Introduction
Ductile iron (DI) can be cast into intricate shapes because of its excellent fluidity and relatively low melting point, and it can be alloyed for improvement of corrosion resistance and strength. Because of the excellent properties obtainable with this low-cost engineering material, ductile iron finds a wide variety of applications, for example, automotive and machine parts, tubes, and applications in environments that demand good corrosion resistance, such as water, soils acids, alkalis, saline solutions, organic compounds, and liquid metals (ASM International, 2009; Roy, 1988). DI can acquire various desirable mechanical properties and corrosion resistance with the addition of proper alloying elements and through heat treatment. Alloying elements can play a dominant role in the susceptibility of ductile iron to corrosion attacks. The alloying elements generally used to enhance the corrosion resistance of ductile cast iron include silicon, nickel, chromium, and copper. Other alloying elements used to a lesser extent are vanadium, titanium, and molybdenum (ASM International, 2009; Roy, 1988). On the other hand, through austempering heat treatment, the microstructure of ductile iron can be modified, so its microstructure is “ausferrite”, which consists of ferrite (α) and high carbon austenite (γHC). The austempered ductile iron (ADI) obtained delivers twice the strength of conventional ductile iron for a given level of elongation, with exceptional wear and fatigue resistance (Brandenberg et al., 2001). Intercritical heat treatments can also be performed on DI to increase the mechanical properties. Intercritical heat treatments can be applied to obtain microstructures of graphite nodules in a matrix of ferrite and austenite particles or graphite nodules in a matrix of ferrite and tempered martensite (Aristizabal et al., 2012; Machado et al., 2020). Corrosion behavior, like mechanical properties, is related to the alloy microstructure. A survey of the literature indicates that some attention has been paid to the corrosion resistance of DI and ADI mainly alloyed with nickel (Cheng-Hsun & Ming-Li, 2010; Minkoff, 1983; Yufu et al., 2012). Also, it is well known that nickel is the most commonly used alloying element because it decreases primary carbide stability while increasing the finesses of pearlite, thus, increasing the strength of the iron (Minkoff, 1983). High-nickel ductile iron has been specified by the ASTM A439 standard for its resistance to heat and corrosion as well as for other special purposes (Yufu et al., 2012). Experiments on the corrosion of cast irons and austempered chilled ductile iron containing nickel indicate that corrosion decreased with increasing nickel content (Hemanth, 2000; Rajagopal & Iwasaki, 1992).
The corrosion behavior of each group of cast iron is very different from that of other groups; however, in general, single phases such as ferrite and austenite tend to be less corrosion resistant than two-phase mixtures (e.g., pearlite and ausferrite) (ASM International, 1992). ADI austempered at low temperatures in the range from 250 °C to 330 °C allow obtaining a lower ausferritic microstructure, while high austempered temperatures (330 °C-450 °C) promote the formation of upper ausferritic microstructures, which consist of broad blades of isolated ferrite; this microstructure results in a high tensile toughness (Blackmore & Harding, 1984). The electrochemical behavior and corrosion resistance of ADI were evaluated by electrochemical techniques in sodium chloride solution. It has been shown that ADI containing an upper ausferritic microstructure attained higher corrosion resistance than an ADI with a lower ausferritic microstructure (Krawiec et al., 2009). Molybdenum was found to contribute substantially towards improving the corrosion resistance of ductile iron, particularly in high silicon ductile irons (ASM International, 2009). Moreover, the nickel and molybdenum content combination of austempered chilled ductile iron, together with chilling and austempering, contribute to its superior corrosion resistance (Hemanth, 2000). The corrosion performance of conventional DI and ADI was evaluated to low sodium chloride concentrations (0.01 M and 0.05 M) by chronopotentiometry and potentiodynamic polarization techniques. Both samples appeared to have comparable corrosion attacks; however, the austempering heat treatment help in the stabilization of nodules improving its corrosion resistance for the corrosive media (Akinribide et al., 2019).
The effects of the molybdenum addition on the microstructure, wear resistance, and corrosion resistance of carbidic austempered ductile iron (CADI) were studied. The corrosion resistance was evaluated by polarization curves in a 3.5 wt % NaCl solution. As the molybdenum content increased, the quantity of austenite and carbides increased, while the acicular ferrite became finer and corrosion resistance increased (Han et al., 2015a). However, higher corrosion resistance of cast iron can be achieved not only by the addition of alloying elements but also by heat treatment (Gonzaga & Fernández, 2005). Vanadium enhances the graphite morphology and matrix structure in ductile iron. Besides this, vanadium can refine grains effectively and promote the formation of acicular ferrite; consequently, better properties are expected. Few published research articles about the vanadium effect on the corrosion behavior in ductile cast iron and austempered ductile iron are available. The present work aims to study the influence of vanadium on the relationship between microstructure and corrosion resistance of ductile iron and austempered ductile iron low alloyed with vanadium and vanadium-molybdenum by potentiodynamic polarization techniques in 0.5 M H2SO4, 0.5 M NaCl, and 0.5 M NaOH corrosive media.
Methods and Materials
Three cast alloys, identified as DI for the unalloyed ductile iron, DI-A for the ductile iron alloyed with 0.5 wt % V, and DI-B for the ductile iron alloyed with 0.5 wt % Mo and 0.49 wt % V, were produced in a 50 kg medium-frequency coreless induction furnace. The base iron was prepared with a mixture of 35 wt % low C and Mn steel, 30 wt % pig iron, and 35% cast iron scrap as the metallic charge. Table 1 shows the chemical composition of the base iron. High purity carbon riser, FeSi (75%), FeMo (62.83%), and FeV (75.34%) were used to adjust the chemical composition of the charge. All the materials were melted and homogenized at 1500 °C-1520 °C. The melt was nodulized with 1.5% nodulizing MgFeSi (45% Si, 8% Mg, 3.3% Ca, 3% Rare Earths) on the bottom of a preheated ladle (20 cm ID, 35 cm deep) covered with low carbon steel scrap at 1440 °C-1460 °C. After skimming the slag of the nodulized melt, 1.0% foundry grade FeSi (75% Si, 1% Ca, 0.9% Al, 1.1% Ba) was added by the ladle inoculation method, where the inoculant was added to the metal stream as it flowed from the nodulized ladle into a preheated pouring type shank ladle (21 cm ID, 20 cm deep).
Table 1 Chemical composition (wt. %) of the base iron.
| Cast alloy | C | Si | Mn | P | S | Mg | Ni | Cr | Mo | V | Al | Cu |
| DI | 2.43 | 0.98 | 0.27 | 0.02 | 0.028 | 0.001 | 0.01 | 0.11 | 0.005 | 0.01 | 0.01 | 0.03 |
| DI-A | 2.41 | 1.19 | 0.20 | 0.03 | 0.027 | 0.001 | 0.01 | 0.11 | 0.005 | 0.005 | 0.01 | 0.02 |
| DI-B | 2.38 | 1.46 | 0.25 | 0.03 | 0.019 | 0.001 | 0.01 | 0.13 | 0.005 | 0.01 | 0.03 | 0.04 |
Source: Authors’ own elaboration.
Each of the three cast alloys were then poured at 1420 °C-1440 °C into three green sand molds to obtain plates of 120 mm x 40 mm and a thickness ranging from 25.4 mm to 4.23 mm by using the pattern shown in Figure 1.
The nominal chemical composition in the castings was analyzed by an Oxford spark emission optic spectrograph. The reported values in Table 2 are the average of three measurements on each cast alloy. Carbon and sulfur content were determined by combustion analysis, using a Leco C/S analyzer.
Table 2 Chemical composition (wt. %) of the cast alloys.
| Cast alloy | C | Si | Mn | P | S | Mg | Ni | Cr | Mo | V | Al | Cu | CE1 |
| DI | 3.43 | 2.41 | 0.30 | 0.02 | 0.019 | 0.037 | 0.01 | 0.10 | 0.005 | 0.01 | 0.02 | 0.06 | 4.24 |
| DI-A | 3.42 | 2.62 | 0.19 | 0.04 | 0.020 | 0.047 | 0.01 | 0.11 | 0.005 | 0.5 | 0.03 | 0.03 | 4.30 |
| DI-B | 3.40 | 2.89 | 0.23 | 0.03 | 0.014 | 0.046 | 0.01 | 0.13 | 0.50 | 0.49 | 0.04 | 0.06 | 4.37 |
1CE: Carbon equivalent. Balance Fe
Source: Authors’ own elaboration.
Samples of the plate of 4.23 mm thick were used for heat treatment to evaluate the microstructural and corrosion behavior of the ductile iron and austempered ductile iron produced. The austempering heat treatment was as follows: the samples were austenitizing at 950 °C for 1 h; then, they were quenched in a salt bath (97.8% NaNO3 and 2.2% Na2CO3) at 300 °C for 1 h, finally they were cooled down with water at room temperature.
Standard metallography techniques (mechanical grinding and polishing followed by etching with 2% nital) were employed to reveal the different microconstituents of the cast alloys. Optical microscopy was performed on polished and etched specimens by using an optical microscope Olympus PMG-3 model, the ASTM A 247 standard, and an image-analyzer with the software Image J version 4.1. The graphite morphology was rated for the nodule count by ASTM standard A 247 and average nodule size on the unetched samples. Phase volume fraction measurements, including nodule graphite, pearlite, ferrite, and carbides, were carried out using the image-analyzer with the software Image J version 4.1. Carbides were revealed by etching 2 min with a water solution of ammonium persulfate (10% vol) (Pedro & Dommarco, 2019). The optical microscopy measurements represent the average of eight different regions on each sample.
Before electrochemical measurements were carried out, DI and ADI samples were embedded in an epoxy resin to control the surface area. The samples were mechanically ground with silicon carbide emery papers down to 600 grit. The samples were ultrasonically rinsed in ethanol between each step. Corrosion behavior of the samples was evaluated by anodic potentiodynamic polarization test in a Potentiostat-Galvanostat AutoPG-UAM equipped with a conventional three-electrode system electrochemical workshop with the DI and ADI samples as the working electrode, graphite as the counter electrode, and a saturated calomel electrode as the reference. All tests were conducted at 25 °C, at molarity held constant in 0.5 M of sulphuric acid, sodium hydroxide, and sodium chloride as corrosive media, and tests were repeated for reproducibility. The electrode potential sweep was raised from -0.25 V to +0.25 V from open circuit potential (OCP), with the scanning rate of 1 mV/s. Corrosion current density values were obtained by the Tafel extrapolation method (McCafferty, 2005; Stern & Geary, 1957).
Results and Discussion
The nominal chemical composition analyzed in the castings is shown in Table 2. The equivalent carbon was hypoeutectic for heats DI and DI-A and hypereutectic for heat DI-B. The silicon content was high to improve graphitization under the high cooling rates imposed by the low thickness of the casting plate used. The residual magnesium content of 0.032%, on average, was obtained in heats for an adequate nodule formation. The amount of vanadium and molybdenum was set at 0.5 wt % for heats DI-A and DI-B.
Figure 2 shows the unetched microstructure of cast alloys manufactured. A homogeneous distribution of spheroidal graphite for the cast alloys with a high nodule count is observed. The largest nodules in low amounts are observed for the cast alloy DI, while the smallest nodules belong to the low alloyed ductile irons (DI-A and DI-B).

Source: Authors’ own elaboration.
Figure 2 Unetched microstructure of cast alloys: a) DI, b) DI-A, and c) DI-B.
Figure 3 shows the etched microstructure of the cast alloys. The microstructure consists mainly of graphite nodules contained in a pearlitic-ferritic matrix. The presence of carbides that may correspond to Fe3C, (Fe2Mo) C, VC, and V4C3 (Han et al., 2015a; Han et al., 2015b) are also evident.

Source: Authors’ own elaboration.
Figure 3 Etched microstructure of cast alloys: a) DI, b) DI-A, and c) DI-B.
The carbides were revealed by etching the as-cast samples with an ammonium persulfate solution, as can be observed in Figure 4. It was determined that cast alloys DI, DI-A, and DI-B contain volume fractions (vol.%) of carbides of 15.53, 3.09, and 2.05, respectively.

Source: Authors’ own elaboration.
Figure 4 Etched microstructure with ammonium persulfate reveals the carbides phase (white regions) of cast alloys: a) DI, b) DI-A, and c) DI-B.
The sample identified as DI for the unalloyed ductile iron shows a higher cementite formation. The thin thickness used (4.23 mm) in the as-cast plate increases the undercooling tendency and promotes metastable eutectic cementite formation. In this case, for the hypo-eutectic chemical composition (CE = 4.24) and the cooling rates imposed by the thin thickness evaluated, the cementite formation was unavoidable for the sample identified as DI. It is recommended (Ruxanda et al., 2001; SORELMETALS, 1990) an equivalent carbon in the range of 4.3 to 4.6 to avoid carbide formation in thin section castings. The samples identified as DI-A and DI-B show a lower amount of carbides than sample DI. This is attributed to the higher carbon equivalent hypereutectic cast irons with carbides that may correspond to (Fe2Mo)C (Han et al., 2015b), and VC and V4C3 types (Han et al., 2015a) that may correspond to the DI-A and DI-B, respectively.
Table 3 shows nodule count, average nodule size, and the volume fraction of phases formed in the ductile irons produced. A higher nodule count with low nodule size was obtained for the ductile irons alloyed with V and V-Mo, while the unalloyed ductile iron shows the lowest nodule count with a large nodule size. The difference in the nodule count for the ductile irons produced is attributed to the effect of the percentage of carbon equivalent (%CE). When the percentage of carbon equivalent is increased, the nodule count is also increased, reaching a maximum value at CE = 4.61, while the nodule size shows the opposite behavior (Fatahalla et al., 1996). According to the results in Tables 2 and 3, the DI-B shows the highest %CE, with the highest nodule count and the lowest nodule size. Pearlite is the primary microconstituent formed in the three cast ductile irons with the highest amount reported for the DI-B sample.
Table 3 Distribution of graphite and volume fraction of phases in the as-cast ductile irons.
| Parameter | Cast alloy | ||
| DI | DI-A | DI-B | |
| Nodule count (nod mm-2) | 218 | 260.48 | 348.95 |
| Average nodule size (µm) | 28.42 | 14.6 | 14.3 |
| Graphite (vol. %) | 14.05 | 10.02 | 13.04 |
| Ferrite (vol. %) | 9.55 | 28.27 | 18.93 |
| Pearlite (vol. %) | 60.84 | 58.6 | 65.99 |
| Carbides (vol. %) | 15.53 | 3.09 | 2.01 |
Source: Authors’ own elaboration.
The microstructure of the samples which were austenitized at 950 °C for 1 h and then austempered at 300 °C for 1 h are shown in Figure 5.

Source: Authors’ own elaboration.
Figure 5 Etched microstructure of the austempered ductile irons of cast alloys: a) ADI-A and b) ADI-B.
The microstructures consist mainly of acicular ferrite in a stabilized high carbon austenite matrix (ausferrite microstructure), carbide, and nodular graphite. It is observed that the nodular graphite is dispersed in the microstructure. In spite that the austempering heat treatment was carried out under the same parameters, it is evident that the microstructure of Figure 5a corresponding to the ADI alloyed with vanadium presents an upper ausferritic microstructure consisting of broad ferrite needles. By contrast, the ADI alloyed with vanadium and molybdenum (Figure 5b) shows a lower ausferritic microstructure with very fine needles of ferrite. The addition of vanadium can refine grains effectively and promote the formation of acicular ferrite (Han et al., 2015a; He & Edmonds, 2002). It is known that molybdenum can distribute both in acicular ferrite and carbide. During the austenitization process, the molybdenum in the austenite decreases the diffusion rate of carbon, and the discrete distribution of carbides has a pinning effect on grain growth. The combined effect of vanadium and molybdenum as carbide formers in the sample ADI-B accelerate the nucleation of acicular ferrite and refine the acicular ferrite grains, as can be observed in Figure 5b.
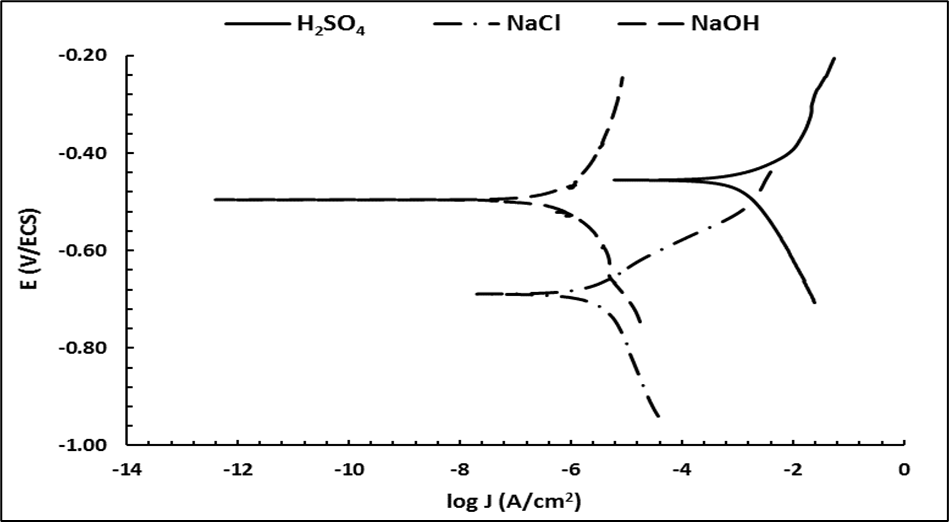
The potentiodynamic polarization curves for DI, DI-A, and DI-B obtained in 0.5 M of NaCl, 0.5 M NaOH, and 0.5 M H2SO4 solutions are shown in Figures 6, 7, and 8, respectively.
Source: Authors’ own elaboration.
Figure 6 Polarization curves of the ductile iron (DI) in 0.5 M of H2SO4, 0.5 M NaCl, and 0.5 M NaOH solutions.

Source: Authors’ own elaboration.
Figure 7 Polarization curves of the ductile iron (DI-A) in 0.5 M of H2SO4, 0.5 M NaCl, and 0.5 M NaOH solutions.

Source: Authors’ own elaboration.
Figure 8 Polarization curves of the ductile iron (DI-B) in 0.5 M of H2SO4, 0.5 M NaCl, and 0.5 M NaOH solutions.
The corrosion potential and corrosion current density are extracted from the polarization curves by fitting the polarization curve. The corrosion current density and the corrosion potential reflect the corrosion resistance of the ductile iron in the environment evaluated. As the corrosion current increases, the rate of corrosion becomes faster, while higher corrosion resistance is indicated by a small corrosion current (Gollapudi, 2012; Stern & Geary, 1957). Table 4 shows the corrosion potential and the corrosion current of the ductile irons evaluated in different corrosive media.
Table 4 Corrosion potential and corrosion current density of the ductile irons and austempered ductile irons.
| Corrosive media (0.5 M) |
Sample | Corrosion potential (V vs SCE) |
Corrosion current density (A cm-2) |
| H2SO4 | DI | -0.453 | 7.11 x 10-4 |
| DI-A | -0.460 | 1.38 x 10-3 | |
| DI-B | -0.461 | 1.09 x 10-3 | |
| ADI-A | -0.203 | 3.32 x 10-4 | |
| ADI-B | -0.505 | 8.71 x 10-4 | |
| NaCl | DI | -0.675 | 2.53 x 10-6 |
| DI-A | -0.666 | 3.75 x 10-6 | |
| DI-B | -0.688 | 2.64 x 10-6 | |
| ADI-A | -0.494 | 4.012 x 10-6 | |
| ADI-B | -0.464 | 1.99 x 10-6 | |
| NaOH | DI | -0.499 | 6.31 x 10-7 |
| DI-A | -0.795 | 2.67 x 10-6 | |
| DI-B | -0.758 | 2.34 x 10-6 | |
| ADI-A | -0.779 | 6.96 x 10-6 | |
| ADI-B | -0.314 | 5.518 x 10-7 |
Source: Authors’ own elaboration.
The potentiodynamic polarization results for ductile irons are reported in Figures 6 to 8 and Table 4. It is observed that DI has the lowest current density for the three corrosive media evaluated compared to the DI-A and DI-B samples, which means that the DI sample has the highest corrosion resistance. Considering the microstructural evaluation observed in Figures 2 to 4 and Table 3, the DI sample shows coarse graphite nodules, while the vanadium and molybdenum addition for DI-A and DI-B samples promotes a refined microstructure and an increase in the nodule count surrounded by ferrite and fine pearlite. This promotes a decrease in corrosion resistance due to the fact that corrosion is promoted by the galvanic graphite-ferrite pair (known as graphitic corrosion) (Logan et al., 2014; Winston & Uhlig, 2008).
However, it must be considered that the DI sample presents few graphite nodules surrounded by ferrite, and the microstructure etched with ammonium persulfate reveals that DI contains a high amount of carbides compared to the DI-A and DI-B samples (Table 3 and Figure 4). The higher volume of carbides tends to increase the corrosion resistance (García et al., 2019; ASM International, 1992; Sain et al., 2016; Sutthiruangwong & Mori, 2005). Nevertheless, the presence of the carbide would not be beneficial for some applications because they cause fragility. In other words, if one takes into account that graphitic corrosion occurs mainly due to the potential difference between the graphite and the surrounding ferrite (Ahmad, 2006; ASM International, 1992; Logan et al., 2014; Winston & Uhlig, 2008), then iron dissolution occurs from the following reactions:
In an acidic medium, iron corrosion is controlled by the cathodic reaction (Winston & Uhlig, 2008), increasing the rate of reaction when the pH decreases, which means that corrosion rate is determined by the rate of hydrogen evolution. This effect occurs for DI-A and DI-B heats, where the corrosion current increase in H2SO4.
In the case of an alkaline medium, the corrosion current decreases, which means that the corrosion rate decreases. This effect is observed in Figures 6, 7, and 8; this response is associated with the formation of a film of Fe(OH)x, which reduces the corrosion rate (Ahmad, 2006; Pourbaix, 1974; Winston & Uhlig, 2008).
Comparing the three media for DI-A and DI-B, and analyzing the corrosion currents shown in Table 4, it is observed that DI-B presents a lower corrosion current, which means that it has greater resistance to corrosion. According to the microstructural evaluation, both have the presence of graphite surrounded by ferrite, which generates the galvanic pair that promotes graphitic corrosion. However, because the phenomenon of corrosion is a process carried out on the metal contact surface, the advantage of DI-B iron is that the fine microstructure that produces smaller graphite nodules also reduces the presence of ferrite surrounding it (Figure 3c and Table 3). In addition, the presence of pearlite is increased, which results in a lower corrosion current in each medium concerning DI-A.
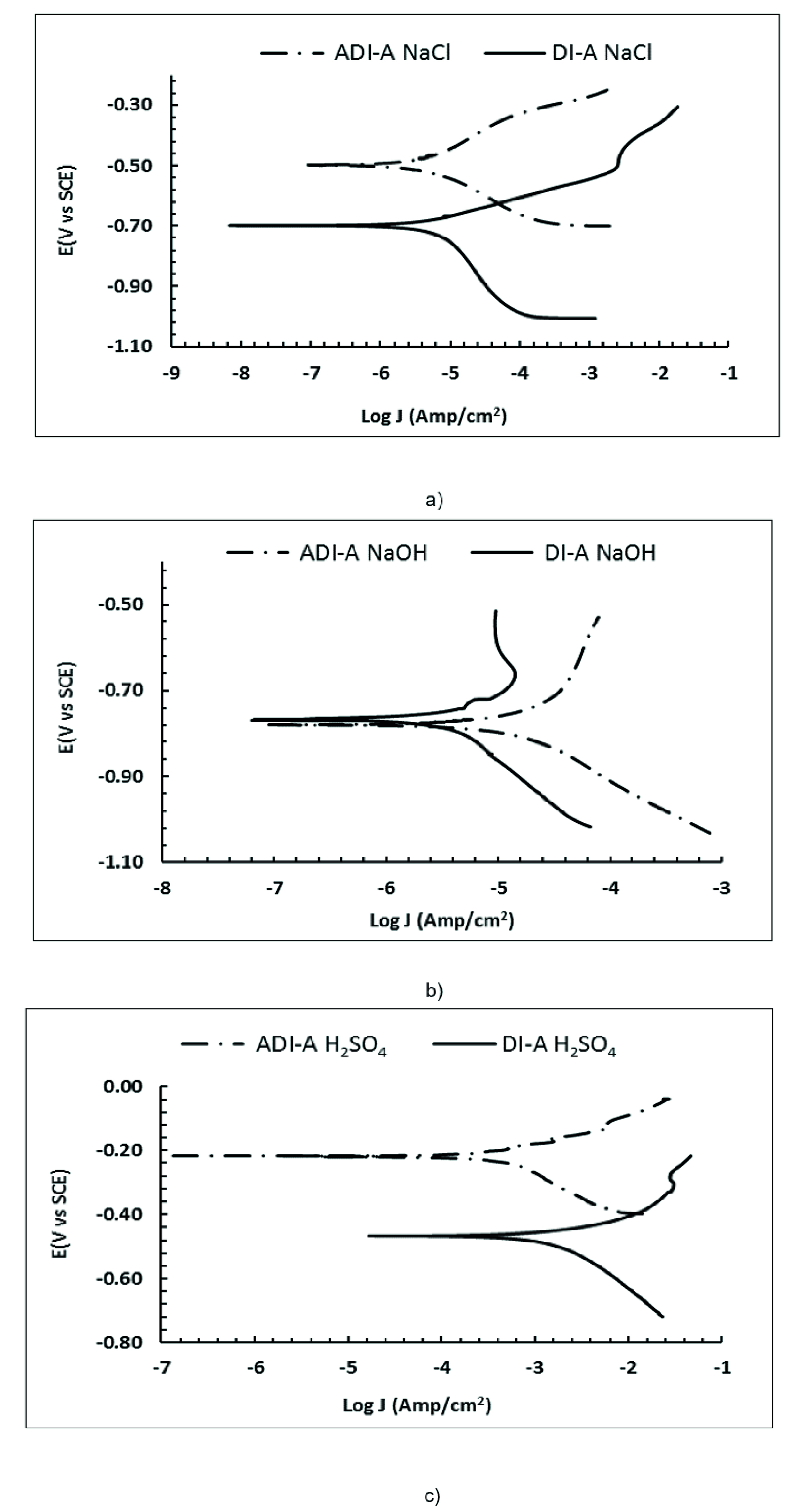
The potentiodynamic polarization curves for ADI-A and ADI-B are shown in Figures 9 and 10, respectively, obtained in 0.5 M of NaCl, 0.5 M NaOH, and 0.5 M H2SO4 solutions.
Source: Authors’ own elaboration.
Figure 9 Polarization curves of the DI-A and ADI-A in a) 0.5 M NaCl, b) 0.5 M NaOH, and c) 0.5 M H2SO4 solutions.

Source: Authors’ own elaboration.
Figure 10 Polarization curves of the DI-B and ADI-B in a) 0.5 M NaCl, b) 0.5 M NaOH, and c) 0.5 M H2SO4 solutions.
Table 4 and Figures 9 and 10 show the corrosion potential and the corrosion current density of the austempered ductile irons evaluated in different corrosive media. In a general way, the results show an increase in corrosion resistance compared to DI-A and DI-B versus ADI-A and ADI-B. However, where an increase in the corrosion current is observed, the corrosion potential tends to shift to positive values, indicating a lower tendency to corrode. This behavior is associated with the presence of a greater amount of acicular ferrite immersed in an austenite matrix, and the presence of these phases inhibit graphitic corrosion mentioned above (ASM International, 1992).
Among the three given aqueous solutions, the sulfuric acid solution is the most aggressive in reducing corrosion resistance. This is because as the pH decreases, the equilibrium potential of reaction 2 becomes nobler; therefore, the potential difference between reactions 1 and 2 becomes larger and, consequently, the corrosion current increases (Ahmad, 2006; ASM International, 1992; Winston & Uhlig, 2008).
Two dissolution mechanisms could occur in acid media (H2SO4); the first happens due to the sulfur ion, and the second one is caused by the oxygen (Banas et al., 2007; Krawiec et al., 2006). Both increase the dissolution rate of ductile irons and austempered ductile irons. Reactions 3 to 5 represent the dissolution mechanism in acid media (Krawiec et al., 2006).
Nonetheless, ADI-A and ADI-B show higher corrosion resistance than DI-A and DI-B and present more positive potentials attributed to the phase transformation obtained by heat treatment, which displaces the corrosion potential toward more positive or noble values.
The polarization curves evaluated in H2SO4 and NaCl media show an anodic current increase related to iron dissolution, unlike NaOH, where a passivation zone attributed the formation of FeOHx (Pourbaix, 1974). This zone is only observed in the ADI-A sample due to the potential scanning zone.
For the NaCl and NaOH corrosive media, reactions 6 and 7 occur (Logan et al., 2014; Pierre, 2000).
Hydroxyl ions (OH-) react with Fe2+ ion to form iron hydroxides according to the following reactions:
And the Fe (OH)3 dehydration allows the formation of Fe2O3 according to reaction 10.
Reactions 6 to 10 show that the hydrogen generated will react with oxygen to form OH- and then with iron, producing an iron hydroxide II, and finally iron hydroxide III. As the reaction mechanisms proceed, a hydroxide layer is formed, which causes that corrosion resistance depends on oxygen diffusion over this layer. In acid media, this layer gets dissolved, increasing the corrosion rate (Winston & Uhlig, 2008).
The austempered ductile irons produced show the presence of high carbon austenite and ausferrite. The dissolution of carbon in austenite will decrease the effect of a potential difference with graphite, decreasing the corrosion rate. The highest corrosion resistance in basic media is greater for the ADI-B. This behavior is due to the fine microstructure of ausferrite obtained by the combined addition of vanadium and molybdenum.
Conclusions
The unalloyed ductile iron shows a high cementite amount due to the cooling rates imposed by the thin thickness and the hypoeutectic chemical composition. This microstructure improves the corrosion resistance in acid media.
The microstructure of the heat-treated samples mainly consists of nodular graphite, acicular ferrite, austenite, and carbides. The acicular ferrite becomes thicker for the austempered ductile iron alloyed with vanadium, while the ADI alloyed with vanadium-molybdenum promotes the formation of a finer microstructure of acicular ferrite. This finer microstructure improves the corrosion resistance in 0.5 M NaCI and 0.5 M NaOH corrosive media.
In acid media (0.5 M H2SO4), the alloyed ADI showed a higher corrosion resistance compared to alloyed DI without heat treatment due to the phase transformation achieved by the heat treatment applied.











 text new page (beta)
text new page (beta)



