Introduction
Fluoride contamination of groundwater by natural as well as anthropogenic sources is a major problem worldwide (Cheng, Meng, Jing & Hao, 2014). The maximum contaminant level (MCL) with fluoride in drinking water, established by the US EPA, is 4 mg/L (Tang, Guan, Su, Gao & Wang, 2009). The World Health Organization (WHO) guideline value of fluoride in drinking water, which was also adopted by the Mexican legislation, is 1.5 mg/L (Diario Oficial de la Federación[DOF], 2001; WHO, 2004). Above 1.5 mg/L, mottling of teeth may occur to an objectionable degree. Concentrations between 3 mg/L and 6 mg/L may cause skeletal fluorosis. The continuous consumption of water with fluoride in exceeding levels of 10 mg/L can result in crippling fluorosis (Yeung, 2008). Fluoride is one of the four most important polluting chemicals, in addition to arsenic, nitrate, and chromium (VI), which cause a large scale of health problems to humans through drinking water exposure (Tang et al., 2009).
It is significant to consider that fluorine content in groundwater is above the WHO standards in many countries and regions. Excessive fluoride concentrations have been reported in groundwaters that belong to more than 10 developed and developing countries (Arora & Maheshwari, 2006; Cheng et al., 2014), including Mexico, where several states are facing fluorosis problems. The most affected states are Aguascalientes, Coahuila, Chihuahua, Durango, Guanajuato, Hidalgo, Jalisco, San Luis Potosi, Sonora, and Zacatecas. In the Bajio region, the fluoride content is found in the range 2.5 mg/L-5.5 mg/L. In this region, about 75% of the people consume groundwater for drinking purposes, exceeding the WHO guidelines (Rosales, Coreño & Nava, 2018). Worldwide, it is estimated that more than 70 million people are suffering from fluorosis (Sehn, 2008).
Fluoride can be removed from water by employing a number of processes, including adsorption and biosorption (Chatterjee & De, 2014); ion-exchange (Rogoziński, Tyszkiewicz, Karasiński, Żelechower & Szala, 2016); chemical precipitation, which may include electro-coagulation/flotation (Drouiche et al., 2012); and membrane processes, such as reverse osmosis (Shen, Richards & Schäfer, 2016), dialysis (Kir & Alkan, 2006), nano-filtration (Jadhav, Marathe & Rathod, 2016), and electrodialysis (Arahman, Mulyati, Lubis, Takagi & Matsuyama, 2016). Most methods for removing fluoride have drawbacks, including high-initial costs, lack of selectivity, low capacity, and difficulty in operation or regeneration.
Activated alumina (AA) has been used as an effective and inexpensive adsorbent for fluoride removal (Leyva-Ramos et al., 2008; Rafique, Awan, Wasti, Qazi & Arshad, 2012), that is because of its strong affinity for fluoride, availability in the market (Shen et al., 2016), high thermal stability (Xu et al., 2017), mechanical strength, porosity, large surface area, and surface chemical properties that can be either acidic or basic, depending on the transition alumina structure and the degree of hydration/hydroxylation of the surface.
Adsorption is one of the most commonly used techniques for fluoride removal from water, and the effectiveness of adsorption techniques is greatly dependent on the physical-chemical properties of the adsorptive materials. A wide variety of adsorption materials has been tested for the removal of fluoride from water, such as montmorillonite (Tor, 2006), bentonite (Ma, Shi, Zheng, Ma & Gao, 2011), hydrocalumite (Guo & Tian, 2013), bauxite and charcoal (Singh, Singh & Singh, 2016), biopolymer (Singh, Singh & Singh, 2016), Al2O3/CNT (Li et al., 2001), activated alumina (AA) (Ghorai & Pant, 2005), bone char (Nigri, Bhatnagar & Ferreira-Rocha, 2017), zeolite (Gómez, Pérez, García, Chebude & Díaz,2013), as well as rice husk and calcium (Bhatnagar, Kumar & Sillanpää, 2011). There are other materials that have been reported as useful for fluoride removal. However, their low adsorption capacities and poor adsorption kinetics demonstrate they still need improvement. An ideal adsorbent should have uniformly accessible pores, a high surface area, and physical-chemical stability. Due to that, adsorption processes involve the passage of the water through a contact bed where fluoride is removed by ion exchange or surface chemical reaction.
The objective of this work is to compare the removal of fluoride ions from water using a γ-Al2O3 synthesized-agglomerate and a commercial AA, as well as the effects of the solution pH and temperature in the adsorption capacity. The average particle diameter of both materials was 3 mm, and they were systematically characterized with XRD, adsorption/desorption of N2, FE-SEM/EDS, 27Al MAS NMR, FT-IR pyridine adsorption, and zeta potential analysis and particle size. That is to understand their physical-chemical properties and the adsorption of fluoride in the alumina.
Materials and Methods
Synthesis and pseudoboehmite agglomeration
In the present study, the starting materials to obtain hydrated alumina (γ-Al2O3) are Al2(SO4)3•18H2O, with a purity of 95% (by weight, wt.), technical grade, Alfa-Omega Chemical S. A., HNO3 (karal 70%), C6H14 (J. T. Baker 99.8%), NH4OH (Karal 30%), and anhydrous NH3 gas (Praxair, 99.98% [wt.]) as precipitant.
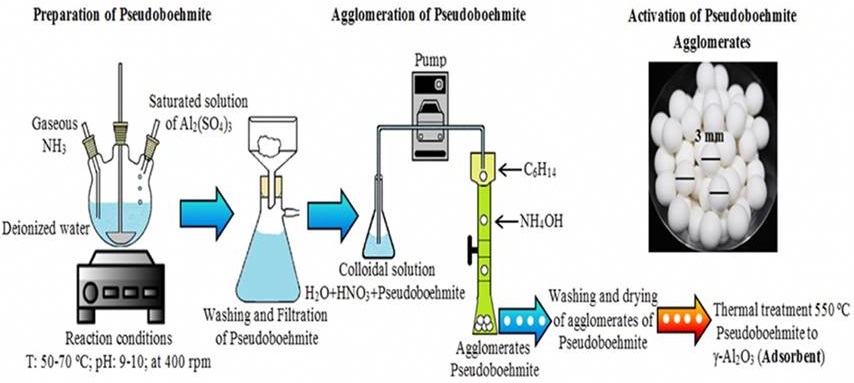
The preparation procedure flow chart is depicted in Figure 1. Al2(SO4)3•18H2O was dissolved into deionized water and filtered for the removal of insoluble impurities. The precursors were prepared by dropping the saturated solution of aluminum sulfate into the solution of water and ammonia gas. That was done under rigorous magnetic stirring at a temperature range of 50 °C-70 ºC. The precipitated solution was filtered, and the precipitate of pseudoboehmite was rinsed with distilled water two times and then dried at 110 °C for 12 h. The agglomeration of pseudoboehmite in solid spheres of 3 mm diameter was obtained from a colloidal solution. The colloidal solution was prepared using pseudoboehmite, nitric acid, and distilled water. This solution was then dripped into a glass column with 15 mL of C6H14 and 25 mL of NH4OH for making the agglomeration. The phase change from pseudoboehmite agglomerates to γ-Al2O3 was performed by a thermal treatment at 550 °C for 6 h. The chemical reactions (1), (2), and (3) occurred during the synthesis and the gamma phase was obtained:
Precipitation step:
Calcination step:
The AA used in this study is commercial spherical with a diameter of 3 mm (Alquimia Mexicana S. de R. L.).
Surface characterization of the adsorbents
The γ-Al2O3 and AA samples were dispersed into distilled water. The solutions pH were adjusted to the following ranges for analysis: between 2 mM and 12 mM when using KCl, and 10 mM when using NaOH. The zeta potential and particle size were analyzed using an electroacoustic technique with a particle size analyzer (AcoustoSizer II, ESA; Colloidal Dynamics, USA) at 25 °C ± 0.2 °C. The textural properties were characterized by N2 adsorption (Micromeritics, ASAP 2010). The samples were vacuum degassed at 200 °C for 3 h. Nitrogen adsorption isotherms were measured at a liquid N2 temperature (77 K) and N2 pressures ranging from 10-6 P/P0 to 1.0 P/P0. The surface area was calculated according to the Brunauer-Emmett-Teller (BET) method, and the pore size distribution was obtained according to the Barret-Joyner-Halenda (BJH) method. The crystalline properties of the samples were determined at room temperature by using X-ray powder diffraction. A Siemens D-500 diffractometer equipped with a CuKα radiation anode was used for these measurements, under the following conditions: a sweep of 5°-80º at an angle of 2θ, with a wavelength of k = 1.54 Å, a current of 30 kV, and a current of 20 mA. Solid-state nuclear magnetic resonance (NMR) measurements were made with a Bruker Avance III HD 400 spectrometer in a nominal field of 9.4 T at a Larmor frequency of 104 MHz. The 27Al spectra were observed using a magic angle spinning (MAS) probe. For this process, rotors of 4 mm were used, as well as a rotation frequency of approximately 11 kHz, using as reference a solid sample of Al(NO3)3• 9H2O (27Al, d = 0.0 ppm). The nature of acid sites was determined by pyridine adsorbed Fourier transformed infrared (pyridine FT-IR) spectroscopy in a FT-IR Nicolet 470 instrument with a resolution of 1 cm-1. The samples were dehydrated at 300 °C for 1 h under vacuum. This was followed by adsorption of purified pyridine at room temperature. Then, the sample was evacuated at room temperature and later at 200 °C. The pyridine-adsorbed IR spectra were recorded. The surface morphology was examined using JEOL JSM-7600-F Field-Emission scanning electron microscopy. This was equipped with an energy dispersive X-ray spectrometer (FE-SEM/EDS) to determine the chemical composition.
Batch adsorption experiments
F− adsorption kinetics experiments were conducted by mixing suspensions containing 10 mg/L, 30 mg/L, 50 mg/L, 100 mg/L, and 150 mg/L of fluoride ion in deionized water with 0.3 grams of adsorbent material to the levels of pH of 5, 7, and 9, which was adjusted with hydrochloric acid. Samples were left for five days at different temperatures (25 °C and 35 °C); this was because the groundwater temperature varies from 25 °C to 50 ºC. The highest temperatures are located near faults and fractures. High temperatures match with the highest groundwater concentrations of As and F- in Guanajuato, Mexico (Morales, Villanueva-Estrada, Rodríguez & Armienta, 2015). The samples in solution were analyzed immediately at the desired mixing time. The residual F− concentration in solution samples was measured using a fluoride selective electrode (Thermo Scientific Orion 4-Star Plus). That was in accordance with the standard method (Wang et al. 2014). After five days, the solution was filtered, and the final concentrations of fluoride ions were determined. It was considered that equilibrium was reached when the fluoride concentration obtained was the same, or that there was a difference of 5% over the earlier recorded period.
The adsorption isotherms were obtained by mixing solutions containing 7.5 g/L of γ-Al2O3 or AA, and 0 to 20 mg/L of F− for 2 h. The suspension also contained 1:1 volume of total ionic strength adjustment buffer (TISAB II) solution to control the suspension of pH at 7.0 ± 0.2 and the ionic strength at 0.04 M., a liter of the TISAB II solution contained, 1% acetic acid (C2H4O2), 8% sodium acetate (C2H3NaO2), 6% sodium chloride (NaCl), <1% Trans-1,2-Diamino cyclohexane tetra acetic acid (CDTA), and >84% deionized water (H2O). After mixing, the solutions samples were analyzed to obtain the equilibrium F− concentration.
Resuls and Discussion
Powder X-ray diffraction (XRD)
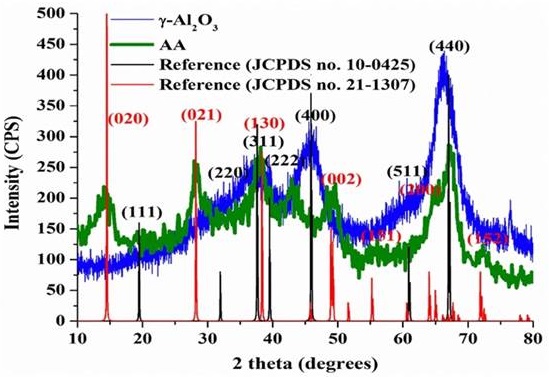
The crystalline properties of synthesized and agglomerate alumina (γ-Al2O3) and AA are shown by the XRD pattern of high angle (10°-80° 2θ) in Figure 2. The material of γ-Al2O3 exhibits some broad crystalline peaks around 35°-40° and especially at 45° and 67º 2θ. This is due to the reflections (of X-rays) from the 311, 400, and 440 crystal planes, in relation with the cubic γ-Al2O3, according to JCPDS Card No. 10-0425 (Renuka, Shijina & Praveen, 2012). This revealed that the γ-Al2O3 presented a low degree of crystallization. However, the XRD pattern for the AA demonstrated a significant number of peaks, which suggests that more than one phase is present. On the other hand, other peaks that do not fit within the phase γ-Al2O3 can be indexed as pseudoboehmite (γ-AlOOH) phase (JCPDS Card No. 21-1307) (Barzegar & Ebadzadeh, 2011). Therefore, the AA material may be composed of γ-Al2O3 and pseudoboehmite such as in a main phase, as shown in Figure 2 (gray). Due to this, the AA material showed a greater degree of crystallinity compared with γ-Al2O3.
Adsorption/desorption of N2
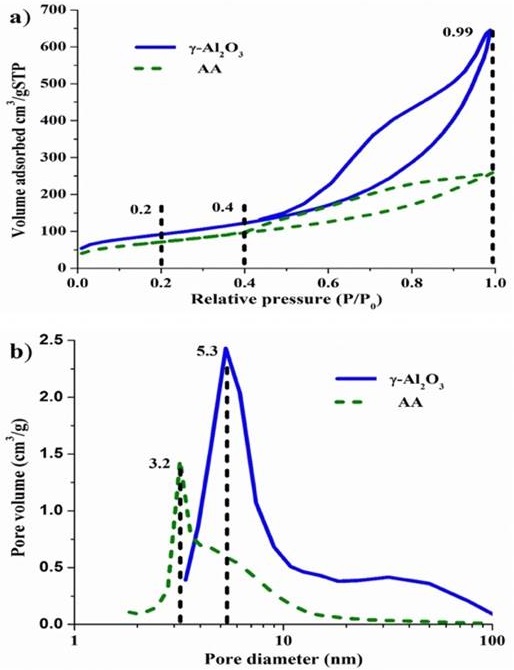
Figure 3 illustrates the N2 adsorption-desorption isotherms and the Barrett-Joyner-Halenda (BJH) pore size distribution curve of γ-Al2O3 and AA. According to the classification made by IUPAC (Sing et al., 1985), the N2 adsorption-desorption isotherms of γ-Al2O3 and AA are found to be of a type IV. Two well distinguished regions in the adsorption isotherm are evident: (i) monolayer-multilayer adsorption and (ii) capillary condensation. The first increase in adsorption at relative pressure P/P0 < 0.2 is due to a multilayer adsorption on the surface, while the second increase at P/P0 = 0.42-0.97 arises from capillary condensation in the mesopores with nitrogen multilayers adsorbed on the inner surface (Gregg, Sing & Salzberg, 1967). These materials show irregular shape isotherms with the hysteresis loop type E, which indicate that the pores in the material have an inkwell-type shape. As seen in Figure 3b, a relatively narrow pore size distribution is detected, and the average BJH pore diameter, calculated by using the adsorption branch data of the N2 adsorption-desorption isotherms, is about 5.8 nm; this measure belongs to the γ-Al2O3, and 3.5 nm is the measure for the AA. These values are congruent due to the higher crystallinity that AA presents, as shown in Figure 2. Table 1 shows the results of the surface area BET, the pore size distribution, as well as the average pore diameter of γ-Al2O3 and AA.
Source: Author’s own elaboration.
Figure 3 Graphs a) N2 adsorption-desorption isotherm and b) pore size distribution curve of the γ-Al2O3 and AA.
Table 1 Specific surface area, average pore volume and average pore size of γ-Al2O3 and AA.
| Sample | SSA (m2•g-1)a | VP (cm3 •g-1)b | DP (nm)c |
|---|---|---|---|
| γ-Al2O3 | 256 | 0.76 | 5.8 |
| AA | 206 | 0.39 | 3.5 |
a Calculated with the BET-equation.
b Measured at p/p0 = 0.99.
c Pore diameter determined using desorption isotherms by the BJH method.
Source: Author’s own elaboration.
Morphology and surface composition of the adsorbents
Figure 4 shows the FE-SEM images of γ-Al2O3 and AA. The powder from the γ-Al2O3 obtained through the process of hydrolysis-precipitation and agglomerates consists of nanofibers, which are about 100 nm in length. That tends to form amorphous agglomerates due to their high surface energy. Therefore, high porosity is generated. In comparison with the morphology of the commercial AA powder (Figure 4b), the powder exhibits a higher degree of agglomeration in the surface and, consequently, it has a lot of small pores.
The FE-SEM images of γ-Al2O3 and AA (Figure. 4) show the spots in places where the electron beam determined the elemental EDS (energy dispersive X-ray spectroscopy) through the analysis. The EDS is employed to identify the elemental compositions from both alumina materials. This technique can be applied to investigate the properties of a broad range of chemicals. Elemental concentrations of aluminum and oxygen are investigated using EDS at one point from both samples. The weight concentrations of both samples are compared in Table 2. The analysis confirms the high purity of the alumina samples. The values obtained in the elemental chemical composition of aluminum and oxygen from both samples are congruent with values reported in the literature related to pure alumina (Ilyas, Pendyala, Narahari & Susin, 2017).
27 Al MAS NMR
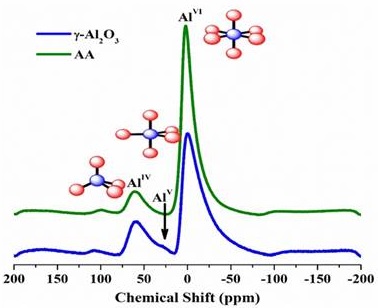
Figure 5 displays the 27Al MAS NMR spectra that belong to the γ-Al2O3 and AA. The first one is Figure 5a, which exhibits characteristic profiles of γ-Al2O3, such as signals between 0 ppm and 5 ppm from the octahedral aluminum AlVI sites. Moreover, the presence of signals in the region that are around 34 ppm is evident. This can be associated to aluminum in pentacoordinate sites AlV, and signals between 58 ppm and 62 ppm from the sites of aluminum in tetrahedral geometry AlIV. The second material (Figure 5b) exhibits signals between 0 ppm and 5 ppm from the octahedral aluminum AlVI sites, and it signals between 58 ppm and 62 ppm from the aluminum in tetrahedral geometry AlIV sites (Hill, Bastow, Celotto & Hill, 2007; Li, Liu & Liu, 2013). For the sample AA, no resonance related to AlV was found. The integration reveals values between 15% and 20% from the tetrahedral sites, which are in accordance with the γ-Al2O3 model (Chagas et al., 2014). The γ-Al2O3, as seen in Figure 5a, exhibited aluminum pentacoordinate sites AlV which, according to the literature, arise from the structure defects from γ-Al2O3. Also, a high AlV amount implies low crystallinity (Tsyganenko & Mardilovich, 1996). The ratio of tetrahedral to octahedral Al is about 1:3, a characteristic from the γ-Al2O3 phase (Sifontes et al., 2014).
FT-IR pyridine adsorption
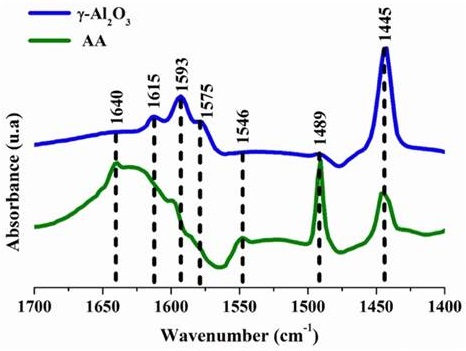
The spectra of the pyridine adsorption-desorption were obtained for the γ-Al2O3 and AA at a temperature of 25 ºC, as shown in Figure 6. This indicates that only Lewis acid sites were detected, but no Brönsted acid sites were observed from the γ-Al2O3. It was possible to observe the IR bands at 1593 cm-1, 1575 cm-1, and 1445 cm-1, which is specific from the vibration mode of pyridine when interacting with medium or weak Lewis acid sites (Liu, 2008; Rana, Huidobro, Ancheyta & Gómez, 2005). Besides the already mentioned bands, other band was observed at 1615 cm-1, associated at pyridine adsorbed on Lewis acid sites. On the other hand, for the adsorbent AA, a band at 1489 cm-1 associated with pyridine adsorbed on both Lewis and Brönsted acid sites was observed (Li et al., 2013; Xue, Li, Wang, Liu & Xu, 2010). The results may be relative to the unsaturated alumina atoms (AlIV and AlV) and the specific surface alumina areas. It is agreed that the weak Lewis acidity mainly arises from pentacoordinate aluminum atoms while the medium strong Lewis acidity mainly relates to aluminum atoms in tetrahedral geometry (Li et al., 2013). This is corroborated in Figure 5. These features influence directly the surface acidity distribution of γ-Al2O3 and AA. As demonstrated in Figure 6, the material AA presents a higher percentage of Brönsted acidity and, according to reported studies, this benefits the fluorine adsorption reaction. That is due to the proton donation.
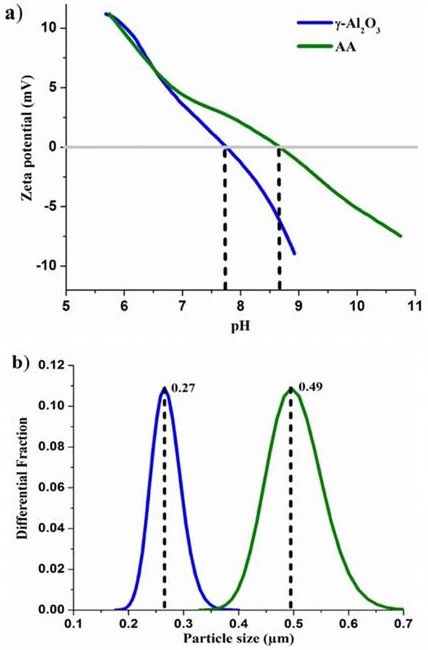
Zeta potential and particle size
The plot of pH versus zeta potential seen in Figure 7 shows the zeta potential measurement of the γ-Al2O3 and AA. Specifically, the adsorption capacity of a material is determined by its chemical composition and distribution in a particle surface charge. Zeta potential (PZ) is widely used for electrical charge magnitude quantification in a double layer (Wang & Keller, 2009). The isoelectric point of AA and γ-Al2O3 is found at pH 8.6 and 7.72, respectively. This is consistent with the reported data in the literature. This indicates that the synthesized and agglomerate alumina surface is slightly basic. This result corroborates that the basic sites concentration is slightly higher for the γ-Al2O3 than AA, as determined by FT-IR pyridine adsorption (Figure 6). The reported values show that the potential zeta for AA or γ-Al2O3 are around pHPZC 8.94 (Cheng et al., 2014). In other researches, different pHPZC values were reported: 8.3 (Bowen, Carry, Luxembourg & Hofmann, 2005), 8.5 (Zamorategui, Ramírez, Martínez & Serafín, 2016), 8.0 for a commercial AA (Ku & Chiou, 2002), and (Choi & Chen, 1979) reported that the pHPZC, which belongs to the adsorbents AA, ranged from 6.2 to 8.9. Therefore, the PZ pertaining to synthesized and agglomerate alumina and commercial AA, which was employed in this work, was within the range of values reported in the literature. These potential changes are related to the flocculation phenomena and the neutralized surface charge. The differences in the values of IEP are attributed to the acidity from both materials. This acidity depends on the coordination of aluminum atoms, as observed in Figure 5; this is due to change in surface charge. The AA presented a higher acidity due to the presence of Lewis and Brönsted acid sites, in comparison with the γ-Al2O3. This can be observed in the FT-IR spectra of the pyridine adsorption. Figure 7b shows the size of particles that belongs to the γ-Al2O3 (0.27 µm) and AA (0.5 µm). The particle size of γ-Al2O3 is smaller than that of the AA. This is due to a combination of phases, such as gamma-alumina and pseudoboehmite as the main phase. Their entirety was not affected by dehydration and agglomeration of crystals.
Adsorption isotherms of fluoride
Adsorption depends on ions in fluid (adsorbate), which diffuses through the solid surface (adsorbent). In this surface, they bond or are held there by weak intermolecular forces (Li et al., 2011). Adsorption studies pointed out the most important characteristics which determined adsorbent suitability for practical applications: the adsorption capacity, selectivity for fluoride ions, regenerability, compatibility, particle and pore size, and low cost. It is important to consider that fluoride removal efficiency always depends on a raw water quality profile, i.e., initial fluoride concentration, pH, temperature, contact time, and adsorbent dosage (Bhatnagar et al., 2011; Mohapatra, Anand, Mishra, Giles & Singh, 2009; Rao, 2003; Tomar & Kumar, 2013). The Langmuir adsorption model is the most common model used to quantify the amount of adsorbate on an adsorbent as a function of partial pressure or concentration at a given temperature. Adsorption isotherms for the γ-Al2O3 and AA are shown in Figure 8, and these go in accordance with the Langmuir equation which is represented as:
In this equation, q e (mg • g-1) is the solution that was adsorbed by the surface, b is the constant equilibrium of adsorption, and q m is the capacity of adsorption in a saturated single layer. Finally, C e (mg • l-1) is the solution in equilibrium state (Rahimi & Vadi, 2014).
Source: Author’s own elaboration.
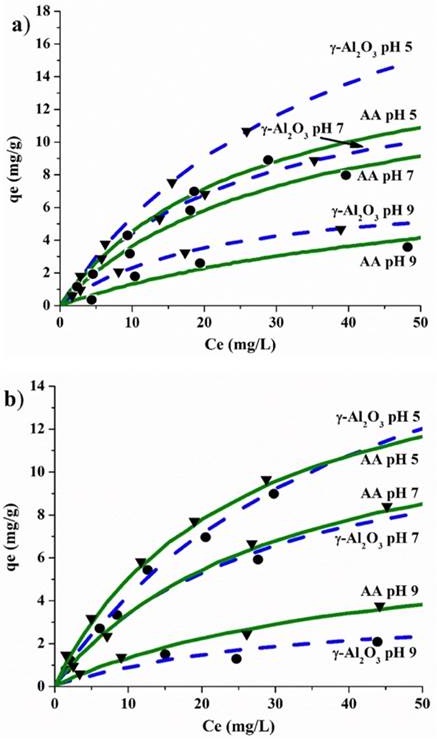
Figure 8 Adsorption isotherms of fluoride of γ-Al2O3 and AA, at a) 25 ºC and b) 35 ºC, at pH different.
Figure 8 illustrated the adsorption isotherms of γ- Al2O3 and AA. Figure 8a shows the adsorption isotherms of F− in the γ-Al2O3 and AA at 25 ºC, at pH 5, 7, and 9. The results indicate a higher capacity of adsorption from γ-Al2O3 than AA at pH 5, approximately 15 mg of F-/g and 11 mg of F-/g, respectively. Figure 8b shows the adsorption isotherms of F− from the γ-Al2O3 and AA at 35 ºC, at pH 5, 7, and 9. These results indicate a slightly higher capacity of adsorption for γ-Al2O3 than for AA at pH 5. That is approximately 12 mg in F-/g and 11 mg in F-/g, respectively. The best-fit parameters are listed in Table 3 and 4. The adsorption capacity results for γ-Al2O3 at pH 7 are approximately 10 mg of F-/g and 9 mg of F-/g for AA at 25 ºC. Moreover, at 35 ºC, the adsorption capacity of 8 mg in F-/g and 9 mg in F-/g was revealed. The qmax value for AA was 16.7 mg/g. The γ-Al2O3 with a qmax (27.5 mg/g) had a much higher level than the AA at 25 ºC. The qmax value for AA was 15.3 mg/g. The γ-Al2O3 presented a qmax (23.3 mg/g), which had a much higher level than the AA, at 35 ºC and at pH 5. In this study, solutions of 50 ppm of fluoride ion were used.
Table 3 Parameters and correlation coefficient of Langmuir equation (pH 5, 7, and 9, at 25 ºC).
| Sample | K (L/mg) |
Qmax
(mg/g) |
R (corr.) |
Langmuir equation |
|---|---|---|---|---|
| (pH 5) | ||||
| γ-Al2O3 | 0.0244 | 27.5200 | 98.99 | qe=(0.0244)•(27.5200Ce)/(1+0.0244Ce) |
| AA | 0.0371 | 16.7319 | 99.57 | qe=(0.0371)•(16.7319Ce)/(1+0.0371Ce) |
| (pH 7) | ||||
| γ-Al2O3 | 0.0421 | 14.9654 | 99.52 | qe=(0.0421)•(14.9654Ce)/(1+0.0421Ce) |
| AA | 0.0319 | 14.8116 | 99.88 | qe=(0.0319)•(14.8116Ce)/(1+0.0319Ce) |
| (pH 9) | ||||
| γ-Al2O3 | 0.0458 | 7.3031 | 98.87 | qe=(0.0458)•(7.3031Ce)/(1+0.0458Ce) |
| AA | 0.0163 | 9.1748 | 99.50 | qe=(0.0163)•(9.1748Ce)/(1+0.0163Ce) |
Source: Author’s own elaboration.
Table 4 Parameters and correlation coefficient of Langmuir equation (pH 5, 7, and 9, at 35 ºC).
| Sample | K (L/mg) |
Qmax
(mg/g) |
R (corr.) |
Langmuir equation |
|---|---|---|---|---|
| (pH 5) | ||||
| γ-Al2O3 | 0.0210 | 22.3138 | 99.12 | qe=(0.0210)•(22.3138Ce)/(1+0.0210Ce) |
| AA | 0.0364 | 15.3703 | 99.21 | qe=(0.0364)•(15.3703Ce)/(1+0.0364Ce) |
| (pH 7) | ||||
| γ-Al2O3 | 0.0332 | 12.5148 | 98.65 | qe=(0.0332)•(12.5148Ce)/(1+0.0332Ce) |
| AA | 0.0293 | 13.7077 | 98.76 | qe=(0.0293)•(13.7077Ce)/(1+0.0293Ce) |
| (pH 9) | ||||
| γ-Al2O3 | 0.0261 | 3.9344 | 98.17 | qe=(0.0261)•(3.9344Ce)/(1+0.0261Ce) |
| AA | 0.0199 | 7.1792 | 99.14 | qe=(0.0199)•(7.1792Ce)/(1+0.0199Ce) |
Source: Author’s own elaboration.
The results indicated that the commercial agglomerate AA presented a higher crystallinity than the γ-Al2O3, synthesized and agglomerate. This is attributed to what is shown in a diffractogram from a hybrid material that belongs to a γ-Al2O3 and a pseudoboehmite as a main phase (> %). On the other hand, the adsorption of fluoride ions occurs on the surface of the adsorbent material. Adsorption process is controlled by the diffusion of the fluoride ion in the liquid, and the fluoride ion must diffuse into the adsorbent agglomerate. This is the reason why the amount of fluoride ion adsorbed is less for the AA than for the γ-Al2O3, since the phenomenon is influenced by the size of the pores through which the fluoride ion is transported. The fluoride was adsorbed in the active sites of the activated alumina that are formed in the aluminum atoms of the alumina framework. No adsorption studies at a lower pH were explored due to the loss of active sites. The reason for this is the dissolution of aluminum present in the alumina.
The capacity of the alumina materials for adsorbing the fluoride was considerably dependent of the solution pH. The effect of the solution pH in the adsorption capacity can be ascribed to the electrostatic or coulombic interactions between the alumina surface and the fluoride ions in the aqueous solution. The γ-Al2O3 and AA surface was charged positively at a pH level lower than the PZC of 7.72 and 8.6, respectively. Hence, the fluoride ion was attracted to both materials’ surface. On the contrary, the surface was negatively charged at a pH level higher than 7.72 and 8.6. Hence, the fluoride ion was repelled from the surface.
Conclusion
The γ-Al2O3 synthesized by the hydrolysis-precipitation method and agglomerate has effective properties to be considered as an adsorbent material, which can be used to remove the fluoride ion present in drinking water from columns of adsorption at industrial sites. The γ-Al2O3 particles had a small zeta potential and a great adsorption capacity compared with the AA particles at the same pH and temperature. The adsorption behavior of γ-Al2O3 and AA was well described with the pseudo-first-order kinetics, and the adsorption isotherms were described with the Langmuir equation. The maximum adsorption capacities of γ-Al2O3 and AA determined by the Langmuir equation were 15 mg/g and 11 mg/g at pH = 5.0, at 25 ºC, respectively. The optimum pH for F− removal by the adsorbents was 5. The γ-Al2O3 synthesized and agglomerate demonstrated to be a more effective adsorbent than the commercial and commonly used AA for F− removal from water.











 nueva página del texto (beta)
nueva página del texto (beta)