INTRODUCTION
Click chemistry is an efficient method for synthesis of new and diverse compounds (i.e. very selective, with high yields, wide scope and without side reactions). It is based in a carbon-heteroatom bond formation and is an innovative functionalization strategy for synthesis of biomolecules. This strategy has been particularly useful for coupling azides with alkynes to get 1,2,3-triazole compounds, small molecules, not found as natural products, which exhibited a broad variety of biological activities such as (Figure 1): 1 against Mycobacterium tuberculosis (Gallardo et al., 2007), 2 as antitumoral agent (Yu et al., 2010), 3 as antifungal (Rajasekaran, Murugesan & AnandaRajagopal, 2006) and 4 as antileishmanial (Ferreira et al., 2007). In particular, 1,2,3-triazole has been used as basic pharmacophore structure for development of antineoplasics (Wu et al., 2009), insecticidals (Boddy et al., 1996), antibacterials (Phillips, Udo, Abdel-Hamid & Varghese, 2009), antituberculars (Gill et al., 2008) and anti-HIV agents (Giffin et al., 2008; Whiting et al., 2006). Moreover, these molecules have been used for labelling biomolecules (Ferro-Flores et al., 2010).
Currently, resistance to first-line antibiotic agents is a severe problem. Infections caused by resistant microbes fail to respond to treatment resulting in prolonged illness and greater risk of death. Thus, these are motors for development of new compounds with potential antibacterial activity.
On the other hand, there is an increasing interest in antioxidants, particularly in those which counteract deleterious effects of free radicals in human body as well as deterioration of fats and other food constituents. Screening of antioxidant activity is commonly done by in vitro assays, such as the ABTS (3-ethylbenzothiazoline-6-sulphonic acid)•+ and DPPH (2,2-diphenyl-1-picrylhydrazyl)• methods. Recently, we reported synthesis of heterocyclic compounds with antiparasitary (Montes-Ávila, Díaz-Camacho, Sicairos-Félix, Delgado-Vargas & Rivero, 2009) and antioxidant (Montes-Ávila, Delgado-Vargas, Díaz-Camacho & Rivero, 2012) activities.
Based on the literature, 1,2,3-triazoles could be the basic structure for designing and synthesis of compounds with antibacterial and antioxidant activities. Antibacterial, antioxidant and toxicity activities of sixteen 1-benzyl-1,2,3-triazole derivatives compounds, are reported in this paper.
MATERIALS AND METHODS
Chemistry
All reagents were purchased in the highest quality available and were used without further purification. Solvents used in column chromatography were obtained from commercial suppliers and used without distillation. Nuclear Magnetic Resonance 1H (200 MHz) and 13C (50 MHz) spectra were recorded on a Varian Mercury 200 MHz Spectrometer in CDCl3 with TMS as internal standard. Chemical Ionization Mass spectra were obtained at a GC-MS Varian Titan 4000 with ion trap, and intensities were reported as a percentage relative to base peak after the corresponding m/z value. All synthesis reactions were carried out under microwave irradiation in heavy-walled Pyrex tubes sealed with aluminum crimp caps fitted with a silicon septum. Microwave heating was carried out with a single mode cavity Discover Microwave Synthesizer (CEM Corp.) producing continuous irradiation at 2455 MHz.
Bacteria and the antibacterial assay
Activity against nine human bacterial pathogens were evaluated; two strains were ATCC (DIFCO Laboratories, MI, U.S.A.) (i.e. Staphylococcus aureus 29213 and Escherichia coli 25922) and seven were clinical isolates provided by the bacteriology laboratory of the Instituto Nacional de Pediatría, Secretaría de Salud in Mexico, City (i.e. Streptococcus group A-4, Staphylococcus aureus 3, E. coli AO11, E. coli AO19, E. coli AO55, Salmonella enterica serovar Typhi and Shigella dysenteriae).
For evaluation of antibacterial activity, 1-benzyl-1,2,3-triazoles were dissolved in an aqueous solution of DMSO (5% v/v) and evaluated at the following concentrations: 3.125 μg/mL, 6.25 μg/mL, 12.5 μg/mL, 25 μg/mL, 50 μg/mL, 100 μg/mL, 200 μg/mL, 225 μg/mL, 250 μg/mL, 275 μg/mL, 300 μg/mL, 350 μg/mL and 400 μg/mL. Antibacterial activity was determined by microdilution assay in 96-well plates (Valgas, Souza, Smânia & Smânia, 2007; Zgoda & Porter, 2001). Strains were cultured in Petri dishes (TSA, tripticase soy agar) at 37 °C for 18 h - 20 h; 2 - 5 colonies were suspended in 1.0 mL of 0.85% NaCl (w/v) and density adjusted to 108 CFU/mL (0.5 McFarland value). Bacterial culture was diluted to 106 CFU/mL and 50 μL were deposited per well and compound at each concentration was added in 50 μL. Gentamicin (0.5 μg/mL, 1 μg/mL, 2 μg/mL, 4 μg/mL, 8 μg/mL, 16 μg/mL and 32 μg/mL) was used as positive control, microorganisms without additives as negative control and bacterial toxicity of dissolving solvents was also evaluated. 96 well plates were incubated (37 °C/18 h- 20 h). Activity inhibition was determined according to Aufort, Herscovici, Bouhours, Moreau & Girard (2008).
Brine shrimp lethality bioassay
The 1-benzyl-1,2,3-triazoles were evaluated for lethality to brine shrimp larvae according to the procedure described by Michael, Thompson & Abramovitz (1956). Briefly, dried brine shrimp eggs were incubated in saline medium with light for 48 h. One-day-old larvae (10 - 12 per vial in 100 µL of saline solution) were transferred into 96-well plates and the 1,2,3-triazoles (100 µL) were added at concentrations of 100 µg/mL, 300 µg/mL, 500 µg/mL, 700 µg/mL and 1000 µg/mL. In each case four replicates of each concentration were assayed. The number of dead larvae was counted at 24 h and the Median Lethal Doses (LD50) with 95% confidence intervals were determined by Probit analysis with SPSS Statistics software v19 (IBM company). Evaluated susbstances were classified by the LD50 values as follows: LD50 ≥ 1000 µg/mL, no toxic; 100<LD50<1000, moderate toxic; and 10<LD50<100, very toxic.
DPPH-scavenging Activity
Scavenging activities of 1-benzyl-1,2,3-triazoles derivatives towards DPPH were assessed by the method described by Scherer & Godoy (2009) with slight modifications. Briefly, a solution of DPPH (0.15 mM) in methanol was prepared. The 1,2,3-triazole derivatives at 100 μg/mL (0.2 mL) were mixed with the DPPH solution (1.8 mL); the mixture was vigorously shaked, incubated in darkness conditions (37 °C/ 30 min), and absorbance was measured at 515 nm by using a UV-visible spectrophotometer, Spectronic Genesys 20. The DPPH-scavenging activity of the 1,2,3-triazoles was calculated as follows
DPPH-scavenging effect (%) = [(A0 - A1)/A0] ×100.
Where: A0 was the absorbance of control; and A1 was the absorbance in the presence of the 1,2,3-triazole derivatives (or the positive control, Trolox) at 100 μg/mL. Calculated values corresponded to the mean of two independent experiments by triplicate.
RESULTS AND DISCUSSION
The 1-benzyl-1,2,3-triazoles compounds were obtained by microwave-assisted click synthesis (Appukkuttan, Dehaen, Fokin & Van der Eycken, 2004; Sarmiento-Sánchez, Ochoa-Terán & Rivero, 2011). Products were analyzed by GC-MS with ion trap in positive chemical ionization; cuasimolecular ion was found for every product. In the proton magnetic resonance spectra of hydroxyalkyltriazoles (5c-e), the chemical shift of position 5 of the heterocyclic ring appeared as a multiplet overlaping with aromatic proton between 7.40 ppm - 7.20 ppm. Carbon 13 NMR signals for carbon 4 and 5 of triazole heterocycle were at 148 ppm and 121 ppm, respectively. When the 4-position of heterocyclic ring was attached to aromatic groups (5f-i, 5n), proton chemical shift of carbon 4 was similar to that of carbon 5 of the heterocycle (δ ∼ 7.60 ppm), contrasting with compounds substituted with non-aromatic groups in carbon 4. In free amine substrates (5g-i, 5k) case, the main products were N-alkylated derivatives, thus, first was necessary to form benzylazide followed by the click reaction.
In biological activities, compounds 5d and 5l showed weak inhibition from 50 µg/mL - 400 µg/mL against S. aureus 3, E. coli AO11, E. coli AO15 and S. enterica serovar Typhi whereas others were inactive against same bacteria. All tested compounds 5a-p were inactive against Streptococcus A-4, E. coli AO19, Shigella dysenteriae, S. aureus 29213 ATCC and E. coli 25922 ATCC. Compound 5l displayed selective weak inhibition against S. aureus 3, this fact suggests that if R2 is replaced by aminophenyl or alkyl-hydroxyl groups and large heterocycles (e.g. naphthyl and c-hexyl), 1,2,3-triazoles derivatives should be inactive against S. aureus 3. The compound 5d showed weak inhibition in range of 50 µg/mL - 400 µg/mL against E. coli AO11, E. coli AO15 and S. enterica serovar Typhi.
It has been registered that 1-phenyl-1,2,3-triazole (Costa et al., 2006) and 1-alkyl-1,2,3-triazole (Gallardo et al., 2007) derivatives are active against M. tuberculosis H37Rv (ATCC 27294) whereas 1-benzyl-1,2,3-triazole (Aufort et al., 2008) derivatives show weak to moderate inhibition against S. aureus isolates (100 µg/mL, 6% to 50% of inhibition). Synthetic compounds 5a-p showed structural similarity with 1-benzyl-1,2,3-triazoles obtained by Aufort et al. (2008) and the registered activities at 100 µg/mL against S. aureus isolates were also similar.
Compounds 5b-i showed hydrogen donating ability on reaction with DPPH radical, being 5g-i those with the highest activity (Table 1). Based on structure-antioxidant activity relationship, activity was associated with the type of substituent. Compounds with alkyl-hydroxyl substituents at C-4 of heterocycle (5c-f) showed similar % DPPH-scavenging activity. Hydroxyl group was important independently of the length of the aliphatic substituent chain at C-4 of 1,2,3-triazole ring. Moreover, derivative 1,2,3-triazole compounds with hydroxyl or free amine group attached to C-4 adjacent carbon position (5k-m) lose their DPPH• inactivating activity. When R2 is ethanoyl substituent (5c and 5f) an increase of DPPH-scavenging activity was observed for the secondary alcohol compared with primary alcohol. Proton transference to the DPPH radical was favored if the substituent of the synthetic 1,2,3-triazole stabilized the aromatic ring, ortho <meta <para; compound 5i showed was the best DPPH scavenger (22.76%), but was lower than the registered for Trolox (80%).
Table 1 Toxicity and antioxidant activity of synthetic 1-benzyl-1,2,3-triazoles.
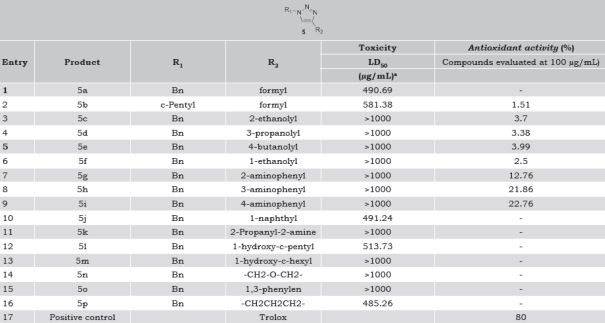
a The toxicity was determined by the Artemia salina bioassay.
Source: Author’s own elaboration.
In the Artemia salina assay, the alkyl-hydroxyl (5c-f) and aminophenyl (5g-i) series were non-toxic. In general, eleven out of sixteen compounds were non-toxic (LDLD50>1000 µg/mL) whereas 5a, 5b, 5j, 5l and 5p were moderately toxic (100<LD50<1000 µg/mL) (Table 1). The null/low toxicity of the synthesized 1-benzyl-1,2,3-triazole derivatives prompted us to carry out new evaluations against different pathogenic bacteria, including M. tuberculosis, and other biological activities.
CONCLUSIONS
Antimicrobial, antioxidant and toxicity activities of sixteen 1-benzyl-1,2,3-triazoles derivatives were evaluated. The 3-(1-benzyl-1H-1,2,3-triazol-4-yl) propan-1-ol and 1-(1-benzyl-1H-1,2,3-triazol-4-yl) cyclopentanol compounds showed weak inhibition against four human pathogenic bacteria. The 5g-i aminophenol derivatives showed the highest DPPH-scavenging activity. Derived from literature and tests evaluated it was observed that small molecules of 1-benzyl-1,2,3-triazoles were not good candidates for antimicrobial drugs due weak inhibition presented. However, it is known that triazole heterocycles have shown good antifungal activity. Synthesized triazole compounds were non-toxic and could be good candidates against human pathogenic fungi.











 nova página do texto(beta)
nova página do texto(beta)