culture of phytoplankton for feeding marine invertebrates. pp. 29-60. En: W. L. SMITH and M. H. CHANLEY (Comps). Culture of marine invertebrate animals. Plenum Press, New York. [ Links ]
HODGSON, P. A., R. J. HENDERSON, J. R. SARGENT and J. W. LEFTLEY, 1991. Patterns of variation in lipid class and fatty acid composition of Nannochloropsis oculata (Eustigmatophyceae) during batch culture I. The growth cycle. Journal of Applied Phycology 3: 169-181. [ Links ]
KATES, M., 1972. Isolation, analysis and identification of lipids. pp. 268-618. En: T. S. WORK and E. WORK (Comps.). Techniques in lipidology. Elsevier, Amsterdam. [ Links ]
LEWIN, R. A., 1989. A simple method for separating chlorophylls a and b from algae. Marine Biology Research Division, Scripps Ins. Ocean 1-2. [ Links ]
NELLYS, M., O. NUSETTI and A. VELEZ, 1988. Producción experimental de fitoplancton para alimento de juveniles del mejillón Perna perna, influencias sobre el crecimiento y metabolismo energético. En: FUNDACIÓN LA SALLE DE CIENCIAS NATURALES (Comp.). Congreso Iberoamericano y del Caribe (Abstracts), Universidad de Oriente, Isla Margarita, Punta Piedra Venezuela: 109 p. [ Links ]
OSTROWKI, A. C. and S. DIVAKARAN, 1990. Survival and bioconversion of n-3 fatty acids during early development of dolphin (Coryphaena hippurus) larva fed oil-enriched rotifers. Aquaculture 89: 273-285. [ Links ]
RAMIREZ-SEVILLA, R., R. RUEDA-JASSO, J. L. ORTÍZ -GALINDO and B. GONZÁLEZ-ACOSTA 1991. Metodología para el cultivo del rotífero Brachionus plicatilis. Investigaciones Marinas, CICIMAR 6: 287-290. [ Links ]
RENAUD, S. M., L. V. THINH and D. L. PARRY, 1999. The gross chemical composition and fatty acid composition of 18 species of tropical Australian microalgae for possible use in aquaculture. Aquaculture 170: 147-159. [ Links ]
RUEDA-JASSO, R., 1996. Nutritional effect of three microalgae and one cyanobacteria on the culture of the rotifer Brachionus plicatilis Müller:1786. Ciencias Marinas 22: 313-328. [ Links ]
RUEDA-JASSO, R. and J. L. ORTÍZ-GALINDO, 1995. Efecto del alimento en el cultivo del rotífero Brachionus plicatilis (Müler:1786). Revista Latinoamericana de Acuicultura 44: 69-77. [ Links ]
SAROKIN, D. J. and E. J. CARPENTER, 1982. Ultrastructure and taxonomic observations on marine isolates of the genus Nannochloris (Chlorophyceae). Botánica Marina 25: 483-491. [ Links ]
SOUTH, G. R. and A. WITTICK, 1987. Introduction to Phycology. Blackwell Scientific Publications, Oxford, 341 pp. [ Links ]
STEIN, J. R., 1973. Handbook of Phycological Methods. Culture methods and growth measurements. Cambridge U.P., Cambridge, 445 pp. [ Links ]
SUKENIK, A. and Y. CARMELI, 1990. Lipid synthesis and fatty acid composition in Nannochloropsis sp. (eustigmatophyceae) grown in a light dark cycle. Journal of Phycology 26: 463-469. [ Links ]
TURNER, M. F. and R. J. GOWEN, 1984. Some aspects of the nutrition and taxonomy of fourteen small green and yellow-green algae. Botanica Marina 27: 249-255. [ Links ]
VOLKMAN, K. J., S. W. JEFFREY, P. D. NICHOLS, G. I. ROGERS and C. D. GARLAND, 1989. Fatty acid and lipid composition of 10 species of microalgae used in mariculture. Journal of Experimental Marine Biology 128: 219-240. [ Links ]
WILHELM, C., G. EISENBEIS, A. WILD and R. ZAHN, 1982. Nannochlorum eucaryotum: A very reduced coccoid species of marine Chlorophyceae. Z. Naturforsch 37: 107-114. [ Links ]
ZAR, J. H., 1995. Biostatistical analysis. 3th edition. Prentice Hall, New Jersey, 662 p. [ Links ]














