INTRODUCTION
Silver is known to intervene in a wide range of biological processes in microorganisms, including the alteration of the cell membrane structure and its functions, as well as the inhibition of proteins associated with Adenosine triphosphate (ATP) production (Pal, Kyung & Myong, 2007; Russell & Hugo, 1994; Sondi & Salopek-Sondi, 2004; Yamanaka, Hara & Kudo, 2005). The different properties it possesses make it useful for medical purposes antibacterial agents (Dominguez-Wong et al., 2014; Liu et al., 2009) and industrial ones (Takai et al., 2002). When the size of the silver is reduced to less than 100 nm, its physicochemical properties can change and evidence the properties that are not seen in a larger scale (Wijnhoven et al., 2017). Silver nanoparticles (AgNP), as a result of their excellent antibacterial activity, are currently one of the most commonly used silver compounds in products currently on the market, including textiles, medical devices, electronic products, detergents and paints (Biffi et al., 2015; Dominguez-Wong et al., 2014; Quadros & Marr, 2010; Wijnhoven et al., 2017).
Some of the radically affects in the agriculture sector are the natural challenges such as changes in the weather and pest infestation, so that the breakthrough of the agriculture will be due to the new technologies (Opara, 2004; Rai & Ingle, 2012; Sunding & Zilberman, 2001). A priority of the agriculture and agribusiness in developed nations is the increasing quantity of food and the value added to the food products (Sunding & Zilberman, 2001). Nanotechnology has become an important field of research and investment for industries, thanks to their unique properties, the nanoparticles (NP) are included in more products. Given the huge increase in the applications of nanomaterials in the consumer products, agriculture and energy sectors, it is essential to understand their role in the environment and its effects on plant life (Cox, Venkatachalam, Sahi & Sharma, 2017). The application of innovative nanotechnology in agriculture is considered as one of the promising approaches to significantly increase crop production (Lal, 2008).
Despite its beneficial potential, its use can be hazardous to health due to the ability to enter the animal cell and plant body and interact with cells. Studies on nanomaterials involve researchers of different areas of knowledge, so there are numerous multidisciplinary reports in the field of nanotechnology; except for some aspects as the interactions with plant biomolecules (Chichiriccò & Poma, 2015).
It is known that AgNPs have bactericidal effects on different phytopathogens (Lamsal et al., 2011; Ouda, 2014; Verma, Kharwar & Gange, 2010), a possible application of AgNP is using them as a disinfectant in seeds. For this reason, it is necessary to study the behavior and effects of nanomaterials in different plant tissues such as seeds. There are no studies on the absorption and effects of AgNP on tomato seeds. The tomato is the second most important vegetable in the world and is a functional food because it provides physiological benefits and meets basic nutritional requirements (Dorais, Ehret & Papadopoulos, 2008).
Some researches in nanotechnology have demonstrated the promising perspective of the development of nanofertilizers and their application. For example, researchers observed that carbon nanotubes and nanoparticles of zinc oxide can penetrate roots and tissues of tomato seeds Lycopersicon esculentum. This indicates that a new nutrient delivery system will be developed through the exploitation of porous domains at the nanoscale surface of plants (DeRosa, Monreal, Schnitzer, Walsh & Sultan, 2010). It is possible that NP can directly enter plant cells through cell wall structures if the particle sizes are smaller than the pore sizes of the cell wall (5 nm to 20 nm). Some authors reported that iron NP penetrate cells of pumpkin plants (González-Melendi et al., 2008). There is an extensive interest in applying NP to plants in the agricultural sector. Seed penetration may be more complicated than cell walls and mammalian cell membranes due to seed thickness, however, it has been shown that the seed coatings of different plant species are selectively permeable to metal ions such as Pb2 + and Ba2 + (Wierzbicka & Obidzinska, 1998).
It is logical to assume that some nanometer-sized materials will be able to penetrate the seed plant cover and affect seed germination. It has recently been shown that exposure of tomato seeds to carbon nanotubes can increase the percentage of germination and improve seedling growth (Khodakovskaya et al., 2009).
The growing demand for analytical information related to artificial inorganic nanomaterials requires the adaptation of existing techniques and methods or the development of new ones. Some well-established techniques, such as electron microscopy and atomic spectrometry, among others, can provide useful information, as is the case with the Atomic Absorption Spectrometry Flame (AAS-F) method, which is fast and simple for the detection and quantification of metal uptake in plants preceded by digestion of the sample.
The study was done using two different concentrations of AgNP and three different exposition times. Second, a reliable method, based on AASF, was developed and tested for the quantification of the metal uptake. To confirm the suitability and reliability of the method (Eurachem Guide, 2014), two Certified Reference Materials (CRM) were used to ensure that the data obtained in the analyses meet the desired quality, providing safety and reliability. The present study was carried out to determine the maximum amount of silver uptake by tomato seeds when they are exposed to AgNP, in such a way that the seeds could have a higher percentage of germination and the seedling growth is improved.
METHODOLOGY
Synthesis of nanoparticles
The synthesis of silver nanoparticles was performed by the chemical reduction method using silver nitrate as the starting material and gallic acid as reducing agent, using the following procedure: silver nitrate and gallic acid were dissolved separately in deionized water and posteriorly the two solutions were mixed and the pH value was adjusted up to 10 by adding ammonium hydroxide dropwise (Martínez-Castañón, Niño-Martínez, Martínez-Gutierrez, Martínez-Mendoza & Ruiz, 2008). The solutions were vigorously stirred for 15 min before and after the mixing. Silver nitrate and gallic acid were purchased from Sigma-Aldrich, and ammonium hydroxide (29.9%, Fermont) was used without any further purification. Mili-Q water (18.2 Ω) was used throughout the experiment. Two sets of samples were prepared with two different silver concentrations of 1 mM and 10 mM, using an Ag:gallic acid molar ratio of 17.2:1. After the synthesis, nanoparticles were filtered and washed 3 times with ethylic alcohol in order to remove residual ionic silver and other byproducts.
Nanoparticles Characterization
UV-Visible spectra of silver dispersion were recorded to verify the presence of the characteristic Surface Plasmon Resonance band of silver nanoparticles by using an Ocean Optics S2000 spectrometer. To determine particle morphology and size, the synthesized nanoparticles were observed in a JEOL model JEM-1230 Transmission Electron Microscope (TEM) at an accelerating voltage of 100 kV. Particle size analysis was performed on a Malvern model Zetasizer Nano ZS Dynamic Light Scattering equipment. Nanoparticles characterization was made using the obtained aqueous dispersion.
Uptake of silver by tomato seeds
Amounts of 0.5 g of tomato seeds (Solanum lycopersicum L.) were weighed. Separately, silver nanoparticles solutions (10 mL) at two different silver concentrations, 1 mM and 10 mM, were prepared. After that, tomato seeds were placed in the previously prepared solutions under vigorously stirring for just 5 min and were left without any stirring during lapses of 1 h, 24 h and 48 h. Each solution sample was triplicate; a total of 18 samples were prepared, 9 for each concentration. In order to quantify the silver uptake by using AAS-F, seeds were filtered from the solutions and washed with deionized water and subject to acidic digestion on a hot plate using an acidic mixture of HCl - HNO3 in a ratio of 1:3 to eliminate organic matter. After digestion, the residue was diluted in 10 ml of deionized water.
Some well-established techniques, such as electron microscopy and atomic spectrometry, among others, can provide useful information related to engineered inorganic nanomaterials, as is the case with the AAS-F method, which is fast and simple for the detection and quantification of AgNP uptake in tomato seeds preceded by digestion of the sample.
RESULTS
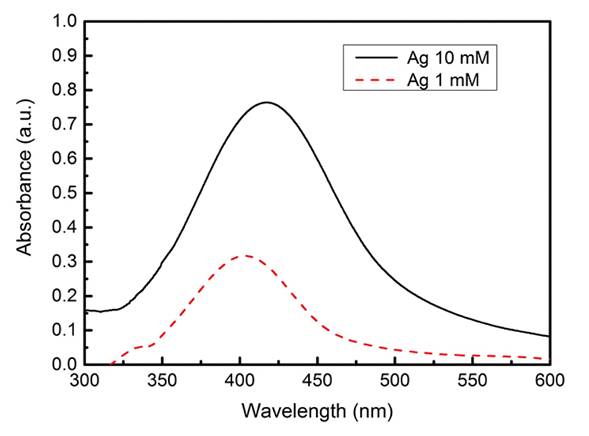
For the synthesis of the AgNP, a dark brown color colloidal dispersion was obtained. The UV-Visible absorption spectrum shows bands at approximately 402 nm and 417 nm for the samples with concentrations of 1 mM and 10 mM respectively; the spectrums can be seen in Figure 1.
The nanoparticles size distributions were determined by DLS as shown in Figure 2.
Figure 3 shows TEM images of the AgNP synthesized in this work, we also include the size distribution histograms taken from the TEM images.

Source: TEM images of the TEM software. Histograms are Author’s own elaboration.
Figure 3 TEM images and size distribution histograms of silver nanoparticles:a) 1 mM and b) 10 mM
To guarantee the reliability of the obtained data, two certified solutions were used, one in preparation of the calibration curve standards of the brand ALPHA SIGMA®, and the other in preparation of two control standards (2.0 μg/ml and 4.0 μg/ml) from an elemental solution of ACC of brand ACCUSTANDARDS®, to verify the instrumental behavior (Figure 4).
The treatments for the experiment with the different concentrations are shown in Figure 5, where the different systems were presented; the time that the seed was in contact with the AgNp, the two concentrations of NP and how much silver was uptaked in mg/Kg.
DISCUSSION
The UV-Visible absorption bands obtained of the AgNP at approximately 402 nm and 417 nm, for the samples with concentrations of 1 mM and 10 mM respectively, agrees with the previously reported by Martínez, stating that silver nanoparticles present an absorption band in a range close to Ultraviolet, approximately between 400 nm and 430 nm, and that they correspond to silver of nanometric size (Martínez-Castañón, Niño-Martínez, Martínez-Gutierrez, Martínez-Mendoza & Ruiz, 2005).
The dispersions of the nanoparticles showed in 0 only present one mode in both samples; the sizes obtained of concentrations 1mM and 10 mM were 21 nm and 29 nm respectively.
From the TEM images (Figure 3), we can see nanoparticles with quasi-spherical shapes and the sizes were obtained with the size distribution histograms showed. For the 1 mM sample, the average size obtained of 100 nanoparticles was 8.5 ± 1.15 nm, while for the sample 10 mM it was 7.7 ± 1.4 nm.
Validation of the Atomic Absorption Spectrometry Flame (AAS-F) method
The performance of the evaluated method can be seen in Figure 4 where it indicated a good performance; the linearity response was evaluated by plotting the absorbance values against its concentration, using 5 standards at a concentration of 0.50 μg/ml -4.0 μg/ml. The analytical response was linear in the working range obtained on the AAS-F calibration curve and the coefficient of determination (r2 = 0.9986).
Limits of detection and quantification LOD and LOQ
The efficiency of the proposed method was performed by the calculation of the Limit of Detection (LOD) and the Limit of Quantification (LOQ). The value obtained by the AAS-F method for the limit of detection was 0.019 μg/ml and for the quantification limit was 0.042 μg/ml. The limits were calculated using concentrations of 3 and 10 times the standard deviation (3SD and 10SD) of the blank for LOD and LOQ respectively (Miller & Miller, 2010; Shrivastava & Gupta, 2011), which corroborates the ability of the method at detection levels lower than analyte concentrations in the sample. LOD and LOQ are expressed in units of concentration (μg/ml Ag+ in solution). The blank was prepared in the same manner as the plant tissue samples, using identical volumes of reagents (Segoviano & García, n.d.). The limits of quantification achieved in the analytical method allow the determination of silver (Ag) in the concentration levels found in the plant tissue samples.
Repeatability and Reproducibility (Accuracy)
Repeatability and reproducibility are generally measured as the Relative Standard Deviation (RSD) of a dataset. Measurements were performed under conditions of repeatability and reproducibility on different measurement days. Repeatability was assessed by measuring two standard solutions (2.0 μg/ml and 4.0 μg/ml) under similar conditions (day, analyst, instrument, samples). The values of % RSD obtained in the repeatability were 0.18% and 0.14%, respectively. Reproducibility was assessed by performing 10 measurements on three different days. The values of % RSD obtained were 0.33% and 0.19%, respectively. The maximum RSD value set by the Association of Official Agricultural Chemists International (AOAC International, 2011) for the analyte level of 1 μg/ml is 11%. Therefore, it can be affirmed that the proposed method showed good repeatability and precision based on the % RSD values obtained.
Absorption of silver in tomato seeds
Figure 5 shows a comparison of different time of treatment: 1 h, 24 h and 48 h, for the two different concentrations, 1 mM and 10 mM, in which it was found that the highest uptake of silver in tomato seeds was obtained for the sample with longer treatment time as well as a higher silver concentration, that is, when the seeds were in contact for 48 h with the AgNP in the maximum concentration of 10 mM.
The low solubility of AgNP used in this experiment suggests that nanoparticles dissolve and penetrate ionically, based on the Larue publication where they reported Ag agglomerates on the surface of lettuce leaves (Lactuca sativa) on the micrometer scale, and on leaf tissues including epidermis, mesophyll, and vascular tissues; thus, AgNP deposited on the surface are able to traverse the epidermis and be transferred to leaf tissue (Larue et al., 2014).
These results are consistent with similar experiments with other types of NP; Au (10 nm - 20 nm), TiO2 (14 nm) and CeO2 (37 nm) NP are shown to accumulate in crop plants, rape and maize respectively after exposure Foliar (Arora et al., 2012; Birbaum et al., 2010; Larue et al., 2012). The mechanisms of foliar transfer of NP are little known. Two pathways have been proposed for hydrophilic compounds through aqueous pores, cuticles and stomas, and for lipophilic diffusion through the cuticle. Although uncoated AgNP are considered as hydrophilic, their possible coating by cuticular waxes may increase their lipophilicity and favor their transfer through the cuticle (Schreck et al., 2012). In the present work, it is shown that the synthesized AgNP penetrate the tomato seeds and, by increasing the concentration of the solution with AgNP, increases the absorption in the seed.
CONCLUSION
According to our results, tomato seeds presented a silver absorption in very small amounts, and those values are below to those established by the Official Mexican Norm for the quantification of heavy metals in foods NOM-130-SSA1-1995. On the other hand, a simple method has been developed for the determination of silver content in nanomaterials of aqueous matrices by acid digestion followed by atomic absorption spectroscopy flame. It was found that the acid digestion is efficient for extraction of the analyte in a single step. The developed method provides limits of detection and quantification of 0.19 µg/ml and 0.42 µg/ml, using criteria of the Eurachem-2016 guide while the limit of detection using the calibration curve was 0.052 µg/ml. The method has been applied to determine the total content of silver in aqueous nanomaterials. The limits of quantification achieved in the analytical method allow the determination of silver in the concentration levels found in the plant tissue samples which gives the highest silver uptake in the tomato seeds to the samples with the highest concentration and longer time of treatment, which were 10 mM and 48 h respectively. It is expected that with the exposure of tomato seeds to silver, the probability of germination and seedlings growth will be increased, as well as the reduction of the use of pesticides; however, it is necessary to further research into the use, effect, and application of nanomaterials in agriculture.











 nueva página del texto (beta)
nueva página del texto (beta)