Introduction
Cyanobacteria make up a very diverse group of prokaryotic organisms belonging to the Bacteria domain, capable of performing oxygenic photosynthesis (Lewis, Joseph & Woodward, 2016; Rantamäki et al., 2016; Whitton, 1992). Recently, researchers from different parts of the world have been interested in working with green biotechnology; they have studied microalgae and cyanobacteria such as Dunaliella salina, Chlorella vulgaris, Anabaena sp., and Fischerella sp., among others (Higo, Katon, Ohmori, Ikeuchi & Ohmori, 2006; Rungaroon, Hakuto & Teruhiro, 2016; Widjaja, Chien & Ju, 2009). Applications have been diverse, examples are Agricultural crops protection, secondary sewage treatment, nanotechnology and pharmaceutics (Dhar, Saxena & Singh, 2009; Ghosh, Paliwal, Maurya & Mishra, 2015; Luangpipat, Beattie, Chisti & Haverkamp, 2011; Singh & Dhar, 2007; Singh, Dhar & Tabassum, 2014). The control of the operating variables in a photobioreactor system is of great importance, in order to recreate the kinetic parameters of reaction. Thus, monitoring the growth rate and production of metabolites of interest is implicit in the control of bioreaction (Su, Mennerich & Urban, 2016). Determining the biomass concentration allows obtaining information to make key decisions about the process conditions such as light intensity, temperature and substrate concentration (Ducat, Way & Silver, 2011; Markov, Weaver & Seibert, 1997; Santos, Fernandes, Wagner, Jacob-Lopes & Zepka, 2015; Taskan, 2016). Nowadays, in situ quantification techniques for microbial cultures can be classified as direct and indirect, impacting from dielectric spectroscopy, dry weight, wet weight, fluorescence, packed cell volume, cells number, infrared spectroscopy, mass of cellular component, measurements of generated heat, and optical methods, among others (Erdle, Mistele & Schmidhalter, 2011; Heidari, Yoon, Park & Lin, 2011; Herbert, 1990; Kiviharju, Salonen, Moilanen & Eerikäiren, 2008; Krommenhoek et al., 2008; Nichol & Sarker, 2011). However, the indirect measurements problem is that the devices do not distinguish between viable and non-viable cells. The electrical impedance is represented as the opposition of a conductor to the flow of alternate current, as the same current is composed by the resistive and reactant vector. Thus, bioelectric impedance is a technique used to quantify body compositions, based on the ability of the organism to conduct an electric current. So, the electrical admittance is a technique to solve the limitations that conductivity techniques present through variations in the frequency. It has been used to validate inter-tissue interactions, to evaluate different biological systems, to generate correlations between their fluids and their conductance, resistivity, and admittance, among others (Boukamp, 1984; Ching, Sun, Jheng, Tsai & Shien, 2015; Chumlea & Guo, 1994; Dimeas & Aspragathos, 2016; Kottam et al., 2006; Kutty et al., 2013; Oh, Lee, Seo, Kim & Woo, 2007; Rodríguez-Sierra & Soria, 2016; Yonamine & Sampaio, 2006). In this work, a technique to determine the concentration of living biomass by electrical admittance for the cyanobacteria Fischerella sp. through the use of graphite electrodes is proposed.
Materials and methods
Photobioreactor system
The bioprocess was taken on 400 mL of photobioreactors with a volume operation of 300 mL, and the strain was maintained under a 12:12 light:dark photoperiod with a light intensity of 73 µmol/m-2s-1 provided by cool white fluorescent tubes (Trademark MAGG) and a constant air flow of 1.5 vvm. No axenic samples from Fischerella sp. were taken.
Samples preparation
There were 105 samples prepared, where 30 were combined with BG-11 medium (Rippka, Dervelles, Waterbury, Herdman & Stanier, 1979; Stanier, Kunisawa, Mandel & Cohen-Bazire, 1971) and distilled water, 60 with living biomass prepared with BG-11 medium, and 15 with inactivate biomass (DBM) or dead biomass prepared with BG-11 medium too. Table 1 specifies the making of each sample analyzed. DBM was obtained from a 250 Ml cyanobacteria culture that was sterilized under the conditions of 15 psi, 15 min, at 121 °C. The supernatant was retired, and dead biomass was washed with distilled water and resuspended in fresh medium BG-11.
Table 1 Liquid samples evaluated using different concentrations of distilled water, fresh medium and biomass. Abbreviations: FM: Fresh medium; DW: Distilled water; BM: biomass; DBM: Inactive biomass.
| # | Fresh medium | Distilled water | Living biomass | Inactive biomass | ||||
|---|---|---|---|---|---|---|---|---|
| FM [%] | V[mL] | DW [%] | V[mL] | BM [%] | V[mL] | DBM [%] | V[ml] | |
| 3 | 0% | 0 | 100 % | 2.8 | X | X | X | X |
| 3 | 75% | 0.7 | 75% | 2.1 | X | X | X | X |
| 3 | 50% | 1.4 | 50% | 1.4 | X | X | X | X |
| 3 | 75% | 2.1 | 25% | 0.7 | X | X | X | X |
| 3 | 100% | 2.8 | 0% | 0 | X | X | X | X |
| 5 | 0% | 0 | X | X | 100% | 2.8 | X | X |
| 5 | 75% | 0.7 | X | X | 75% | 2.1 | X | X |
| 5 | 50% | 1.4 | X | X | 50% | 1.4 | X | X |
| 5 | 75% | 2.1 | X | X | 25% | 0.7 | X | X |
| 5 | 100% | 2.8 | X | X | 0% | 0 | X | X |
| 3 | 0% | 0 | X | X | X | X | 100% | 2.8 |
| 3 | 75% | 0.7 | X | X | X | X | 75% | 2.1 |
| 3 | 50% | 1.4 | X | X | X | X | 50% | 1.4 |
| 3 | 75% | 2.1 | X | X | X | X | 25% | 0.7 |
| 3 | 100% | 2.8 | X | X | X | X | 0% | 0 |
Source: Author's own elaboration.
Electric system preparation
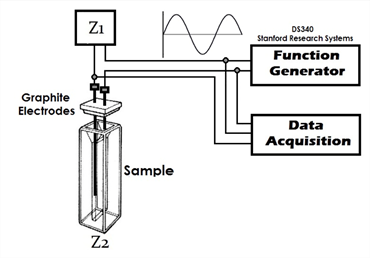
The characterization of the system was achieved using graphite electrodes with a length of 3 cm and a diameter of 0.07 cm. The electrodes were placed on a plastic cover which has an area of 0.81 cm2. The cover was used to seal the plastic cuvette containing the sample to evaluate. The cells volume was 2.8 mL and a signal generator (Stanford Research Systems, Mod. DS340) was used to supply a sine signal with an amplitude of 5 Vpp to the electric circuit varying the frequency of the sine signal in 10 Hz, 50 Hz, 100 Hz, 500 Hz, 1000 Hz, 5000 Hz, 10 000 Hz, 50 000 Hz, 100 000 Hz, 250 000 Hz, 500 000 Hz, 750 000 Hz and 1 000 000 Hz. In Figure 1, the used experimental setup is shown.
Data characterization and processing
All samples were run in triplicate, both at the electric system and at its dry weight determination. A comparative analysis of each sweep was performed with different concentrations of biomass; this was done in order to observe the response of the system in each frequency sent by the function generator. The data obtained were processed through the software Origin version 8.0.
Living Biomass dry weight measurements
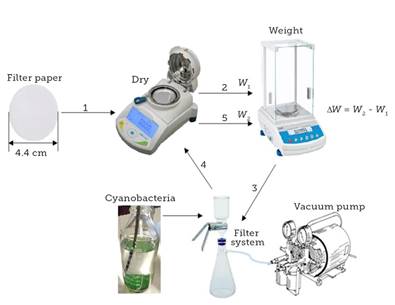
Dry weight measurements were given through drying the filter with a humidity balance PMB (Adam Equipment, Mod.17250) at 140 °C for 5 min; next, it was weighed in an analytical balance (RADWAG Wagi Elektroniczne, Mod. AS 220.R2). The biomass was filtered with the equipment KG 47 GLASS SUPPORT (Advantec MFS, Inc., Mod. 311400) of 300 mL and a Kitasato flask (KIMAX, Mod. 27060) of 1000 mL, as well as with a vacuum pump (WACO, Mod. 73021-00). The filter with living biomass was dried using the humidity balance PMB at 140 °C for 5 min, then it was weighed in the analytical balance. Figure 2 shows the flow of the drying process of the biomass evaluated by electrical admittance.
Results and discussion
Samples scanning
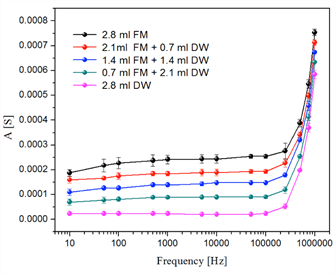
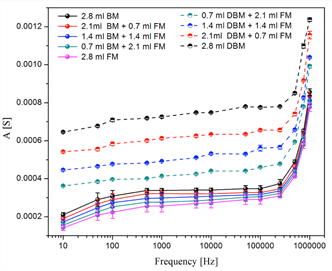
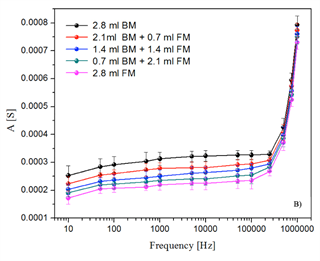
All samples were evaluated with different frequencies included in the range from 1 × 10-1 Hz to 1 × 10-6 Hz. All the sweeps reflect the resistive, capacitive and inductive regions. Figure 3 shows the electrical behavior of the solutions without Fischerella sp. biomass (only fresh medium and distilled water, with different concentrations), and where the electrical response was relatively very low. On the other hand, Figure 4 shows the response of the electrical system for living and inactive biomass solutions prepared with fresh medium at different concentrations. In the comparative analysis between non-active biomass and living biomass, Figure 4 shows a higher variability with non-activated biomass with respect to living or activated biomass; that is, the electrical impedance values were higher when the dead cyanobacteria Fischerella sp. was analyzed. However, the only focus of this research is the quantification of the living biomass. For this, in Figure 5 only the electrical response of the living biomass for the different frequencies evaluated is shown.
Source: Author's own elaboration.
Figure 3 Sample’s experimental scanning with graphite electrodes for fresh medium with distilled water scanning. Abbreviations. FM: Fresh medium; DW: Distilled water.
Source: Author's own elaboration.
Figure 4 Comparative response of the electrical system for the evaluated Fischerella sp. samples of inactive biomass and live biomass. Abbreviations. FM: Fresh medium; BM: Living biomass; DBM: Inactive biomass.
Source: Author's own elaboration.
Figure 5 Response of the electrical system for the samples evaluated from Fischerella sp. living biomass with fresh medium scanning. Abbreviations. FM: Fresh medium; BM: Living biomass.
Our results are supported by studies (Deurenberg, 1992) which postulate that when the frequency results to be lower or equal to 1 kHz (<10 kHz), the current does not pass through the cell membrane, and therefore the resistance obtained is only related to the extracellular mass. Otherwise, with 50 kHz-100 kHz the current crosses the membrane and allows determining the total cell mass (intra and extracellular components).
Therefore, for the calibration curves preparation, it was necessary to select a frequency under which the sweeps did not show high variability, whereby the frequency of 50 kHz was chosen. In Figure 5, the electrical behavior of the activated biomass prepared with fresh medium is shown. In comparison with the response of the system in the samples of distilled water and fresh medium, a higher electrical response was presented for the living biomass.
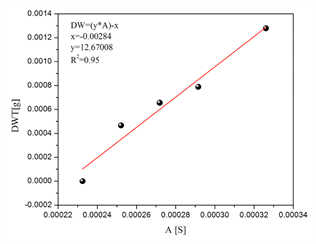
Considering the admittance values obtained from the response of the electric system at 50 kHz, we adjusted to a linear system to determine concentrations of biomass in fresh culture medium. The linear behavior reflects an R2 = 0.95. In Figure 6, the constants for the slope and the intercept for the calibration system are verified.
Conclusions
The scale measurement system used sinusoidal, electrical signals provided by a function generator where, by modifying the excitation frequencies from 1 × 101 Hz to 1 × 106 Hz, it was capable to generate transduction responses in Fischerella sp. along the sweeps, generating correlated voltage variations in the different percentages of living biomass concentration. Derived from these sweeps, the frequency with the best response for the characterization of the biomass concentrations was selected, allowing to quantify, by an indirect method, the existing cell density in the sample under study. The design opts for low-cost materials by the use of graphite electrodes with adequate polarization, allowing suggesting the integration of more robust embedded electrical systems, capable of adapting to the industrial needs that demand it. In future research work, it is necessary to study the behavior of the electrical system with non-activated biomass when it interacts with the activated biomass, in addition to transferring this quantification system to other species of cyanobacteria.











 text new page (beta)
text new page (beta)