INTRODUCTION
World’s human population has increased nearly fourfold in the past 100 years. It is projected to exceed 9 billion by 2050 (Food and Agriculture Organization [FAO], 2011). Nearly 40% of the world’s potential crop production is lost each year because of weeds, pests and diseases effects (FAO, 2011), an argument for use of pesticides for crop protection.
Besides agricultural applications, pesticides are used to mitigate presence of organisms that are potentially harmful to human health, especially in countries with tropical climates (World Health Organization [WHO], 2010). Pesticides are systematically used in agriculture, industry, households, gardens, and veterinary medicine (Comisión Intersecretarial para el control de proceso y uso de plaguicidas, fertilizantes y sustancias tóxicas [Cicoplafest], 2004). Benefits of pesticide use are many; however, dispersion of large amounts to environment adversely affects ecosystems as well as human health (Aktar, Sengupta & Chowdhury, 2009).
Culiacan city, capital of the state of Sinaloa in northwestern Mexico, lies on a plain that includes more than 130 000 ha of crops (42 000 km2 of intensive agriculture) representing 42% of Sinaloa State and 4% of farmland in Mexico. Large quantities of fertilizers and pesticides are applied there (Ruiz-Fernández, Páez-Osuna, Hillaire-Marcel, Soto-Jiménez & Ghaleb, 2001). Sinaloa State is a semiarid region, with rainfall events from June to September (mean annual precipitation 640 mm) and dryness rest of year (Instituto Nacional de Estadística y Geografía [INEGI], 2011).
Culiacan River Basin is in Hydrological Region 010, in northwestern of Mexico, with a surface area of 18 800 km2, and is formed by Tamazula and Humaya rivers confluence in Culiacan city. Culiacan River crosses Navolato city and empties into Altata-Ensenada del Pabellon Lagoon (AEPL) in El Castillo village, Navolato (Comisión Nacional del Agua [Conagua], 2004), where intensive farming of shrimp (Penaeus spp.), oysters (Crassostrea spp.), mussels (Chione spp.) and fish (e.g. Mugil spp., Gerres spp., Lutjanus spp.) produces about 3000 t per year. This lagoon system has been extensively studied and high levels of phosphorus, heavy metals and pesticides have been documented (Carvalho, Fowler, González-Farías, Mee & Readman, 1996; Carvalho et al., 2002; Galindo-Reyes, Villagrana-Lizárraga, Guerrero-Ibarra & Quezada-Urenda, 1992; Galindo- Reyes, Fossato, Villagrana-Lizarraga & Dolci, 1999a; Galindo-Reyes, Villagrana-Lizárraga & Lazcano-Álvarez, 1999b; Green-Ruiz & Páez-Osuna, 2001; Páez-Osuna, Osuna-López, Izaguirre-Fierro & Zazueta-Padilla, 1993a; b; Páez-Osuna, Osuna-López, Izaguirre-Fierro & Zazueta-Padilla, 1994; Páez-Osuna, Bojórquez-Leyva & Green-Ruiz, 1998; Ruiz-Fernández et al., 2001; Ruiz-Fernández, Hillaire-Marcel, Ghaleb, Soto-Jiménez & Páez-Osuna, 2002; Ruiz-Fernández, Hillaire-Marcel, Páez-Osuna, Ghaleb & Soto-Jiménez, 2003), including clear evidence of eutrophication problems (De la Lanza & Flores-Verdugo, 1998).
Region exports vegetable crops to USA each year, particularly tomatoes and bell peppers. The lower basin of Culiacan River combines the highest economic returns of the two most important economic activities of the State of Sinaloa, crop production and livestock: approximately 47 848 ha are under crop production (tomato, cucumber, bell pepper, and eggplant) and there are more than 30 cattle farms (INEGI, 2011). Irrigation system on these farms is provided by a series of canals derived from Humaya and Tamazula rivers conjunction into Culiacan River, which crosses Culiacan city (population, approximately one million) before reaching agricultural fields, subjecting it to various opportunities for pesticide contamination (Junta Municipal de Agua Potable y Alcantarillado de Culiacan [JAPAC], 2001).
It is important to monitor pesticides in nearby ecosystems to agricultural areas to know the impact of agriculture on the environment. The objective of this study was to monitor presence and levels of pesticides in Culiacan, Humaya and Tamazula rivers from July 2008 to June 2009. This is one of the first monitoring of pesticides in Culiacan Valley’s water, an extremely important national agricultural region.
MATERIALS AND METHODS
Sampling sites
Six sampling sites (figure 1) were selected on basis of existence of farming activities and/or natural runoff (streams, canals, etc.).

Source: Author’s own elaboration.
Figure 1 Sampling sites in rivers of Culiacan River basin, Sinaloa, Mexico.
Sites A and B lay on upper reaches of Humaya River (Agua Caliente town, 24°52’07’’ N 107°23’28’’ W) and Tamazula River (Jotagua town, 24°51’52’’ N 107°16’12’’ W) respectively, where effects of agricultural activity were minimal. Sites C, D, E and F represented agricultural land that forms Culiacan Valley (20°40’N 107°30’W) from confluence of Humaya and Tamazula rivers: C, sub basin of Culiacan River [Puente Negro (24°48’33’’ N 107°24’28’’ W); D, Cofradía de Navolato (24°48’01’’ N 107°36’60’’ W); E, Iraguato (24°37’27’’ N 107°39’52’’ W); and F, El Castillo (24°29’60’’ N 107°42’28’’ W).
Sample collection
Fresh surface water was collected, to a 30 cm depth, twice a month from each sampling site over a period of one year (June 2008 to July 2009) (Mejias & Jerez, 2006). Samples, in 1 L amber glass containers, were placed in an ice chest that excluded light, and were transported to the National Food Safety Research Laboratory at the Center of Research for Food and Development (CIAD, for its acronym in spanish Culiacan Unit) and stored at 4 ºC until pesticide extraction. In order to avoid biological degradation of pesticides, extraction was performed within 48 h of sample collection.
Analysis of pesticides
Samples were analyzed in two gas chromatographs Varian 3800 and CP3800 (GC), equipped with; electron capture detector (ECD), thermionic specific detector (TSD), electrolytic conductivity detector (ELCD) and pulsed flame photometric detector (PFPD). In all cases, certified reference materials (analytical standards of pesticides) were used (Chem Service, USA). Solvents (acetone, dichloromethane, hexane and petroleum ether) were pesticide grade (Burdick & Jackson®) purchased from a local supplier (Sumilab, Mexico). Chromatographic conditions of analysis for each pesticides group are shown in table 1.
Table 1 Chromatographic conditions for pesticides groups.
| Chromatographic Conditions | Organophosphates | Organochlorines and Pyrethroids | Organonitrogens |
|---|---|---|---|
| Detector | PFPD | ECD | TSD |
| Column | CP SIL 5CB de 30 m × 0.53 mm | CP SIL 5CB de 25 m × 0.32 mm | HP 5 de 25 m × 0.53 mm |
| Temperature of detector | 250 ºC | 300 ºC | 300 ºC |
| Temperature of injector | 240 ºC | 230 ºC | 250 ºC |
| Gas carrier | Nitrogen | Nitrogen | Nitrogen |
| Volume injected | 2 μL | 1 μL | 2 μL |
Source: Author’s own elaboration.
Pesticides were detected in an initial chromatographic run and confirmed with another detector. Methods 507 and 508 reported by US Environment Protection Agency (US EPA) were used for pesticide quantification.
Sample preparation
Water samples were filtered through a Whatman #2 filter paper. Filtrate and 50 mL PBS 2M were combined with samples and pH was adjusted to 7.0 by addition of HCl or NaOH (solution 0.1 M) when was necessary (Environmental Protection Agency [EPA], 1995a; b).
Extraction of pesticides
Each 1 L water sample was combined with 150 g of sodium chloride and stirred in a separatory funnel until dissolved. Mixture was extracted with dichloromethane (3 mL × 60 mL), shaken for 2 min and allowed to stand for 10 minutes to achieve phase separation. Extracts were combined, dried with anhydrous sodium sulfate and collected in a 500 mL Kuderna-Danish concentrator, then attached to a 10 mL collection tube (EPA, 1995a; b) and concentrated in a steam bath. Later, azeotropic distillation used petroleum ether (150 mL) and acetone (30 mL). Finally, extract was concentrated until 1 mL of acetone (McMahon & Hardin, 1998).
Method Validation
Quality control of analytical method was based on recovery rates of fortified matrix samples (distilled water) with groups representing pesticide organochlorines, organophosphates, synthetic pyrethroids and methylcarbamates. Fortification levels ranged from 0.065 μg/L to 5.58 μg/L. Recovery rates ranged from 70% to 114% and relative standard deviation (RSD) was <13% for all added analytes. Limits of detection (LOD) and quantification (LOQ) were calculated by radio signal/noise ratio (S/N or Np-p) method proposed by the International Conference on Harmonization (ICH, 1996). LOD were between 0.001 μg/L and 0.059 μg/L, and quantification limits were three times LOD (table 2).
Table 2 Parameters used for the evaluation of the analytical method.
| Pesticide | Recovery (%) | RSD (%) | LOD | LOQ |
|---|---|---|---|---|
| Dimethoate | 80.3 | 5 | 0.015 | 0.045 |
| Diazinon | 70 | 12.9 | 0.015 | 0.045 |
| Malathion | 81.1 | 8.2 | 0.015 | 0.045 |
| Propachlor | 71.5 | 5.8 | 0.001 | 0.003 |
| Atrazine | 78.45 | 12.9 | 0.01 | 0.059 |
| Metalaxyl | 78.5 | 9.6 | 0.01 | 0.059 |
| Lindane | 106.65 | 5.8 | 0.001 | 0.003 |
| Heptachlor | 89.325 | 9.6 | 0.001 | 0.003 |
| Aldrin | 70.1 | 1.9 | 0.001 | 0.003 |
| Dichloropropanilide | 95.775 | 12.9 | 0.001 | 0.003 |
| pp DDE | 108.575 | 9.6 | 0.001 | 0.003 |
| Endosulfan beta | 113.825 | 12.6 | 0.001 | 0.003 |
| Cis permethrin | 78.225 | 9.6 | 0.001 | 0.003 |
| Trans permethrin | 81.275 | 9.6 | 0.001 | 0.003 |
| Carbaryl | 79.125 | 5 | 0.059 | 0.177 |
Source: Author’s own elaboration.
Results were within range suggested by EURACHEM Guide (Magnusson & Örnemark, 2014).
RESULTS
Frequencies and concentration levels of pesticides in water samples
Of 144 water samples analyzed, 24 were taken from Tamazula River, 24 from Humaya River and 96 from Culiacan River. Pesticides were present in 81% (116/144) of analyzed samples. Their frequencies are shown in figure 2.
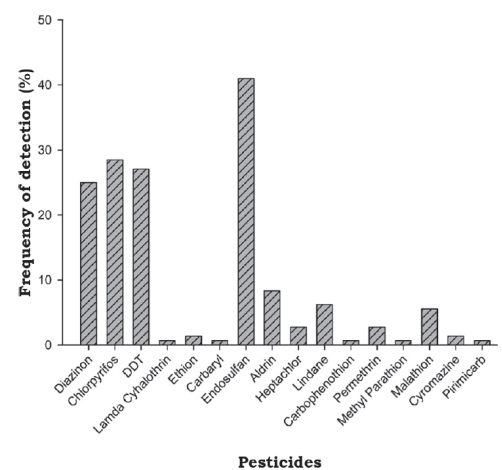
Source: Author’s own elaboration.
Figure 2 Frequency of individual pesticides detected in 144 water samples from six sites in Culiacan River basin.
Around the world, pesticides monitoring in natural environments are important by allows early detection of problems with products toxicity used to control pests and alterations or disturbances generated in environment. Previous studies show that pesticides presence in water is very variable, however most published studies show that >50% of samples contain at least one pesticide. For example, Picó et al. (1994) found pesticides (organochlorines, carbamates, triazines and organophosphates) in 62.5% (25/40) of water samples from rivers Canyoles, Albaida, Claria, Serpis, Polop, Belcaire, Turia and Xuquer, Lake Albufera and irrigation canals on south coast of Spain, in the most important agricultural area in Valencia. Besides, Darko, Akoto & Oppong (2008) reported presence of organochlorines pesticides in water samples from Bosomtwi Lake, Ghana. DDT was detected in 66% (33/50), lindane in 64% (32/50) and endosulfan in 56% (28/50) at mean concentrations of 0.012 ng/g, 0.071 ng/g and 0.064 ng/g, respectively.
Presence of pesticides in water samples is highly variable and may depend on many factors including sample size, number of samples analyzed; extraction method used and most important use of pesticides in study area. Similarly, most studies show a positive sample percentage above 50% for at least one pesticide (Darko, Akoto & Oppong, 2008; Picó et al., 1994).
In the present study, 15% of positive samples (21/144) were from Tamazula River, 12% (17/144) from Humaya River and 73% (106/144) from Culiacan River. The highest percentage of samples containing pesticide residues corresponded to Culiacan River. This may be related to the large area of intensive agriculture in Culiacan River lower basin.
Concentrations of pesticides (table 3) were within range permitted by ecological standards of water quality in Mexico (Diario Oficial de la Federación [DOF], 1989), and were below levels permitted by US EPA regarding protection of freshwater aquatic life (EPA, 2006).
Table 3 Concentrations of pesticides in river water samples from the Culiacan river basin.
| Pesticide | Tamazula River (μg/L)a ± S.D. | Humaya River (μg/L)a ± S.D. | Culiacan River (μg/L)a ± S.D. | CE-CCAb (μg/L) | EPAc (μg/L) |
|---|---|---|---|---|---|
| Diazinon | 0.0296 ± 0.01 (6) | 0.0211 ± 0.01 (4) | 0.0403 ± 0.02 (28) | _ | 0.17 |
| Chlorpyrifos | 0.0296 ± 0.01(2) | 0.0163 ± 0.007 (8) | 0.0157 ± 0.01 (32) | _ | 0.083 |
| DDT | 0.05102 ± 0.04(7) | 0.0525 ± 0.05 (10) | 0.0425 ± 0.05 (32) | 1 | 1.1 |
| Endosulfan | 0.03601 ± 0.03(18) | 0.0201 ± 0.01 (12) | 0.0198 ± 0.01 (44) | 0.2 | 0.22 |
| Ethion | N.D. | N.D. | 0.0162 ± 0.006 (4) | _ | _ |
| Aldrin | 0.0229 ± 0.01(6) | 0.0099 ± 0.00 (1) | 0.1023 ± 0.16 (7) | 3 | 3 |
| Heptachlor | 0.0377 ± 0.02(4) | N.D. | 0.0059 ± 0.01 (4) | 0.5 | 0.52 |
| Lindane | 0.0104 ± 0.001(5) | 0.0041 ± 0.00 (1) | 0.0045 ± 0.003 (7) | 2 | 0.95 |
| Carbaryl | N.D. | N.D. | 0.1113 ± 0.00 (1) | _ | _ |
| Carbophenothion | N.D. | N.D. | 0.0099 ± 0.00 (1) | _ | _ |
| Permethrin | N.D. | N.D. | 0.2351 ± 0.18(7) | _ | _ |
| Methyl Parathion | 0.0036 ± 0.00(1) | N.D. | 0.0098 ± 0.004 (6) | 0.04 | 0.65 |
| Cyromazine | 0.1177 ± 0.00(1) | N.D. | 0.1961 ± 0.00 (1) | _ | _ |
| Pirimicarb | N.D. | N.D. | 0.0772 ± 0.00 (1) | _ | _ |
| Malathion | 0.0059 ± 0.0302(5) | N.D. | N.D. | _ | 0.1 |
| Lamda Cyhalothrin | 0.0059 ± 0.0302(5) | 0.0212 ± 0.00 (1) | N.D. | _ | _ |
Mean concentration; [ ]: indicates number of concentrations; S.D.: Standard Deviation; b: CCE-CCA, Ecological Criteria for Water Quality, DOF (1989); c: EPA, Environmental Protection Agency; N.D.: Not Detected. Source: Author’s own elaboration.
The highest concentration of pesticides in Tamazula River occurred during July and corresponded to DDT (0.156 μg/L) and endosulfan (0.156 μg/L). For Humaya River, the highest concentration also occurred in July and corresponded to DDT (0.167 μg/L). In Culiacan River, the maximum concentration of pesticides differed among sampling sites. At site C the highest concentrations were cyromazine (0.196 μg/L) in September and DDT (0.286 μg/L) in July; at site D the highest was aldrin (0.462 μg/L) in January; at site E the highest was carbaryl (0.111 μg/L) in July; and at site F the highest was diazinon (0.124 μg/L) in September.
Concentration of pesticide residues did not differ significantly among sampling sites, mainly because constant movement and exchange of water masses through system caused mixing and homogenization of pesticides dissolved and suspended in water.
The highest incidence of different types of pesticide residues in the months of July, August and September concur with the end of spring-summer crop season in Culiacan Valley and rainy season arrival. This suggests that pesticides are retained in soil -considering that application peak is February and March (Leyva-Morales et al., 2014)-, and are released and transported by runoff or agricultural drains to river. Some studies have suggested that DDT is associated with agricultural soil erosion, and may be settled in bodies of water in a stream of detritus and then undergoes microbial degradation to become DDE (dichlorodiphenyldichloroethylene) (Aislabie, Richards & Boul, 1997; Miglioranza, Aizpún & Moreno, 2003).
Organochlorines (OCs)
Frequencies of detection of organochlorine pesticides in samples were, in descending order, endosulfan (41%)> DDT (27.1%)> aldrin (8.3%)> lindano (6.3%)> heptachlor (2.8%) (figure 2).
Persistence and movement of pesticides in environment are determined by characteristics such as water solubility, octanol-water partition coefficient (Kow), organic carbon sorption constant (Koc), Henry’s law constant and half-life (DT50) (table 4).
Table 4 Physicochemical characteristics of pesticides identified in water of rivers in the Culiacan valley.
| Pesticide | Chemical Class | Water Solubility at 20 °C (mg/L)a | Henry's Law Constant (Pa m3/mol)a | cKOC (mL/g)a | dKow (Log P)a | Half-Life (days) in Watera |
|---|---|---|---|---|---|---|
| Aldrin | Organochlorine | 0.027 | 1.72 × 1001 | 17 500 | 6.5 | ND |
| Carbaryl | Carbamate | 9.1 | 9.20 × 10-05 | 300 | 2.36 | 12 |
| Carbophenothion | Organophosphate | 0.34 | 5.80 × 10-01 | 50 000 | 4.75 | ND |
| Cyromazine | Triazine | 13,000 | 5.80 × 10-09 | 756b | 0.069 | 28b |
| Chlorpyrifos | Organophosphate | 1.05 | 0.478 | 8151 | 4.7 | 29.6 |
| DDT | Organochlorine | 0.006 | 8.43 × 10-01 | 151 000 | 6.91 | 28e |
| Diazinon | Organophosphate | 60 | 6.09 × 10-02 | 609 | 3.69 | 50 |
| Endosulfan | Organochlorine | 0.32 | 1.48 | 11 500 | 4.75 | 20 |
| Ethion | Organophosphate | 2 | 3.85 × 10-02 | 10 000 | 5.07 | 146 |
| Heptachlor | Organochlorine | 0.056 | 3.53 × 1002 | 24 000 | 5.44 | 1 |
| Lamda Cyhalothrin | Pyrethroid | 0.005 | 2.00 × 10-02 | 283 707 | 5.5 | 40 |
| Lindane | Organochlorine | 8.52 | 1.483 × 10-06 | 1270 | 3.5 | 28 |
| Malathion | Organophosphate | 148 | 1.00 × 10-03 | 1800 | 2.75 | 6b |
| Methyl Parathion | Organophosphate | 55 | 8.57 × 10-03 | 240 | 3 | 9 |
| Permethrin | Pyrethroid | 0.2 | 1.89 × 10-01 | 100 000 | 6.1 | 1 |
| Pirimicarb | Carbamate | 3,100 | 3.30 × 10-05 | 388b | 1.7 | 6 |
University of Hertfordshire (2007); b: Kegley, Hill, Orme & Choi (2011); cKoc = Organic Carbon Sorption Constant; dKow = Octanol-Water Partition Coefficient; e: Oregon State University (1998); ND = No Data. Source: Author’s own elaboration.
OC pesticides have a relatively short half-life in water (<30 days), are practically insoluble in it (<50 mg/L) and immobile (Koc> 4000 mL/g), and volatility per Henry’s law constant is moderate (0.1 Pa m3/mol -100 Pa m3/mol) for aldrin, DDT and endosulfan and high (>100 Pa m3/mol) for heptachlor. They have good affinity for organic matter, so they are deposited in aquatic ecosystems sediment (Instituto Nacional de Ecología y Cambio Climático [INECC], 2007; University of Hertfordshire, 2007; Yang, Lv, Shi & Jiang, 2005). These characteristics may account for their low concentrations in water samples. However, owing to their high Kow (Kow> 3) OCs tend to bioaccumulate in environment and may be expected to be found in higher concentrations in sediment and fish or other aquatic organisms.
DDT and its metabolites are ubiquitous and persistent compounds that have been detected in marine water and sediment as well as in shrimp and clams in AEPL near Culiacan Valley (Galindo-Reyes et al., 1992; Galindo-Reyes et al., 1999a; b). Persistence of DDT may be associated with extensive use throughout the world, including Culiacan Valley, for both domestic and agricultural purposes (Ecobichon, 1995; Carvalho et al., 2002). Most countries prohibited its use in early 1970s owing to its persistence in environment and accumulation in food chain (Bolt & Degen, 2002). Mean concentrations of DDT were 0.051 μg/L, 0.0525 μg/L and 0.0425 μg/L for Tamazula, Humaya and Culiacan rivers, respectively. Carvalho et al. (2002) attributed presence and high persistence of DDT in this region to heavy seasonal application in past and discharge of residues to irrigation water.
Endosulfan is an insecticide-acaricide of few OCs authorized in Mexico for use in cultivation of cucumber, pear, melon, snuff (Nicotiana sp.), tomato, wheat and grapes, and in industry exclusively for plants formulators of pesticides (Cicoplafest, 2004). Commercial product of endosulfan is a mixture of stereoisomers, designated "α" (CAS No. 959-98-8) and "β" (CAS 33213-65-9), in a ratio of 70:30, and it may further contain small amounts of endosulfan sulfate and others related chemicals (Fan, 2007). It is liposoluble, with low water solubility (0.32 mg/L) and high solubility in most organic solvents. It has a cyclic structure, low vapor pressure (Henry's law constant of 1.48 Pa m3/mol), a high chemical stability, and a remarkable resistance to attack by microorganisms. It tends to accumulate in fatty tissue of living organisms (Kow =4.75), and in soil (Koc = 11 500 mL/g) and groundwater (Oregon State University, 1998). Mean concentrations of endosulfan were 0.03601 μg/L, 0.0201 μg/L and 0.0198 μg/L for Tamazula, Humaya and Culiacan rivers, respectively (table 3). In water, half-life of α-endosulfan is approximately one to three months whereas that of β-endosulfan and endosulfan sulfate (a transformation product of both isomers) is more than two years (Fan, 2007; United Nations Environment Programme [UNEP], 2007), which explains the high frequency of this pesticide in river water. Also, its use as a commercial and domestic insecticide might contribute to its presence in water. This condition is associated with water run-off and attachment to soil particles. This varies from season to season and year to year, depending on weather conditions including dry and rainy seasons (Said, El Moselhy, Rashad & Shreadah, 2008).
Lindane (γ-HCH) constitutes about 10% - 15% of technical product of hexachlorocyclohexane, and is authorized in Mexico for treatment of seeds of oats, barley, corn, sorghum and wheat, for industrial use in plants formulators of pesticides, for control of some pests of livestock (mites, lice, etc.) and for urban use exclusively in health campaigns (Cicoplafest, 2004). Lindane is strongly adsorbed to soils with high levels of organic matter, but there are indications that volatilization is an important route of dissipation under tropical conditions and high temperatures. It rapidly degrades to tetrachlorociyclohexenes and pentachlorociyclohexanes when exposed to ultraviolet radiation (Instituto Nacional de Ecología [INE], 2004). Lindane is included in various international conventions such as Stockholm Convention, Rotterdam Convention and Aarhus Protocol on Persistent Organic Pollutants (POPs) to Convention on Long-range Transboundary Air Pollution (INE, 2004). Mean concentrations of lindane were 0.0104 μg/L, 0.0041 μg/L and 0.0045 μg/L for Tamazula, Humaya and Culiacan rivers, respectively and hence lower than those of other OCs (table 3).
Organophosphates (OPs)
Frequencies of detection of organophosphate pesticides (OPs) in samples were, in descending order, chlorpyrifos (28.5%)> diazinon (25%)> malathion (5.6%)> ethion (1.4%)> carbophenothion (0.7%)> methyl parathion (0.7%) (figure 2).
Solubility in water is low (≤50 mg/L) for carbophenothion, chlorpyrifos and ethion, and moderate (50 mg/L- 500 mg/L) for diazinon, malathion and methyl parathion. Half-life in water also varies: <30 days for chlorpyrifos, malathion and methyl parathion, 30 - 100 days for diazinon, and 100 - 365 days for ethion. With respect to their bioaccumulation, methyl parathion and malathion are moderately bioaccumulative (Kow between 2.7 and 3), while other OPs are highly bioaccumulative (Kowow> 3). Organic carbon sorption constant varies, with methyl parathion being slightly mobile (Koc between 75 mL/g and 500 mL/g), diazinon and malathion moderately mobile (Koc between 500 mL/g and 4000 mL/g) and carbophenothion, chlorpyrifos and ethion highly mobile (Koc> 4000 mL/g) (table 4) (INECC, 2007; University of Hertfordshire, 2007). Mean concentrations of diazinon were 0.0296 μg/L; 0.0211 μg/L and 0.0403 μg/L and of chlorpyrifos were 0.0296 μg/L, 0.0163 μg/L and 0.0157 μg/L for Tamazula, Humaya and Culiacan rivers, respectively. Other OPs were detected infrequently or only in one river (e.g. ethion in Culiacan River or malathion in Tamazula River) (figure 2 and table 3). Low concentrations of chlorpyrifos and diazinon are explained by their short half-life in environment.
Pyrethroids
Lambda cyhalothrin and permethrin were detected with low frequency (0.7% and 2.8%, respectively). Their water solubilities are low (0.005 mg/L and 2.0 mg/L, respectively). Half-life in water is 1 day for permethrin and 40 days for lambda cyhalothrin. Both compounds have a high Koc (Koc> 4000) and Kow (Kow> 3), and for this reason can be fixed in soil, sediment, biota and organic matter and/or move to surface waters and, also bioaccumulate in body fat of animals. Food chain is the main route of exposure for animals and humans (table 4) (INECC, 2007). Lambda cyhalothrin was detected only in Humaya River (at 0.0212 μg/L) and permethrin was found only in Culiacan River (at 0.2352 μg/L). Low concentrations and frequencies indicate a recent application in the area (figure 2 and table 3).
Carbamates (CBs)
Carbaryl and pirimicarb were also present although with very low frequency (approximately 1% of all analyzed samples). Whereas solubility of carbaryl in water is low (9.1 mg/L), for pirimicarb it is high (3100 mg/L). Both compounds have a low half-life in water (12 and 6 days, respectively) and also are considered moderately mobile (Koc between 75 mL/g - 500 mL/g) and their bioaccumulation is low (Kow< 2.7). Therefore, presence of these compounds suggests a recent application. Pirimicarb and carbaryl were detected solely in Culiacan River, at concentrations of 0.1113 μg/L and 0.0772 μg/L, respectively (figure 2 and table 3); agricultural use is authorized in the area for a variety of crops (Cicoplafest, 2004).
Other pesticides
Cyromazine, a compound of the triazines group, was detected in 1.4% of samples. Its solubility in water is very high (13 000 mg/L), its bioaccumulation (Kow< 2.7) and half-life in water (28 days) are low, and it is considered slightly mobile (Koc = 756 mg/L) (INECC 2007; University of Hertfordshire, 2007). Cyromazine was detected in Tamazula and Culiacan rivers at concentrations of 0.1177 μg/L and 0.1961 μg/L, respectively (table 3). Cyromazine was detected in Tamazula and Culiacan Rivers at 0.1177 μg/L and 0.1961 μg/L, respectively (table 3).
CONCLUSIONS
Pesticides detected in Culiacan River lower basin were at concentrations below the maximum allowed by national (DOF, 1989) and international (EPA, 2006) laws that enforce protection of freshwater aquatic life. They indicate a low influence of agricultural activities, as well as, proper use of agricultural pesticides in studied region.
Some compounds of recent use are not covered by any of regulations (e.g. cyromazine) so it would be instructive to compare their presence here with records from other parts of the world.
Most of compounds found were organochlorines indicating historical contamination, mainly associated with DDT metabolites not necessarily parent compound. Use of some of these compounds (e.g. lindane) has been restricted in Mexico, whereas in some countries they have already been banned. However, comparatively low concentrations and frequencies suggest that pesticide contamination derived from runoffs toward river from contaminated soil particles and not from use of pesticides in intensive agriculture of region.











 nova página do texto(beta)
nova página do texto(beta)


