Introduction
Banana peel makes up about 30% of the fruit and is discarded after harvesting, marketing, and consumption. However, it could be used for human consumption due to its chemical composition (González-Montelongo, Gloria & González, 2010). Banana peel has been used as medicine (to treat swelling, itching, bruising, wrinkles, and sunburn) (Nagarajaiah, 2011), cattle feed, and in soap manufacturing (Baskar, 2011). Moreover, banana peel is rich in dietary fiber, protein, essential amino acids, polyunsaturated fatty acids, and potassium (Happi, Rado, Wathelet, Tchango & Paquot, 2007). It is also rich in phenolic compounds (Someya, Yoshiki & Okubo, 2012) which have positive effects on certain types of cancer and heart disease (Anantharaju, Gowda, Vimalambike & Madhunapantula, 2016). The aim of this research was to evaluate the bromatological and antioxidant composition of different varieties of banana peels to develop a food product and, then, determine its sensory acceptance.
Materials and Methods
Chemicals and reagents
Hexane, sulfuric acid, and sodium hydroxide were purchased from AYT chemicals and High Purity, respectively. Ascorbic acid was supplied by Zave; acetone and methanol were purchased from Hycel. Folin-Ciocalteu reagent and sodium carbonate were acquired from Golden Bell, and 2,2’-azino-bis(3-ethylbenzothiazoline)-6-sulfonic acid (ABTS•+), 2,2-diphenyl-1-picrylhydrazyl (DPPH•), gallic acid, and potassium persulfate were supplied by Sigma-Aldrich.
Plant material
Five cultivars of banana (Musa spp.), roatán, morado, dominico, macho, and manzano, were obtained from a local market in Xalapa, Veracruz, Mexico.
Organic acid treatment and convection drying
The banana peels were manually separated and sumerged in different organic acids (15% w/v for 10 min, 20 min, and 30 min) (erythorbate, ascorbic, citric, and sorbic acid) to identify the organic acid that improves banana peel colorimetric conditions and prevents enzymatic browning. Colorimetric parameter as luminosity (L), which can acquire values from 0 to 100 (L meaning how light or dark a color is; the closer to 100 value, the darker the color is); saturation (C), which can also acquire values from 0 to 100 (C meaning how weak or intense a color is; the closer to 100 value 100, the more intense the color is); and hue angle (°h), this parameter acquires values from 0° to 360º (ºh tells us the color of the sample) were evaluated by using a Cielab colorimeter in conjunction with a Cielab color chart; mathematical formulas are shown below (Figure 1). The best treatment for subsequent uses of banana peel was then selected. After selecting the best treatment, the banana peels were cut into small pieces and convection dried at 60 °C using a drying oven (Binder) and ground in a coffee mill (Krups). The powder was vacuum sealed and stored at 4 °C until analysis.
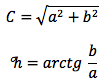
Source: Authors’ own elaboration.
Figure 1 Equations used for the calculation of C and ºh, where L, coordinates a and b are given by the equipment (López & Di Sarli, 2016).
Bromatological analysis of dry samples
Compositional analysis of dry sample was conducted. Samples were sent to Laboratorio de Alta Tecnología in Xalapa, Veracruz, Mexico, where protein values were determined following Mexican standard NMX-F-068-S-1980 (Diario Oficial de la Federación[DOF], 1980). Ether extract was determined by the Sohxlet method, according to Mexican standard NMX-F-089-S-1978 (DOF, 1978b). Crude fiber was determined by Mexican standard NMX-F-090-S-1978 (DOF, 1979), and ashes were quantified following Mexican standard NMX-F-066-S-1978 (DOF, 1978a).
Determination of essential fatty acids and amino acids in obtained banana peel flours.
The fatty acid profile was determined in five varieties of banana peel flour at Unidad de Desarrollo De Alimentos of the Instituto Tecnológico de Veracruz, Mexico. Lipids purication was carried out according to the modified method by Sánchez-Machado, Nuñez-Gastelum, Reyes-Moreno & López-Cervantes (2010): 1 μL of purified sample was injected into a gas chromatograph (Mod. HP 6890) equipped with a flame ionization detector and a column (HP-INNOWAX). The injection was done in split mode 20:1 at an initial furnace temperature of 120 °C, which was maintained for 2 min and then a ramp of 10 °C/min was programmed until reaching 220 °C and was maintained for 13 min.
The amino acid profile of the selected banana peel flour was determined at the Laboratorio de Química Analítica, Facultad de Ciencias Químicas, Benemérita Universidad Autónoma de Puebla. Gas chromatography with mass spectrometry was used to carry out the determination using a capillary column of 20 m and 0.18 mm ID, 0.18 μm. The temperature schedule was 100 °C (1 min), 35 °C/min to 290 °C (3 min), 40 °C/min to 360 °C; injector temperature was 250 °C.
Solvent extraction
The method used for solvent extraction was that proposed by González-Montelongo et al. (2010). Flour of each variety of banana peel (0.25 g) was extracted with 5 mL of a mixture of acetone solvent and water (1:1, v:v). The mixture was shaken by hand to homogenize samples, and the extractions were carried out in sealed tubes at room temperature (23 (C) during 2 h. Extracts were centrifuged at 5000 g for 20 min in a Labnet Prim R C-2500-R centrifuge. Antioxidant potential of each extraction was estimated instantly.
Extract antioxidant activity
To measure the quantity of compounds that have antioxidant activity present in banana peels, radical cation decolorization assay (ABTS(+), free radical decolorization assay (DPPH(), and total extractable phenol method were performed. All measurements to determine antioxidant activity were done with a LW Scientific UV-200-RS spectrophotometer.
Total extractable phenols
Total extractable phenols were determined following the method of Gallegos-Infante et al. (2010), modified. This characteristic was estimated by mixing 6 mL of deionized water, 50 (L extract, and 100 (L Folin-Ciocalteu reagent. After 6 min, 2 mL of 5% sodium carbonate solution were added to the mixture, which was adjusted to 1.4 mL with deionized water and allowed to stand at room temperature for 60 min in a dark room. Then, the absorbance was measured at 760 nm. Gallic acid was used as the standard.
2,2-diphenyl-1-picrylhydrazyl DPPH(.
Modified Blois (1958) assay was performed: 3 mL of a methanolic solution of DPPH( (with an absorbance of approximately 1.000) was added to 75 (L of the different extracts. Absorbance was measured at 515 nm after 15 min. Ascorbic acid was used as the standard.
2,2’-azino-bis(3-ethylbenzothiazoline)-6-sulfonic acid ABTS((.
Quantity of compounds with antioxidant activity was determined with the assay described by Re et al. (1999). ABTS(( radical was generated by mixing 50 mL of 7 mM ABTS solution with 0.88 mL of 140 mM potassium persulfate (both solutions were prepared with extract solvent). This solution was left to stand at room temperature without light for 16 h (absorbance of the prepared solution must be approximately 1.000). The antioxidant extracts (76 (L) were added to 3 mL of the ABTS(( radical once it formed, and absorbance was measured at 730 nm after 7 min. Trolox was used as the standard.
Product preparation and antioxidant and microbiological determination
At this stage, selected banana peel flour was mixed with corn, amaranth, and wheat flour, concentrations used in this work are shown in Table 1. Cookies, tortilla, and nacho style chips, hot cakes and tortillas were prepared.
Table 1 Mixtures used in percentage by weight for the preparation of different products.
| Banana Peel Flour (%) |
Corn Flour (%) |
Amaranth Flour (%) |
Wheat Flour (%) |
|
| Preparation 1 | 10 | 90 | 0 | 0 |
| Preparation 2 | 25 | 75 | 0 | 0 |
| Preparation 3 | 50 | 50 | 0 | 0 |
| Preparation 4 | 75 | 25 | 0 | 0 |
| Preparation 5 | 100 | 0 | 0 | 0 |
| Preparation 6 | 75 | 0 | 25 | 0 |
| Preparation 7 | 50 | 0 | 50 | 0 |
| Preparation 8 | 25 | 0 | 75 | 0 |
| Preparation 9 | 75 | 0 | 0 | 25 |
| Preparation 10 | 50 | 0 | 0 | 50 |
| Preparation 11 | 25 | 0 | 0 | 75 |
Source: Authors’ own elaboration.
The chosen product was subjected to antioxidant and microbiological determination. The methods performed were the same as in flours for the case of antioxidant compounds. To determine the presence of mesophilic aerobic organisms, the Mexican official standard NOM-092-SSA1-1995 (DOF, 1994) was used; and to determine fungi and yeasts, NOM-111-SSA1-1994 (DOF, 1995a) was carried out. Finally, to determine total coliforms NOM-113-SSA1-1994 (DOF, 1995b) was utilized.
Sensory evaluation of the selected product
The levels of consumer acceptance of the nacho snack product were determined at Unidad de Ciencias de la Salud de la Universidad Veracruzana, campus Xalapa (n = 141). Each participant evaluated four characteristics of the product (smell, color, taste, and texture) based on a 9-point scale (1 - I dislike extremely, 2 - I dislike a lot, 3 - I dislike moderately, 4 - I dislike slightly, 5 - I do not like nor dislike, 6 - I like it slightly, 7 - I like moderately, 8 - I like very much, 9 - I like it extremely).
Statistical analysis
Data analysis was carried out with Minitab software 16. An analysis of variance was applied to compositional and antioxidant determination results to identify differences among banana peel varieties. Analyses of variance were also carried out on data resulting from the product analysis. In both cases the Tukey test was applied using a 5% significance level.
Results and Discussion
Organic acid treatment and convection drying
This step was useful to know which organic acid improved the colorimetric conditions of the banana peels by controlling the browning process that undergoes in a natural way in them and also to improve their appearance for later uses given to the peels in this research. Immersing banana peels in solutions containing organic acids produced a variation in the values of the colorimetric parameters evaluated. Table 2 shows the results of the colorimetric analysis of roatán banana peel flour obtained in the different treatments with organic acids.
Table 2 Results obtained by colorimetry of L, C, and ºh for roatán banana peel flour in different organic acid immersions.
| Tratamiento | L | C | ºh |
| Control 10 min | 37.760 ± 0.330c | 18.142 ± 1.186ef | 181.233 ± 0.012cde |
| Control 20 min | 34.980 ± 0.170de | 17.243 ± 1.785fg | 181.244 ± 0.016abcd |
| Control 30 min | 40.450 ± 0.060b | 21.054 ± 1.124de | 181.309 ± 0.034a |
| Ascorbic acid 10 min | 37.060 ± 0.100c | 23.311 ± 0.934bcd | 181.246 ± 0.016abcd |
| Ascorbic acid 20 min | 35.420 ± 0.390d | 28.813 ± 0.220a | 181.170 ± 0.002efg |
| Ascorbic acid 30 min | 35.300 ± 0.220d | 26.040 ± 0.799ab | 181.098 ± 0.015hi |
| Sorbic acid 10 min | 32.170 ± 0.210g | 14.357 ± 1.714gh | 181.198 ± 0.003def |
| Sorbic acid 20 min | 27.250 ± 0.250h | 13.345 ± 1.751h | 181.160 ± 0.039fgh |
| Sorbic acid 30 min | 31.580 ± 0.370g | 13.561 ± 0.842h | 181.242 ± 0.040bcd |
| Citric acid 10 min | 31.480 ± 0.140g | 15.520 ± 0.666fgh | 181.061 ± 0.036i |
| Citric acid 20 min | 34.360 ± 0.360ef | 15.173 ± 1.137fgh | 181.106 ± 0.024ghi |
| Citric acid 30 min | 33.750 ± 0.330f | 15.588 ± 1.880fgh | 181.151 ± 0.013fgh |
| Erythorbate 10 min | 42.840 ± 0.120a | 22.827 ± 0.927bcd | 181.294 ± 0.003abc |
| Erythorbate 20 min | 42.940 ± 0.250a | 24.763 ± 1.028bc | 181.306 ± 0.006ab |
| Erythorbate 30 min | 40.510 ± 0.110b | 21.306 ± 0.413cde | 181.239 ± 0.009cd |
Different letters indicate that there are significant differences (p < 0.05) between columns.
Source: Authors’ own elaboration.
For ascorbic acid L value, differences are shown as immersion time increases: parameter C for this treatment has high (C) at 20 min and is higher than with the other treatments. The ºh values for the three treatments with this acid indicate that hue becomes greener with increasing immersion time. Using ascorbic acid, values of L, C, and h are significantly different from control values as immersion time decreases.
Citric acid generated L values lower than those of the control. Chroma values were significantly different among the three treatments, but not between 10 min and 20 min. No significant differences in h are shown as time of citric acid immersion increases.
According to Table 2, erythorbate L values are closer to 50 as time immersion decreased, while for parameter C higher values were observed than those of the control, but they were not significantly different from ascorbic acid after 10 min immersion. There were no significant differences in ºh among three treatments of both erythorbate and ascorbic acid. Despite the good performance of erythorbate, its use is not permitted in flour by Codex Alimentarius and Mexican official standard NOM-147-SSA1-1996 (DOF, 1999). Ascorbic acid presented similar results to erythorbate; besides, it is more economical and its use in foods is allowed by Mexican normativity.
Compositional analysis of dry samples
The results obtained for protein are shown in Table 3. Based on analysis of variance, there were significant differences in the percentage of proteins among the five varieties of analyzed banana peels. The macho variety showed the highest value (7.3%), exceeding the values for pulp of the same variety (3.3%) obtained by Juarez-Garcia, Agama-Acevedo, Sayago-Ayerdi, Rodríguez-Ambriz & Bello-Perez (2006). Banana pulp has a protein content of 1% (Tobin & Muller, 1988), and flours made from banana pulp, according to Da Mota, Lajolo, Ciacco, Cordenunsi & Brazil (2000), had protein percentage values between 2.5%-3.3%. These results reveal that flours made from banana peel have a higher concentration of protein than flour made from the pulp. The production of flour by mixing pulp and peel has been studied by Gil et al. (2011), who observed a protein content of 5.15%. The values obtained in our study are similar to those obtained by Granda et al. (2005) of nearly 7% protein in ripe banana peel. Happi et al. (2007) reported the presence of 8% to 11% protein, of which the main essential amino acids present were leucine, valine, phenylalanine, and threonine.
Table 3 Results of compositional analysis performed on different varieties of banana peel.
| Analysis | Roatán | Macho | Dominico | Manzano | Morado |
| Moisture WB (%) |
89.679 ± 0.115a |
83.378 ± 0.451c |
87.579 ± 0.947b |
84.087 ± 0.526c |
86.338 ± 0.283b |
| Protein (%) |
6.9c | 7.3a | 7.1b | 6.2d | 5e |
| Fiber (%) |
9.729 ± 0.556b |
8.293 ± 0.341c |
12.332 ± 0.293a |
8.102 ± 0.958c |
8.828 ± 0.010bc |
| Ash (%) | 13.078 ± 0.091a |
11.626 ± 0.137b |
11.496 ± 0.201b |
10.474 ± 0.104c |
9.457 ± 0.217d |
| Ether extract (%) |
12.554 ± 0.057b |
15.550 ± 0.274a |
12.289 ± 0.173b |
15.662 ± 0.086a |
7.908 ± 0.345c |
| Carbohydrates (%) |
57.738c | 57.229d | 56.781e | 59.561b | 68.804a |
Moisture values were obtained on wet basis (WB), while the other analyses were obtained on dry basis. Different letters indicate that there are significant differences between rows.
Source: Authors’ own elaboration.
In the case of crude fiber, there are significant differences (p < 0.05) among the peels of macho, roatán, and dominico banana varieties. Morado banana peel flour was not significantly different from roatán, macho, and manzano, but it was different from dominico. These values range between 8.8%-12%. Flour from dominico bananas variety had the highest content of fiber. In general, banana peel has a higher percentage of fiber than other foods such as corn (Jin-Shun, Xiao-yan, Xiao-pan & Lin-Shuang, 2017).
Banana peel has a high content of ash. Islas-Hernández et al. (2007) found a percentage in banana pulp flour of 4.4%. Ripe banana peel contains a higher concentration of ash than green banana peel (Da Mota et al., 2000); this may be due to the migration of moisture from the pulp to the skin during the ripening process, serving as a vehicle for the minerals present in the pulp (Casallas, 2014). Wheat flour contains percentages of ash below 1% (Aydin, Paulsen & Smulders, 2009), which is less than this study’s results. Ugye, Nyiaatagher & Studies (2009) obtained 8.5% ash for banana peel; they also determined high percentages of minerals: potassium, calcium, sodium, iron, and manganese.
Table 3 shows that ether extract was not significantly different between dominico and roatán flours but was significantly different from macho and manzano, (p < 0.05), and morado flour. According to the analysis of variance, macho and morado flours contain a higher percentage of ether extract. It was observed in all samples that the solvent turned yellow due to the presence of pigments from banana peels (Londoño, 2012). Results obtained in our study are higher than those presented by Granda, Mejía & Jiménez (2005) for green and ripe banana peels, with values of 8.3% and 7.0%, respectively. Wachisasiri, Julakarangka & Wanlapa (2009) obtained a value (13.1%) similar to those listed here for banana peel. Banana peel is rich in polyunsaturated fatty acids, especially linoleic acid and α-linolenic acid (Happi et al., 2007).
The percentage of carbohydrates was obtained by difference for each one of the banana peel varieties. The samples, however, did not have significantly different (p < 0.05) percentages. The presence of carbohydrates is higher in peel than in pulp and is composed mainly of sugars and starch (Arora, Choudhary, Agarwal & Singh, 2008). Ugye et al. (2009) report 59% carbohydrates in banana peel; this value was also obtained for the manzano variety in this study. The flour of the banana peel varieties studied contains a lower percentage of carbohydrates than wheat flour (73.15%), given by Horsfall, Eboh & Nwaojigwa (2007).
Fatty acid composition of the different varieties of banana peel is shown in Table 4. Linoleic acid (ω-6) and linolenic acid (ω-3) occupy over 40% of the total amount of fatty acids in all varieties. The results of contents of linoleic and linolenic acids results are similar to those obtained in three varieties of banana peel by Happi et al. (2007). A high presence of palmitic acid is also observed, which is considered to play an important role in the responses by the hypothalamus to high fat diets (Cheng et al., 2015). In order to maintain a membrane phospholipids balance, an optimal intake of palmitic acid may be crucial (Carta, Murru, Banni & Manca, 2017); besides, saturated fats may favorably increase blood HDL-cholesterol levels without significant changes of the total HDL-Cholesterol ratio. Finally, palmitic acid, among all, may have special structural and functional roles in utero and in infancy (Agostini, Moreno & Shamir, 2015).
Table 4 Percentage of fatty acids present in different banana peel flours (n = 3).
| Banana Peel Variety | |||||
| Fatty Acid (%) | Roatán | Macho | Morado | Manzano | Dominico |
| Lauric Acid C12:0 |
1.08±0.05a | 0.89±0.04ab | 0.53±0.27c | 0.56±0.1bc | 0.55±0.04bc |
| Miristic Acid
C14:0 |
1.34±0.01a | 0.91±0.06bc | 1.05±0.11b | 0.8±0.05c | 0.89±0.01bc |
| Palmitic Acid C16:0 |
41.46±0.93a | 38.23±1.39b | 38.67±1.07b | 39.76±0.7ab | 35.04±0.76c |
| Stearic Acid C18:0 |
3.78±0.66a | 2.6±0.22b | 2.22±0.05b | 2.16±0.01b | 2.11±0.27b |
| Oleic Acid C18:1 |
4.51±1.15a | 3.77±0.06a | 2.72±0.09a | 3.2±0.4a | 3.29±0.94a |
| Linoleic Acid C18:2 (ω-6) |
25.65±0.08ab | 22.89±0.23b | 34.24±0.94a | 33.71±7.35a | 30.62±0.13ab |
| Linolenic Acid C18:3 (ω-3) |
22.19±0.74cd | 30.85±1.24a | 20.59±0.33d | 24.82±0.18bc | 27.51±1.79b |
Different letters indicate significant differences between rows (n = 3).
Source: Authors’ own elaboration.
Macho banana peel flour (MBPF) was selected to determine essential amino acids, almost all are present in the flour analyzed; the exceptions are tryptophan and histidine. The essential amino acids with greater presence were threonine, valine, isoleucine, and leucine (Table 5). Macho banana peel contains eight out of 10 essential amino acids which are less than the essential aminoacids present in other foods, for example, corn (nine essential amino acids) (El-Shafei, Abbassi, Bassily & Said, 1983) and wheat (nine essential aminoacids) (McElroy, Clandinin, Labay & Pethybridge, 1949). Macho banana peel protein could be consisered as a high biological value protein, because it contains almost all the group of aminoacids (Instituto Tomás Pascual Sanz, 2010), but more studies should be performed in order to confirm this information.
Table 5 Amino acids present in flour made from macho banana peel variety (n = 2).
| Non-essential amino acids |
g of amino acid/100 g of proteín |
Essential amino acids |
g of amino acid/100 g of proteín |
| Aspartic acid | 0.291(0.06 | Threonine | 0.137(0.03 |
| Serine | 0.165(0.03 | Valine | 0.217(0.04 |
| Glutamic acid | 0.450(0.09 | Methionine | 0.045(0.09 |
| Proline | 0.166(0.03 | Isoleucine | 0.123(0.02 |
| Glycine | 0.217(0.04 | Leucine | 0.223(0.02 |
| Alanine | 0.251(0.05 | Phenylalanine | 0.064(0.01 |
| Cysteine | 0.060(0.01 | Lysine | 0.098(0.02 |
| Arginine | 0.077(0.02 |
Source: Authors’ own elaboration.
Extract compunds with antioxidant activity
Total phenol values of the different varieties of banana peel in this research ranged from 9.53 mg GAE/g - 67.4 mg GAE/g sample; dominico banana peel had the highest value, followed by manzano>macho>roatán>morado (Table 6). According to Someya et al. (2002), phenolic compounds are more abundant in peel than in pulp, because they accumulate in dermal tissues of the plant body due to their role as protection against ultraviolet radiation. They also act as attractants during fruit dispersion and as chemical advocates against pathogens and predators.
Table 6 Antioxidant capacity results for the different varieties of banana peel.
| Total phenols | DPPH+ | ABTS˙+ | |
| Variety | mg GAE/ g of sample | mg AAE/ g of sample | mg TE/ g of sample |
| Dominico | 67.4 ± 0.2a | 63.4 ± 6.5a | 203.49 ± 17a |
| Roatán | 13.43 ± 0.7c | 6.7 ± 0.9c | 32.54 ± 0.14c |
| Macho | 13.84 ± 0.04c | 51.2 ± 0.9b | 55.46 ± 8.92b |
| Manzano | 24.2 ± 1.3b | 65.5 ± 5.6a | 74.59 ± 13.73b |
| Morado | 9.53 ± 0.6d | 55.4 ± 1.7b | 58.61 ± 0.64b |
Note: GAE = Gallic acid equivalents, AAE = Ascorbic acid equivalents, and TE = Trolox equivalents. Different letters indicate significant differences between columns.
Source: Authors’ own elaboration.
DPPH( and ABTS(+ decolorization assays (Table 6) showed higher concentrations of antioxidants in dominico and manzano banana peels. Using acetone in the extraction mixture increased solvent polarity, helping extraction of antioxidant compounds present in the peel, such as flavanones, flavonoids, and polyphenols (Saif & Hashinanda, 2005) due to the large number of hydroxyl groups in the molecular structure which are responsible for capturing free radicals (Kumarappan, Thialagam & Mandal, 2012). According to studies by Kanazawa & Sakakibara (2000), the main water-soluble antioxidant present in the banana peel is dopamine (100 mg/100 g of simple) whose antioxidant capacity is considered like that of ascorbic acid. The presence of dopamine in banana peel can help protect humans against damage to the intestinal mucosa by modulating eicosanoid synthesis (Ayano, 2016).
ABTS(+ radical bleaching occurs due to transfer of hydrogen atoms or transfer of electrons in order to stabilize this free radical (Londoño, 2012). ABTS(+ values obtained for the different varieties are higher than those obtained using the DPPH( method because the molecule of ABTS(+ is a completely flat structure that, unlike DPPH radical, reacts readily with reducing agents (Rojano et al., 2009).
Someya et al. (2002) attributes antioxidant capacity of banana peel to the gallocatechin compound. This molecule belongs to the group of catechins and exerts a strong antioxidant effect against lipid peroxidation and protective effects against cardiovascular disease and cancer as well (Kondo, Kurihara, Miyata, Suzuki & Toyoda, 1999).
In the specific case of morado banana peel, antioxidant capacity can be attributed to anthocyanins, which are responsible for the characteristic purple color of this variety, specifically delphinidin and cyanidin, which were determined in a variety similar to morado banana (Seymour, Taylor & Tucker, 1993).
Product making and antioxidant and microbiological determination
Based on the compositional and antioxidant results of the different varieties of banana peel studied, MBPF was selected for its use in preparing food products. Moreover, 80 g flour per kg of macho banana can be obtained. Formulations were made in conjunction with amaranth, wheat, and corn flours (CF). Five different products were developed: cookies, tortilla and nacho style chips, hot cakes and tortillas. No favorable or suitable sensory properties were observed at concentrations of 50%, 75%, and 100% MBPF in products made in conjunction with corn and wheat flour. However, with concentrations of less than 25%, organoleptic properties were improved, with the exception of flavor which was still perceived as bitter. This feature is ascribed to the presence of phenolic compounds in banana peel; they are also responsible for bitterness in other products such as wine (Drago, 2007).
In the formulations with 10% banana peel flour combined with CF, organoleptic properties of color and texture were more acceptable. Carboxymethylcellulose (CMC) 0.5% was used to improve crispiness of nacho chips; this additive also helped to form a stronger dough as well due to the polymers of starch present in the CF (Bello-Pérez, Contreras-Ramos, Romero-Manila, Solorza-Feria & Jiménez-Aparicio, 2002). Also, water retention improved (Bersuder, Hole & Smith, 2001), and sesame at concentrations between 10% - 25% was added to provide a better taste. A range of products, whose stability and organoleptic properties favor consumption of banana peel flour in functional foods, was obtained.
Products developed with amaranth flour in the formulation mixtures had a chewy consistency which prevented the proper development of different types of foods cooked with other formulations, unlike the other two flours.
Results obtained for DPPH(, ABTS(⁺ assays, and total phenol method in the nacho snack product are shown in Table 7. For the case of total phenols, 10% MBPF nacho snack product showed higher presence of these compounds than commercial nachos and nachos made with CF. The antioxidant capacity of MBPP nachos is attributed to tannins, specifically gallocathechin (Someya et al., 2002). Another group of substances with antioxidant capacity present in the final product may be flavonoids, because they have proven to be stable at high temperatures and with water deficit (Barron-Yañez, García-Mateos, Soto-Hernández, Colinas-León & Kite, 2011). Flavonoids have been linked to treatment of cardiovascular disease and for liver protection, and used as antiallergic, anti-cancer, and anti-thrombotic (Gracia, 2007). In the case of DPPH( radical, the nacho snack made with 10% MBPF showed the highest value relative to the other two samples. The same behavior is observed with ABTS˙⁺ assay, except that, for this trial, the commercial nacho snack and CF nacho were not statistically different. Increased antioxidant capacity is due to the use of banana peel flour in the product.
Table 7 Antioxidant capacity results for the different varieties of nacho snack product.
| Total phenols | DPPH+ | ABTS˙+ | |
| Sample | mg GAE/ g of sample | mg AAE/ g of sample | mg TE/ g of sample |
| Nacho MBPF (10%) |
2.56 ± 0.04a | 2.14 ± 0.13a | 5.31 ± 0.02a |
| Commercial nacho snack |
0.78 ± 0.005b | 0.62 ± 0.03b | 0.79 ± 0.3b |
| Nacho CF | 0.68 ± 005c | 0.2 ± 0.07c | 1.02 ± 0.15b |
Note: MBPF = macho banana peel flour, CF = corn flour, GAE = Gallic acid equivalents, AAE = Ascorbic acid equivalents, and TE = Trolox equivalents. Different letters indicate significant differences between columns.
Source: Authors’ own elaboration.
Determinations made in the 10% MBPF nacho snack product did not show presence of aerobic mesophiles, indicating that there was no contamination during processing. Molds and yeasts were also absent so that product decomposition by such microorganisms would not occur, and toxigenic fungi that may be present in processed dried products such as flour would not be developing (Doolotkeldieva, 2010). Absence of total coliforms confirms correct disinfection during banana peel washing, as these microorganisms indicate efficiency, sanitation and sanitary quality in water and vegetables. Overall, there were no bacteria of the genera Escherichia, Enterobacter, Klebsiella, and Citrobacter (Ashbolt, Grabow & Snozzi, 2001).
Sensory evaluation of the nacho snack showed that texture was the most important characteristic, and its results were I dislike slightly, while the other characteristics such as odor, flavor, and color received a score corresponding to I do not like nor dislike. This result occurred because the nacho product became moist during the sensory evaluation.
Conclusions
Banana peels immersed in a solution of 10% ascorbic acid for 20 min maintain the most suitable colorimetric qualities after drying by convection.
The studied flours have high protein, fat, and fiber contents as well as presence of fatty acids (linoleic and linolenic acids) and amino acids (threonine, valine, leucine, and isoleucine). Macho banana peel showed the highest fiber, protein, and ether extract values.
Banana peel flours have compounds with antioxidant capacity as does the nacho product made from MBPF as compared with commercial nachos.
With banana peel flour different products can be developed. This can enhance food availability in the Mexican market and can be consumed by a wide range of the population. It would also reduce pollution caused by this by-product.











 nueva página del texto (beta)
nueva página del texto (beta)


