Introduction
The smooth venus clam Chionista fluctifraga (G. B. Sowerby II 1853) is a bivalve mollusk of commercial importance in northwestern Mexico. This species is normally found as a benthic resident from the intertidal zone at depths around 50 m in bays and coastal lagoons of southern California (USA), the Pacific coast of the Baja California Peninsula, and the Gulf of California (Fischer et al., 1995). The clams live buried (10 cm - 15 cm) in substrates composed mainly by muddy and sandy sediments, showing an aggregate distribution and densities from 2 ind.m-2 to 21 ind.m-2 (Martínez-Córdova, 1987). Clam fishermen extract the species directly from the substrate using their hands or scoops, and the fishery is developed mainly under recreational and artisanal regimes, with an annual production around 1600 t (Castillo-Durán et al., 2013; Tinoco-Orta & Cáceres-Martínez, 2003).
Production sites for this clam such as El Sargento, Santa Rosa, El Soldado, and La Cruz lagoons are located in the Gulf of California (Martínez-Córdova, 1996), where the extracted clams are sold in the local and regional markets. Nevertheless, the most important production site is at Bahía de San Jorge (Sonora, Mexico), which have certified waters, and this allows the harvested clams to be exported to the United States of America (USA). The bay is managed by the cooperative San Jorge, and harvested clams from this area are shipped to Ensenada B.C., Mexico, cleaned and selected by size and then distributed by road to USA. The cooperative has a relative constant production of ~450 t by year and has been exporting since 2007 (Castillo-Durán, Hoyos-Chairez, Arreola-Lizárraga, Martínez-Córdova & Chávez-Villalba, 2016).
Benthic bivalve populations are susceptible to over-exploitation or to collapse of fishery stocks due to the ease with which they can be harvested from coastal ecosystems (Gorman, Mayfield, Ward & Burch, 2011). Regulatory authorities demand studies considering the biological characteristics of target species to develop suitable harvest strategies for the effective management of fisheries, particularly for those in emerging status (Hartill, Cryer & Morrison, 2005). Thus, information on species composition, distribution, abundance, biomass, and basic biological parameters (Mayfield, McGarvey, Carlson & Dixon, 2008), as the condition of organisms, is required for a proper administration of resources. In principle, the development of any species used for fishery or aquaculture practices is related to its physiological state (Villarroel, Acosta & Arrieche, 2016). This can be revealed indirectly, using condition indices (CI), which may serve to know both ecophysiological and economic characteristics of the species (Lucas & Beninger, 1985). In the first case, the indices determine long-term fluctuations of the physiological activity (health) of the exploited stocks under given environmental conditions (Sokolowski, Bawazir, Sokolowska & Wolowicz, 2010). Normally, variations in condition index of any species are related to changes of environmental variables, particularly of temperature and food supply that have a major influence on biological activities (De Montaudouin et al., 2016). In the second case, the indices help as indicators of the quality of products to be sold.
CI are used in studies of many bivalve species, and in the case of C. fluctifraga our laboratory estimated the CI in relation to reproductive processes in adult clams sampled from a natural population located in a closed unexploited area of the Bahía de San Jorge (Castillo-Durán et al., 2013). Similarly, the CI of juveniles produced in hatchery was calculated to determine their performance under cultivated conditions in this same location (Castillo-Durán et al., 2016). Nevertheless, there are no studies about the physiological state of clams coming from the commercial fishery. This information may serve to improve management procedures such as detecting best seasons for marketing, identifying differences in condition between juvenile and adults and, in general, recognizing the status of the fishery itself. Therefore, the objective of this study was to determine the CI of different sizes of clams C. fluctifraga, harvested during one year from the commercial fishery grounds of Bahía de San Jorge (Sonora, Mexico). The condition of clams and its relationship with biological processes and environmental variables is described.
Material and Methods
Study site
This study was accomplished in the commercial grounds of the Bahía de San Jorge, which is situated in an arid region with a semi-warm climate northeast of the Gulf of California (latitude N: 30° 55’ 48” to 31° 13’ 48”; and longitude W: 113° 01’ 48” to 113° 10’ 12”) (Figure 1). This area presents an air temperature range between 12 °C - 33 °C, high evaporation rates (~1900 mm yr-1), and low rainfall (annual mean <100 mm yr-1) (Stensrud, Gall & Nordquist, 1997). The 130 km2 bay is characterized by extensive intertidal flats with smooth reliefs, showing an average depth of 3 m and is influenced by semidiurnal mixed tides with a tidal range of ~7 m (Marinone & Lavin, 1997).
Environmental variables
Environmental variables as well as water samples were taken in a monthly basis, from April 2009 to March 2010, in a point located approximately in the middle of the bay. Temperature, salinity, and dissolved oxygen were recorded with a multi-parameter probe (YSI® 556 MPS, Yellow Springs, Ohio) and, at the same time, seawater samples (2 L) were collected to measure concentrations of chlorophyll a, and seston. Chlorophyll was determined spectrophotometrically at 665 nm and 750 nm after the filtering (GF/C Whatman Filter, 1.2 μm, Pall Corporation, Port Washington, NY) and the 90% acetone extraction method (Parsons, Takahashi & Margrave, 1984). The concentration of chlorophyll a was used as an indicator of phytoplankton biomass (Ren & Schiel, 2008). Similarly, for seston determinations, water was filtered (GF/C Whatman Filter, 1.2 μm, Pall Corporation, Port Washington, NY), and the filters with samples were placed in an oven at 80 °C for 24 h, weighed to obtain dry weight (inorganic + organic fractions), and then placed in a muffle furnace at 450 °C for 4 h. The filters were weighed again to obtain the inorganic seston (PIM), which, by difference with the total weight, estimates the particulate organic matter fraction (POM).
Fishery and sampling of clams
Most of the bay is concerted to the cooperative for the fishery of the species. The grounds are separated into three types of fishing areas: open areas to commercial fishery, temporary closed areas, and closed unexploited areas. In the open areas, clam fishermen work during low tides, harvesting the animals from the upper to the lower zones as far as the seawater level permits suitable yields. In general, fishermen extract all the clams they can detect into the sediments, and when there are no more clams to collect, they move to another open area to start the harvesting again. Harvested clams are assembled in a lodge where they are separated by size, and then they are put in sacks to be transported in refrigerated trucks to Baja California (Mexico). The cooperative uses three sizes for commercial operations: (1) clams < 30 mm height (small), which are reintroduced in marine areas in Baja California until marketable size is reached; (2) clams between 30 mm - 40 mm (medium), which is the range preferred for exportation; and (3) clams > 40 mm (large) that are used for regional markets.
Sampling of clams for CI determinations was performed in a monthly basis (April 2009 -March 2010). Every month, 20 clams per size group were sampled randomly from commercial captures, placed in coolers containing ice, and then transported 600 km to the laboratory (Cibnor-Guaymas, Sonora). The CI of clams was determined using the formula by Walne & Mann (1975): CI=(TW×1000)⁄SW, where TW is the dry weight of soft tissue, and SW is the dry weight of the shell. For this, the clams were cleaned and opened to separate soft tissues from shells; soft tissues were placed in pre-weighed aluminum cases and then in an oven for 48 h at 80 °C, and later they were re-weighed to determine dry weight. The shells were put in marked aluminum cases, which were placed in an oven (48 h at 80 °C), and then weighed in a balance.
Data analysis
Data are presented as mean ± standard deviation. A two-way analysis of variance (Anova) was used to examine the effect of size and time (month) on CI. The Tukeys’ Honest Significant Difference method (Tukey HSD) was computed to find differences of condition index according to size for each month. Pearson correlation tests were applied to analyze possible relationships between the CI of the three sizes of C. fluctifraga and records of temperature, salinity, oxygen, as well as CI and content of chlorophyll a, POM, and PIM-POM in water. Statistic tests were analyzed at a significance level of p < 0.05.
Results
Environmental variables
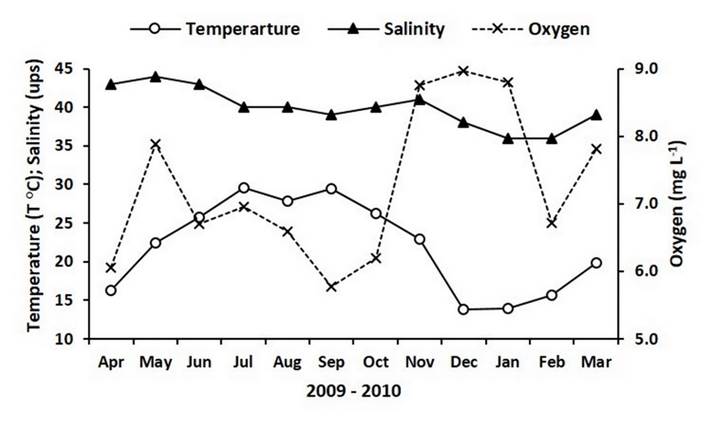
Temperature records in the Bahía de San Jorge during the study period started with 16.3 °C in April 2009 and then increased to reach 29.6 °C in July, descending in August (27.9 °C), and peaking again in September (29.4 °C). Later, the temperature declined and reached the lowest record (13.8 °C) in December to start increasing from January 2010 and reaching 19.8 °C by the end of the study (Figure 2). Hypersaline conditions were observed during most of the experimental time, with salinity records showing a descending pattern; the maximum value (44) was detected in May and the minimum value (36) was detected during January and February 2010 (Figure 2). Oxygen concentrations were always higher than 5.5 mg L-1 along the experiment, indicating well-oxygenated conditions in the study site. Excluding May, oxygen values oscillated between 6 mg L-1 and 7 mg L-1 from April to October, and then they increased and were > 8 mg L-1 during November, December and January 2010. Oxygen values in February and March were 6.7 mg L-1 and 7.8 mg L-1, respectively (Figure 2).
Source: Author’s own elaboration.
Figure 2 Temperature and salinity records as well as oxygen concentrations in Bahía de San Jorge (Sonora, Mexico).
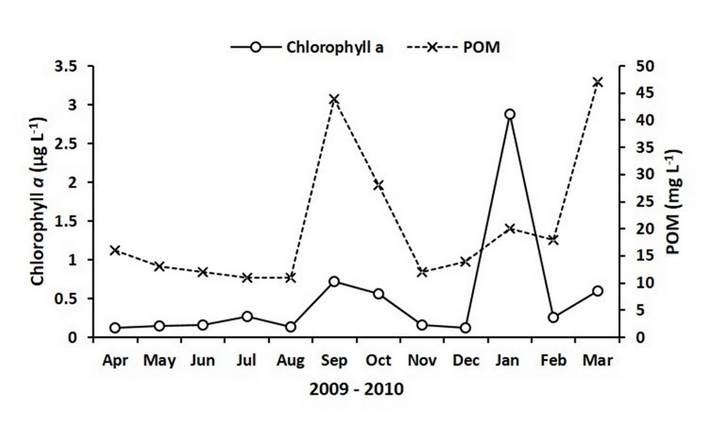
Concentrations of chlorophyll a in the study area ranged between 0.1 µg L-1 and 0.3 µg L-1 for most of the experimental period. Nevertheless, three peaks were detected; the first in September (0.72 µg L-1), the second, and most important, in January 2010 (2.87 µg L-1), and a third in March 2010 (0.6 µg L-1) (Figure 3). For this study, seston was represented by the POM. The concentrations of POM showed a similar pattern to that of the chlorophyll a, remaining relatively constant (11 mg L-1 - 16 mg L-1) from April to August and showing peaks in September (44 mg L-1), January 2010 (20 mg L-1) and March (47 mg L-1) (Figure 3). PIM-POM values along the study showed an average of 5.2 mg L-1 ± 1.5 mg L-1, representing a mean concentration of 21.5% of POM in relation to PIM.
Condition index (CI)
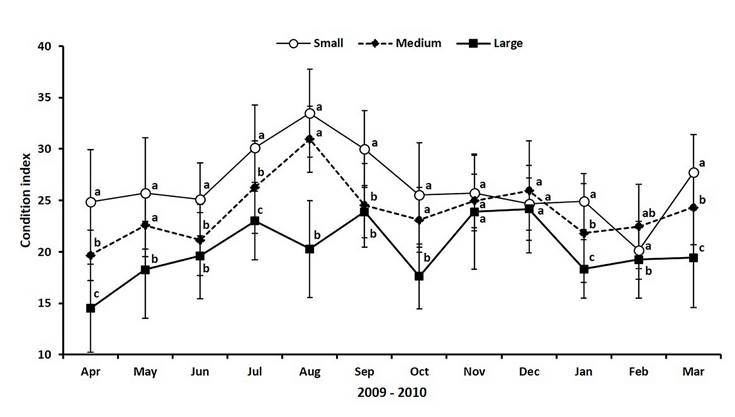
Values of CI in small clams oscillated between 24 and 28 for most of the year, except in August when the CI peaked reaching its maximum value (33.5), and during February 2010 when CI decreased to the lowest record (20.2) (Figure 4). The CI of medium clams followed a similar pattern of small ones, but the values were lower along the study period, except in December and February 2010. The maximum CI registered in medium clams was 31 (August), while the minimum was 19.7 at the beginning of the study (April). Large clams showed a different CI pattern, with more variation, but without showing a clear peak. Moreover, the condition of clams was lower all along the experimental time than the CI obtained for small and medium clams. The highest and lowest records for large clams were 24.2 and 14.5 in December and April 2009, respectively (Figure 4).
Source: Author’s own elaboration
Figure 4 Values of condition index for three different size ranges of the venus clam Chionista fluctifraga sampling from the commercial harvesting in Bahía de San Jorge (Sonora, Mexico). Different superscripts indicate significant differences in a Tukey HSD test (p < 0.05).
The two-way Anova test showed that the CI of clams was significantly different (F = 115.74; gl = 2; p < 0.0001) among sizes (Table 1); the mean CI was higher for small clams (26.5 ± 3.9), followed by medium clams (24.0 ± 3.7), and the lowest was for large individuals (20.2 ± 4.1). Moreover, statistics revealed a significant effect (F = 19.42; gl = 11; p < 0.0001) of time (month) and the interaction of size and time on CI (Table 1). Results of Pearson correlation tests between CI and environmental variables showed a statistically significant relationship (F = 9.88; gl = 1; p < 0.0104) between small clams and temperature (Table 2). The rest of the variables showed low or very low positive and negative relationships.
Table 1 Results of two-way Anova test for condition index of the venus clam Chionista fluctifraga according to size and time (month).
| Source of variation | Degrees of freedom | Mean Square | F | Probability |
|---|---|---|---|---|
| Main effect | ||||
| Factor A (size) | 2 | 1818.23 | 115.74 | <0.0001 |
| Factor B (month) | 11 | 305.16 | 19.42 | <0.0001 |
| Interactions | ||||
| Factors A × B | 22 | 67.9626 | 4.33 | <0.0001 |
Source: Author’s own elaboration
Table 2 Results of Pearson correlation tests for condition index of the venus clam Chionista fluctifraga and environmental variables.
| Environmental variable | Condition index (size - clams) | ||
|---|---|---|---|
| Small | Medium | Large | |
| Temperature | 0.70505 (p=0.0104)* | 0.43267 (p=0.1601) | 0.31203 (p=0.3235) |
| Salinity | 0.18006 (p=0.5755) | -0.19144 (p=0.5512) | -0.25939 (p=0.4156) |
| Oxygen | -0.24107 (p=0.4504) | 0.08469 (p=0.7936) | 0.31904 (p=0.3121) |
| Chlorophyll a | -0.08199 (p=0.8000) | -0.22664 (p=0.4787) | -0.15647 (p=0.6272) |
| POM | 0.14449 (p=0.6541) | -0.08975 (p=0.7815) | 0.02315 (p=0.9431) |
| PIM-POM | -0.23633 (p=0.4596) | 0.00351 (p=0.9914) | -0.11052 (p=0.7324) |
*Significant relationship (F = 9.88; gl = 1)
Source: Author’s own elaboration
Discussion
The results in this study showed that the condition of C. fluctifraga coming from the fishery varied significantly, indicating that animals respond in a distinctive way in relation to size. CI in bivalves may reflect various aspects (including nutritive value, somatic condition, effect of environmental variables) and can be used as indirect means to verify reproductive status (Sokolowski et al., 2010). It was observed that small clams (< 30 mm) showed the highest condition throughout the study period, with a significant relationship with temperature. It is known that one of the major factors controlling growth and reproduction of bivalves is temperature (Adkins, Marsden & Pirker, 2016). According to literature, small clams of this study would be young organisms with less than 1.5 years old (Drover, 1974), showing a fast growth and reproductive activity (Martínez-Córdova, 1987). Therefore, it appears that CI variability could be associated to changes in nutriment storage and gamete developing, which are eventually influenced by temperature, as noted before for this species (Castillo-Durán et al., 2013). In C. fluctifraga, the fastest growth occurs in clams between 10 mm and 30 mm associated to increasing temperatures and in terms of reproduction; these clams present small gonads, which suggest a limited contribution to reproduction (Martínez-Córdova, 1987). Nevertheless, it was found that variation of CI in small clams coincided with the reproductive cycle of the species (Castillo-Durán et al., 2013), showing high values during gametogenesis and reduction during spawning. Similarly, the CI pattern corresponded with the CI reported in the previous study for individuals between 35 mm and 55 mm, but the values are higher in the present study. This may indicate that contribution to reproduction from these organisms could be higher than expected. Thus, small clams could be considered as developing young individuals that use energy for both somatic growth and reproduction, but it is difficult to know which of the requirements is most demanding.
According to Drover (1974), the medium size clams here correspond to animals between 1.5 and 3 years old, which can be considered as adult organisms presenting a high reproductive activity (Martínez-Córdova, 1987). These clams displayed intermediate values of CI with a low relationship with temperature. As indicated before, temperature is an important factor affecting bivalve growth, but it seems to have a lesser effect in medium clams since growth tend to be slower than in younger individuals. Nevertheless, the effect of temperature on nutriment storage and gamete developing could be more important for these organisms. For instance, the CI in these clams followed the reproductive pattern defined for the venus clam, where reproduction showed a strong relationship with CI (Castillo-Durán et al., 2013). It seems that medium clams use the energy from food supplies for storage and reproduction but, since they have slow growth patterns, most of their energy seems to go for gametogenesis.
Although the CI of large clams reflected, to some extent, part (April-July) of the reproduction period described for the species, the values were inferior particularly during August, when it was the moment of high reproductive activity and greater IC (Castillo-Durán et al., 2013). In addition, these clams exhibited the minor CI along the study period, and this showed a different pattern than that observed in the previous study. These results suggest that production of gametes and nutriment storage is fewer than patterns detected for small and medium clams, which could indicate that the influence of large clams on reproduction is lower than it was supposed. These clams are relatively old specimens (approximately 3.5 to 5 years of age), which means that they are approaching the end of their life span, which is around five years (Drover, 1974). Moreover, these clams show little or no growth because they are approximating asymptotic growth (Martínez-Córdova, 1987), which could be also related to the effect of temperature, since this parameter showed a low relationship with CI. If there is a minimal contribution to reproduction and there is no growth in these clams, it appears that they use most of their energy for physiological maintenance only.
In terms of environmental conditions, although no relationship of CI with salinity and oxygen was obtained, salinity indicated typical marine conditions and concentrations of oxygen were always > 5.7 mg L-1, indicating a well-mixed water column at the study site. These parameters were not restrictions for the normal development of clams, and it appeared that they did not directly affect the CI. Nevertheless, temperature showed different grades of relationship with CI, which seemed to be expressed through processes such as reproduction and nutrient storage. In the case of this last process, bivalves with good condition are the result of high concentration and quality of the food supply (Navarro & Iglesias, 1993). In this study, chlorophyll a content (phytoplankton biomass) exhibited relatively low values in the bay and the relation PIM-POM showed that POM was present in low proportions when compared to average values registered by the laboratory (chlorophyll = 3.0 µg L-1 ± 1.5 µg L-1; POM = 7.8 µg L-1 ± 1.9 mg L-1) in the lagoon La Cruz in the Gulf of California (unpublished results), which is within the geographic range of the species. Moreover, chlorophyll and POM did not show any relationship with CI. This could indicate low food availability, but infaunal species, such as the venus clam, are suited to deal with high concentrations of low quality and inorganic particles, and they can feed on particles suspended in the water column and ingest deposits available at the water-sediment interface (Bacon, MacDonald & Ward, 1998). These feeding strategies seem to allow the clams, making the most with the accessible food sources and, thus, satisfactorily cover their physiological needs.
Conclusion
In view of previous observations, it seems that the main factor affecting the CI of clams is temperature, which regulates processes such as reproduction and nutrient storage that at the same time are expressed through the CI. These processes seem to have different effects on CI, depending on the size of the clams, which is directly related to the organisms age. According to our results, and based on literature of C. fluctifraga, the small clams are young individuals in development that seem to use energy for both somatic growth and reproduction, which represents animals with a high nutritional value, being advantageous for commercialization. Medium clams are adult specimens with slow growth, directing most of their energy to gametogenesis; they are healthy organisms but with moderate alimentary use. Finally, large clams are old organisms that are reaching the asymptotic growth and their contribution to reproduction is uncertain; this represents individuals with low nutritious rate. These results could be useful for producers when selecting harvesting seasons as well as clams based on their size for differentiated commercial strategies.





![Radiación gamma para inducción de mutagénesis en pasto rosado [Melinis repens (Willd.) Zizka]](/img/es/next.gif)





 nueva página del texto (beta)
nueva página del texto (beta)