INTRODUCTION
Boron is an element required for the development of plants, animals and humans (Khaliq et al. 2018); however, higher concentrations can induce adverse effects on human health and biota. Particularly, it has been reported (Weir and Fisher 1972, Price et al. 1996, Hilal et al. 2011) that high concentrations generate reproductive toxicity in animals (rats, mice, rabbits). Although negative effects on human reproduction have not been found (Bolt et al. 2020), boron causes nausea, diarrhea, headache, kidney damage, and even death due to circulatory collapse (Nasef et al. 2014). Recently an in vitro study reported that a high boron dose promotes the transforming activity of nontumorigenic cells (Xu et al. 2020). The World Health Organization (WHO 2017) has established a critical limit of 2.4 mg/L for boron concentration in human drinking water, while for irrigation waters the critical levels vary from 0.3 to 3 mg/L depending on the sensitivity of the crops (Ayers and Westcot 1989, Landi et al. 2019). The presence of high concentrations of boron has been detected in groundwater, wastewater, and rivers (Koç 2007, Xu et al. 2010, Velázquez et al. 2011, Palmucci and Rusi 2014). This situation reduces the availability of water resources, which is crucial due to water scarcity in different regions of the world (Boretti and Rosa 2019).
Among the techniques reported for boron removal, adsorption is a simple process whose effectiveness depends on the selectivity of the adsorbent, the boron concentration, and pH (Ezechi et al. 2012). These characteristics determine the usefulness of adsorption for low boron concentrations (Guan et al. 2016, Weidner and Ciesielczyk 2019). In this sense, different materials have been evaluated as adsorbents of boron: resins, alumina, activated carbon, low-cost sorbents, oxides and hydroxides, mesoporous silica, layered double hydroxides, among others (Ezechi et al. 2012, Theiss et al. 2013, Guan et al. 2016).
Zeolites are highly crystalline aluminosilicates with a nanoporous structure which makes them useful for gas separation as catalysts, ionic exchangers, and adsorbents (Byrappa and Yoshimura 2013), characteristics that diversify their application in industrial processes, agriculture, and environmental protection. The adsorption properties of zeolites depend on their framework type, Si/Al ratio, and chemical surface (Yu and Han 2015). This last one is decisive for the anion or neutral species removal because the zeolites require to be modified in order to increase their affinity for these chemical species.
Diverse studies have evaluated the adsorption capacity of anions on modified zeolites, varying the zeolite type, the adsorption conditions and the type of modifier for nitrates (Schick et al. 2010, Batubara et al. 2018), molibdates (Verbinnen et al. 2012), cromates (Barquist and Larsen 2010), arsenates and arsenites (Medina-Ramírez et al. 2013, 2019, Noroozifar et al. 2014) and selenium oxyanions (Jevtić et al. 2014).
Boron adsorption on modified zeolites has been studied on HDTMA-Br modified clinoptilolite reaching a removal efficiency higher than 60 % at a pH of 8.5 (Demirçivi and Nasün-Saygili 2010). Dionisiou et al. (2013) reported a boron removal of 24 % at a pH of 9.5. Kluczka et al. (2013) reported the boron adsorption on ZrO2 modified-clinoptilolite; they evaluated the effect of pH, temperature, dose, concentration, and adsorption time, achieving a boron removal of 75 %.
Chen et al. (2020) studied the boron removal on magnetite nanoparticles and they found evidence of the formation of a Fe-O-B bond that enhances the boron adsorption. Jalali et al. (2016) evaluated modified FeCl3 mineral and organic sorbents for boron adsorption; their best results were obtained using the modified organic sorbent. Additionally, the formation of a nickel complex with boric acid and polyborates was documented by Graff et al. (2017), who showed the thermodynamic stability of the complex. These results suggest the viability of evaluating nickel as a modifier of sorbent for boron removal.
In addition, the modification of nanoparticles by aminosilanes has been reported as an efficient strategy to improve the adsorption of chromate ions (Hozhabr et al. 2015). For these reasons, the effect of nickel chloride, ferric chloride, and aminopropyltriethoxysilane (APS) as modifier agents of LTL and FAU X zeolites, was studied in the present work, and the boron removal capacity of the modified zeolites was evaluated. The zeolites were chosen considering the difference in their topology and the pore channel systems, where FAU X is characterized by three-dimensional channel systems while LTL poses unidimensional channel systems, characteristics that influence the diffusion for modification and adsorption processes. Additionally, the boron adsorption capacity of the modified zeolites was evaluated using groundwater samples and their performance was compared using a synthetic boron solution. The groundwater samples were obtained from a well located in the geothermal area of Ixtlán de los Hervores, Michoacán, Mexico. In this area, the groundwater contains high concentrations of boron, a condition associated to geothermal activity, being the zone near the Ixtlán geyser where the highest boron concentration (11.268 mg/L) has been detected (Velázquez et al. 2011).
MATERIALS AND METHODS
Materials
For zeolite synthesis, sodium aluminate (Al2Na2O4, 99 %) and aluminum hydroxide (Al(OH)3, 99 %) were used as alumina precursors; Ludox HS-40 as silica precursor, sodium hydroxide (NaOH, 97 %) and potassium hydroxide (KOH, 90 %) as mineralizing agents, and deionized water as reaction media. Salts of nickel (NiCl2, 99.9 %) and iron (FeCl3, 99.9 %), as well as 3-APS (99 %) were used as modifying agents of the zeolites. Boric acid (H3BO3, 99.5 %) was used for the synthetic boron solution. All reagents were purchased from Sigma Aldrich.
Synthesis and modification of the zeolites
The faujasite type X and LTL (Linde Type-L) zeolites were synthesized by the hydrothermal method. The LTL zeolite was obtained following the procedure reported by Mintova (2016). Briefly, an alumina solution of molar composition 0.01096 M Al(OH)3, 0.06896 M KOH and 0.5277 M H2O was prepared. Afterwards, a silica solution of molar composition 0.1142 M SiO2, 0.034 M KOH and 0.4861 M H2O was obtained. The alumina solution was added drop to drop to the silica solution under stirring. The obtained gel was aged at room temperature for 40 h. Subsequently, the slurry was transferred to a Parr Teflon-lined stainless-steel autoclave and it was submitted to crystallization at 170 ºC for 20 h. The final product was recovered by centrifugation, and it was washed with deionized water. The solid was dried at 60 ºC for 24 h.
On the other hand, the X zeolite was synthesized according to the procedure described by Lechert and Staelin (2001). First, an alumina solution of molar composition 0.0022 M Al2O3, 0.0146 M Na2O and 0.5733 M H2O was prepared, then a silica solution was obtained from a molar composition of 0.0188 M SiO2, 0.0175 M Na2O and 0.694 M H2O. Subsequently, the alumina solution was slowly added to the silica solution. The slurry was kept under stirring for 20 min. Afterwards, the suspension was transferred to a Parr Teflon-lined stainless-steel autoclave and it was submitted to crystallization at 90 ºC for 8 h. The final product was recovered, washed with deionized water and dried at 100 ºC for 6 h.
Modification of the LTL and X zeolite was carried out by chemical exchange. For this purpose, three modifying solutions with a 10 mM concentration were prepared by dissolution of nickel chloride, ferric chloride and APS in deionized water. The zeolite (LTL or X) was added to the modifying solution, and it was kept under stirring for 24 h at 40 ºC. The modified zeolite was recovered, washed and dried at 100 ºC for 12 h. Six modified zeolites were obtained, which resulted from modifying zeolites X and LTL, each one with three modifiers: NiCl2, FeCl3 and 3-APS. The label of each sample corresponded to the name of the zeolite followed by the metallic ion, for instance LTL-Ni. For zeolites modified with APS, the zeolite name was followed by APS.
Groundwater samples collection and analysis
The groundwater samples were obtained from a well within the geothermal area of Ixtlán de los Hervores, Michoacán, Mexico (20º10’05” N, 102º 22’ 52” W; Fig. 1). This well is used as a source of drinking water and it has an average boron concentration of 5.5 mg/L. The water samples were directly collected from the well in polypropylene bottles previously washed with a solution of sulfuric acid and deionized water. During the sampling, pH, electric conductivity, temperature and total dissolved solids were measured. The samples were kept at 4 ºC until their chemical analysis.
For the groundwater analysis, pH, electric conductivity, temperature and total dissolved solids (TDS) were measured using a Hanna potentiometer model H198129. The concentration of ions present in the groundwater samples was determined by standard methods (Eaton et al. 2005): carbonates and bicarbonates by H2SO4 titration; sulphates by BaCl2 precipitation; chlorides by AgNO3 precipitation ; phosphorus by the persulfate methosd, and calcium, magnesium, sodium and potassium with a Perkin Elmer 3100 spectrophotometer. The boron concentration was determined by colorimetry using athe azomethine-H method and a UV-vis spectrometer (420 nm) (Rodier et al. 2011).
Evaluation of boron adsorption on modified zeolites
The evaluation of boron removal on modified zeolites was performance using: (a) a boron solution prepared by dissolution of boric acid in deionized water, labeled as synthetic boron solution, and (b) groundwater from the well of Ixtlán de los Hervores, Michoacán, Mexico. A solution of boric acid with (5 mg/L) was prepared as synthetic boron solution.
The boron adsorption was carried out in a batch system, where 50 mL of the boron source either synthetic (5 mg/L) or groundwater (5.56 mg/L) were added to an Erlenmeyer flask, then 1 g (pH experiments) or the selected amount (zeolite dosage experiments) of modified zeolite was added. The slurry was kept under stirring at 25 ºC for 24 h. Afterwards the solid was recovered, filtered and dried at 40 ºC for 24 h. The boron residual concentration in the solution was determined by the azomethine-H method (Rodier et al. 2011). The experiments were performed by triplicate for the trials using groundwater, while for the trials using synthetic boron the experiments were carried out by duplicate.
The adsorbed boron was calculated as follows:
where %B ads is the percentage of adsorbed boron onto zeolite; and C i and C f are the initial and final boron concentrations in the solution (mg/L), respectively.
Effect of pH and zeolite dosage on boron removal
To evaluate the effect of pH on the boron adsorption capacity of modified zeolites, the pH of the groundwater (boron concentration = 5.56 mg/L; Table I) was adjusted to pH values of 7, 8.5 and 10 using NaOH and HNO3 1M. The modified zeolite was added to groundwater and kept under stirring at 25 ºC for 24 h. Afterwards, the residual boron concentration was determined. The optimum pH was selected considering the highest boron removal on each modified zeolite. At this pH, three different zeolite dosage were evaluated: 5, 10 and 20 g/L. The conditions of these trials were carried out at 25 ºC for 24 h.
TABLE I CHEMICAL COMPOSITION OF GROUNDWATER USED IN BORON ADSORPTION EXPERIMENTS.
| Variable | Unit | Value |
| pH | 7.14 | |
| Electrical conductivity | µS/cm | 1450 |
| Temperature | ºC | 47.5 |
| PO4 3- | mg/L | 6.88 |
| B | mg/L | 5.56 |
| HCO3 - | meq/L | 3.23 |
| Cl- | meq/L | 5.97 |
| SO4 2- | meq/L | 4.09 |
| Ca2+ | meq/L | 0.82 |
| Mg2+ | meq/L | 0.56 |
| Na+ | meq/L | 10.56 |
| K+ | meq/L | 0.74 |
Characterization techniques
The zeolites were analyzed by X-ray diffraction (XRD) using a PANalytical X-ray diffractometer with a CuK α radiation source (λ = 1.5406 Å). The trans mission electronic microscopy (TEM) (JEOL 1010 field emission microscope, operated at 80 kV) and scanning electronic microscopy (SEM) (JEOL scanning electron microscope, model JSV-6610LV), were used to determine the particle size and morphology of the zeolites, respec tively. The particle size distribution was performed using ImageJ software. The textural properties of the zeolites were determined by nitrogen physisorption using Micromeritics ASAP 2010 equipment. The specific surface area was calculated by the Brunauer-Emmett-Teller (BET) method in the relative pressure range (0.05 < P/Pº < 0.3), the total pore volume being obtained at P/Pº = 0.99. Microporous and external surface areas were obtained using the t-plot method. The modified zeolites were analyzed by FT-IR using a Frontier model PerkinElmer spectrophotometer.
RESULTS AND DISCUSSION
Physical and chemical characterization of the zeolites
From the X-ray diffraction (XRD) patterns of the X (Fig. 2a) and LTL (Fig. 2d) zeolites it can be observed that both of them were obtained as unique crystalline phases. The X zeolite was identified as faujasite type X (JCPDS 39-0218), while the LTL zeolite was identified as Linde Type L (JCPDS 043-05060). Regarding their textural properties, the zeolites presented adsorption isotherms (Fig. 2b, e) corresponding to type IVa. In this kind of isotherm, the capillary condensation occurs, and the hysteresis is observed. This behavior is due to the interactions between the adsorbent and adsorbate as well as the interactions between the molecules in the condensed state (Thommes et al. 2015). Differences were observed in the hysteresis loops of the synthesized zeolites’ isotherms. The LTL zeolite exhibited a hysteresis loop type H1, which is associated to materials with a narrow range of mesopores or due to pores of ink-bottle geometry. On the other hand, the X zeolite presented type an H3 hysteresis loop corresponding to non-rigid aggregates of plate-like particles. The pore size distribution of the zeolitic materials is depicted in figure 2c, f. It is observed that the X zeolite presented a bimodal distribution in the range of micro and mesopores, whereas for the LTL zeolite its pore size distribution was narrower belonging to the mesopores range.

Fig. 2 X-ray diffraction patterns, nitrogen adsorption isotherms and pore size distribution of the (a, b, c) X (FAU X) and (d, e, f) LTL (Linde type L) zeolites. au: area unit; dv(d): derivative of pore volume with respect to pore diameter.
The textural properties of the zeolites are summarized in table II. It was observed that the X zeolite presented a lower specific surface area compared to other reports (Medina-Ramírez et al. 2018, 2021). This behavior can be attributed to the low Si/Al ratio (1.39) of the synthesized X zeolite. According to Shirazi et al. (2008) the BET area is increased as the Si/Al increase. In agreement with the pore size distribution, the X zeolite exhibited a mesoporous area corresponding to 68 % of the total specific surface area. In contrast, the LTL zeolite presented a higher specific surface area, which was mesoporous. Additionally, the average pore size of the LTL zeolite was larger than in the X zeolite. The difference in textural properties of the evaluated zeolites is associated with their topologies, which possess specific building units that determine the type and dimensions of the cavities of the zeolite structure. Particularly, the X zeolite possesses a framework constituted by a sodalite-type cage and a supercage of double 12-member rings. Even though the LTL zeolite presents a similar supercage, it exhibits a cancrinite-type cage. Additionally, the particle size of the zeolite influenced on its pore size distribution. LTL was obtained as nanocrystals while the X zeolite presented crystals in the range of microns. The presence of nanoparticles enhances the formation of mesopores (2 nm < pore size < 50 nm) and increases the specific surface area.
TABLE II TEXTURAL PROPERTIES OF THE X AND LTL ZEOLITES.
| Si/Al* | SSA (m2/g) | SMicro (m2/g) | SMeso (m2/g) | Total pore volume (cm3/g) | VMicro (cm3/g) | VMeso (cm3/g) | Average pore size (nm) | |
| X zeolite | 1.39 | 57.18 | 18.14 | 39.04 | 0.1242 | 0.0094 | 0.1148 | 6.59 |
| LTL zeolite | 2.95 | 181.6 | - | 181.6 | 0.6195 | - | 0.6195 | 13.64 |
*Si/Al: Si and Al ratio, SSA: specific surface area, SMicro: microporous area, SMeso: mesoporous area, VMicro: microporous volume, VMeso: mesoporous volume.
The morphology of the X and LTL zeolite is depicted in figure 3. The X zeolite presented crystalline agglomerates, although the characteristic morphology of this zeolitic phase is octahedral crystals (Parsapur and Selvam 2018). The variation on batch composition and the synthesis led to different morphology. The particle size distribution indicated that the X zeolite exhibited a size in the range of 0.5-2.2 mm.
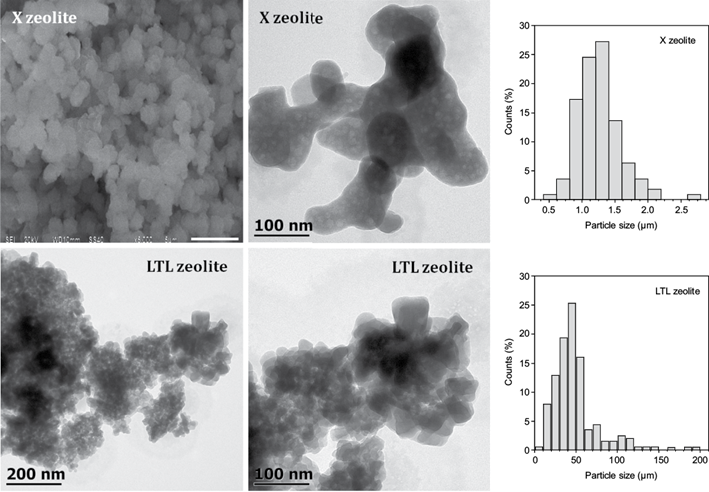
Fig. 3 Scanning electron microscopy and transmission electron microscopy micrographs and particle size distribution of the X (FAU X) and LTL (Linde type-L) zeolites.
On the other hand, the LTL zeolite crystals were observed as tablet-like morphology nanocrystals, in accordance with the findings reported by Wong et al. (2012). The particle size distribution of LTL nanocrystals was in the range of 10-120 nm, its average particle size was of 49 nm.
The pristine and modified zeolites were characterized by FT-IR. The spectra are shown in figure 4. It can be observed that for the X zeolite (Fig. 4a), two bands were identified associated to symmetric and asymmetric stretching vibration of internal tetrahedral at 674 and 962 cm-1, respectively (Byrappa and Kumar 2007). The T-O bend of internal tetrahedral was attributed to the band at 447 cm-1. Regarding the vibration of the external linkage, the band observed at 674 cm-1 corresponded to the symmetric stretching while the band associated to D6R, characteristic of faujasite structures, was detected at a 563 cm-1, which is closer to that reported in the literature (Novembre et al. 2011). The presence of adsorbed water on the zeolitic structure was observed around to 1640 cm-1. With respect to the LTL zeolite (Fig. 4b), the asymmetric stretching of the Si-O-T (T = Si, Al) tetrahedral was associated to a band at 1094 and 1002 cm-1 (Mozgawa et al. 2011) while the external symmetrical stretching of tetrahedral groups was attributed to the band at 728 cm-1. The presence of double six-member ring was detected at 586 cm-1. The T-O bending and pore opening were associated to the band at 460 and 435 cm-1, respectively (Byrappa and Kumar 2007). For modified zeolites, a slight displacement of the X and LTL zeolite characteristic bands to a lower wavenumber, as well as a decrease in its intensity, were observed. This behavior is due to the difference in ionic radii and occupied sites by the exchanged ions (Krol et al. 2021).

Fig. 4 Fourier transform infrared spectroscopy spectra of the pristine and modified (a) X (FAU X) and (b) LTL (Linde type-L) zeolites.
Additionally, bands associated with these ions were identified in zeolites modified with Ni, Fe and APS. Ni was associated with the presence of a Ni-O stretching vibration mode in the region of 600-700 cm-1 (Quiao et al. 2009); these bands were observed at 681 cm-1 for X-Ni (Fig. 4a) and 601 cm-1 for LTL-Ni zeolites (Fig. 4b). The presence of iron was associated to the band around 579 cm-1, which corresponds to the Fe-O stretching vibrational mode (Zhang et al. 2017). For the APS modifier, the bands corresponding to the amine group vibration are reported around 1650-1560 cm-1 (N-H deformation vibrations) and 3500-3300 cm-1 (N-H stretching bands) (Heacock and Marion 2017); however, these regions were overlapped with the bands attributed to the stretching vibration of water molecules (4000-3000 cm-1) and the signals corresponding to bending vibration water (1700-1500 cm-1) in the zeolites (Byrappa and Suresh 2007), so it was not possible to identify them.
Figure 5 shows the SEM micrographs and energy dispersive spectroscopy (EDS) spectra of the modified zeolites. After modification treatment the morphology of the zeolites did no present relevant changes. The presence of Fe and Ni in the zeolites modified with FeCl3 and NiCl2, respectively, was detected by EDS, indicating the effectiveness of the ionic exchange. This was corroborated to observe a decrease in the content of sodium in the X zeolite and in potassium content in the LTL zeolite (Table III). Additionally, the modification treatment led to an increase in the Si/Al ratio for modified X zeolites, a behavior associated to a dealumination process during the modification (Sato et al. 2003). In contrast, the Si/Al for modified LTL zeolites exhibited slight variations and these findings can be related to the fact that the pristine LTL zeolite possesses a higher Si/Al ratio than the pristine X zeolite, which conferred to the former a higher structural stability at the modification treatment conditions. On the other hand, the difference on the iron and nickel content of the modified zeolites is associated to the number and accessibility of additional framework sites of these topologies as well as the ionic radius of the exchanged ions.
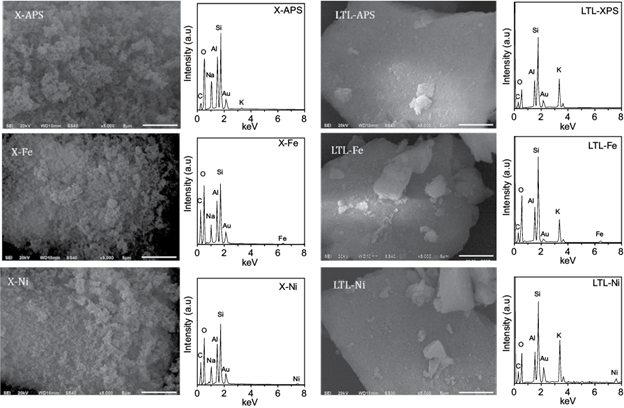
Fig. 5 Scanning electron microscope micrographs and energy dispersive spectroscopy spectra of the modified X (FAU X) and LTL (Linde type-L) zeolites.
TABLE III CHANGES IN THE COMPOSITION OF THE MODIFIED ZEOLITES (TOTAL WEIGHT %).
| Sample | Si/Al | Na | Fe | Ni | Sample | Si/Al | K | Fe | Ni |
| X zeolite | 1.39 | 12.44 | - | - | LTL zeolite | 2.95 | 19.00 | - | - |
| X-APS | 1.67 | 10.56 | - | - | LTL-APS | 3.01 | 19.48 | - | - |
| X-Fe | 1.62 | 9.40 | 1.96 | - | LTL-Fe | 2.82 | 12.00 | 1.26 | - |
| X-Ni | 1.67 | 8.30 | - | 2.63 | LTL-Ni | 2.97 | 18.05 | - | 5.16 |
X zeolite: X zeolite without modification, X-APS: X zeolite modified with aminopropyltriethoxysilane (APS), X-Fe: X zeolite modified with FeCl3, X-Ni: X zeolite modified with NiCl2; LTL zeolite: LTL zeolite without modification, LTL-APS: LTL zeolite modified with APS, LTL-Fe: LTL zeolite modified with FeCl3, LTL-Ni: LTL zeolite modified with NiCl2 .
Boron adsorption
Figure 6 shows the boron adsorption removal on the modified zeolites, using a synthetic boron solution and groundwater samples at different pH values. As can it be observed, all the modified zeolites achieved their highest boron adsorption capacity when the synthetic boron solution was used. Nevertheless, the boron removal capacity on the modified zeolites was reduced when the groundwater was evaluated. This effect was influenced by the zeolite framework and the modifier agent, which are susceptible to the pH and electric conductivity generated by the presence of other chemical species. Regarding the X zeolite, the boron adsorption was higher in the synthetic solution compared to the LTL zeolite; the opposite case was observed in groundwater solutions where the highest adsorption occurred in the LTL zeolite.

Fig. 6 Boron (B) adsorption on X (FAU X) and LTL (Linde type L) modified zeolites using a synthetic boron solution and groundwater samples at different pH values.
It was observed that the equilibrium pH (final pH) increased in the synthetic and groundwater solutions. Shevade and Ford (2004) observed that surface protonation-dissociation reactions of the zeolitic materials can play an important role in the final pH in this kind of experiments. In synthetic solutions, the LTL-APS and X-APS zeolites generated pH > 9, while LTL and X zeolites modified with NiCl2 and FeCl3 generated pH < 9.0 (Fig. 7); at the same time, the lowest boron adsorption was observed at pH > 9.0, onto APS modified zeolites. The APS modifier contains amino groups (-NH) and it is known that in acid pH these amino groups on the adsorbent surface are protonated (-NH3 +), whereas a deprotonation occurs when the pH increases. In this last condition, a greater number of surface negative charges is generated (Hozhabr et al. 2015). On the other hand, at pH > 9.0 the main chemical species of boron is B(OH)4 -, therefore the lower boron adsorption observed at pH > 9.0 can be attributed to an electrostatic repulsion between particles (Irawan et al. 2011, Wei et al. 2011, Adeyemi and Gazi 2016).
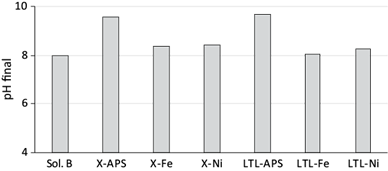
Fig. 7 Final pH (equilibrium pH) of synthetic boron solutions and six modified zeolites. Sol. B: pH of the boron synthetic solution without zeolites; X-APS, X-Fe, X-Ni, LTL-APS, LTL-Fe, and LTL-Ni: X and LTL zeolites modified with aminopropyltriethoxysilane (APS), FeCl3 and NiCl2, respectively.
In groundwater solutions, the final pH varied depending of the initial pH and type of modified zeolite (Fig. 8). As in synthetic boron solutions, the highest final pH was observed on APS modified zeolites (X-APS, LTL-APS).

Fig. 8 Change of pH in groundwater solutions at different initial pH values for (a) X (FAU X) and (b) LTL (Linde type L) zeolites.
It is known that pH controls adsorption in the water interface adsorbent, and it is therefore an important factor in the boron adsorption process (Yuksel and Yurum 2009). The pH also determines the boron chemical species in solution: at low boron concentration (< 25 mM) and pH < 9.1, boron exists mainly as B(OH)3, while at pH > 9.1, B(OH)4 - predominates (Goldberg 1993, Liu et al. 2009, Dionisiou et al. 2013). In this work, the optimal pH values for boron adsorption in both synthetic solutions and groundwater were higher than 8 and less than 9, indicating the presence of the two species of boron (boric acid B(OH)3 and borates B(OH)4 -). These pH values are slightly lower than the pKa of boric acid (9.2), indicating a greater adsorption when this chemical species predominates in the solution (Weidner and Ciesielczyk 2019). Although the chemical species of boron was not determined in this work, according to the boron speciation diagram reported in the literature (Graff et al. 2017) and the concentration of boron for the synthetic solution and groundwater samples used (5 mg/L), as well as the pH evaluated (pH = 8-9), it was assumed that the species to be removed by the zeolites were borates and boric acid.
Effect of electrical conductivity
Figure 9 shows the relationship of saline concentration, measured as electrical conductivity (EC) in equilibrium solutions and adsorbed boron onto modified zeolites using: (a) a synthetic boron solution and (b) groundwater at three pH values. The EC ranged from 168.6 to 269.1 µS/cm in the synthetic solution and from 1588 to 2153 µS/cm in groundwater solutions. Correlation was highly significant r = -0.829, R2 = 0.687), showing an inverse relationship between EC and adsorbed boron. The decrease in adsorbed boron with the increase in saline concentration has been explained as a result of the compression of the diffuse double layer between the adsorbent and the surrounding ion solution, which reduces adsorption sites for boron (Adeyemi and Gazi 2016). On the other hand, the ionic strength has also been related to the bond type established between the chemical boron species and the adsorbent. This property has been used to distinguish between inner-sphere complexes, with strong covalent bonds and low dependence of boron adsorption of ionic strength, and external sphere complexes with weaker bonds (hydrogen bridges, electrostatic interactions or hydrophobic attraction) and high dependence of boron adsorption on the ionic strength of the solution (Goldberg 2005, Liu et al. 2009). The decrease in adsorbed boron with increase in electric conductivity observed in this work (Fig. 9) could indicate that boron interactions with modified zeolites are specific to the type of modifier, therefore the chemical species present influencing its selectivity.
Effect of ions
The ions present in the solutions influence boron adsorption. In this work, the groundwater used is Na-HCO3, and Na+, Cl-, SO4 2- and HCO3 - ions predominate (Table I). The anions Cl-, SO4 2- and HCO3 - contribute to a lower boron adsorption by competing for adsorption sites onto modified zeolites. This adverse effect of saline ions such as sulfate, chloride, nitrate and carbonate on boron adsorption has been observed by Nasef et al. (2014) onto exchange resins, where the originally adsorbed boron was quickly replaced by the anions present in the solution. However, Senkal and Bicak (2003) mentioned that cations Ca2+ and Mg2+ do not exert significant interference in boron adsorption when using the iminodipropylene glycol polymer for the removal of boron in water.
Effect of the modifier
Regarding the modifier, it was observed that boron adsorption in the synthetic solution followed this order: NiCl2 > FeCl3 > APS (Fig. 6). In these low saline solutions, the main factors affecting boron adsorption are pH and the type of bonds between boron species and the modifier. Ni- and Fe-modified zeolites generated a final pH > 8 and < 9, while APS-modified zeolites produced a final pH > 9.0, which was related to the highest and lowest boron adsorption, respectively. On the other hand, it is known that nickel forms inner-sphere complexes with boron (Weidner and Ciesielczyk 2019) and strong covalent bonds, which favors the selective adsorption of the chemical forms of boron. Boron adsorption has been observed to be unaffected by co-existing salts in a solution when the adsorption mechanism is based on the formation of such complexes (Liu et al. 2009). In the case of FeCl3, the adsorption mechanism was probably the formation of external sphere complexes (physical adsorption), since saline concentration affected boron adsorption (Fig. 10). External sphere complexes form weaker bonds such as hydrogen bridges, electrostatic attraction, or hydrophobic attraction (Liu et al. 2009).

Fig. 10 Final electrical conductivity (EC) and adsorbed boron (B) on six modified zeolites. Synthetic boron solution. X-APS, X-Fe, X-Ni, LTL-APS, LTL-Fe, and LTL-Ni: X (FAU X) and LTL (Linde type L) zeolites modified with aminopropyltriethoxysilane (APS), FeCl3 and NiCl2, respectively.
In regard to the APS modifier, the main factor affecting boron adsorption was the final pH > 9.0, and boron adsorption was not affected by EC. In relation to adsorption mechanisms, the formation of complexes should be considered based on the observations of Liu et al. (2009), who observed that boron adsorption is significantly unaffected by co-existing salts in a solution when the adsorption mechanism is based on the formation of inner-sphere complexes. Other researchers (e.g., Hozhabr et al. 2015) that have studied Cr(VI) ions adsorption onto silica magnetite nanoparticles modified with 3-APS mention that the electrostatic and hydrogen bond interactions between surface functional groups and HCrO4 - ions have an important role in adsorption process. These three adsorption mechanisms (inner-sphere complexes, electrostatic and hydrogen bond interactions) could be involved in boron adsorption onto LTL modified zeolites, although more research is required.
Effect of the zeolite dosage
The effect of the dosage of modified zeolite on boron adsorption was tested in three adsorbent concentration (5, 10 and 20 g/L) using groundwater at optimal pH determined for each material. Boron adsorption ranged from 5.6 to 17.9, 10.5 to 21.5 and 16.1 to 35.2 % in adsorbent doses of 5, 10 and 20 g/L, respectively (Fig. 11). It is observed that the amount of adsorbed boron was a function of zeolite concentration, an effect derived from increased adsorbent surface area (Demirçivi and Nasün-Saygili 2010, Demetriou and Pashalidis 2012). The highest percentage of adsorbed boron was obtained with LTL-Ni zeolite at 20 g/L. Because no higher concentrations were explored, it was not possible to determine the maximum adsorption capacity of these modified zeolites, which is suggested to be explored in future research.

Fig. 11 Adsorbed boron (B) according to modified zeolite concentration. (a) X (FAU X) and (b) LTL (Linde type L) zeolites. Modifiers: APS: aminopropyltriethoxysilane, Fe: FeCl3, Ni: NiCl2.
The highest boron adsorption onto LTL-Ni zeolites could also be influenced by its textural properties. With regards to the zeolites studied in this work, the specific surface area (SSA), total pore volume, mesoporous area (Smes), mesopore volume (Vmeso) and average pore size were higher in the LTL zeolite than in the X zeolite (Table II). A high specific surface determines, in part, a high adsorption capacity of zeolites by increasing adsorption sites (Batubara et al. 2018), while large cavities or channels in these minerals (identified here by mesopore surface and volume, and mean pore size) were associated with increased ion exchange capacity and molecular adsorption (Byrappa and Yoshimura 2013). In agreement with these observations, we found that the LTL zeolites exhibited highest average boron adsorption, although only in groundwater solutions with high EC; however, in synthetic boron solutions with low EC, the highest average boron adsorption was obtained with the X zeolite. Both zeolites form type IV isotherms, which are characteristic of multilayer adsorption.
Statistical analysis
The ANOVA for boron adsorption (%) onto six modified zeolites in groundwater solutions with different pH values is shown in table IV. Since the probability value (Pr) indicates highly significant differences between treatments, a means separation was performed using the Tukey test (Pr ≤ 0.05). Results of the Tukey test showed seven groups (Table V). Considering the “a” and “ab” groups presented in table V, the highest boron adsorption was obtained with LTL-Ni zeolite at pH = 7.0 and LTL-Ni zeolite at pH = 8.5, whereas the LTL-Fe zeolite at pH = 7.0 showed the lowest boron adsorption capacity (“d” group, Table V). For adsorbent dosage, ANOVA indicated highly significant differences between treatments (Table VI). For the Tukey mean separation test (Pr ≤ 0.05), 10 groups were obtained (Table VII), where the highest boron adsorption corresponded to LTL-Ni zeolite at a dose of 20 g/L (“a” group, Table VII), followed by X-Ni zeolite at 20 g/L (“b” group, Table VII).
TABLE IV ANOVA FOR BORON ADSORPTION TREATMENTS ON SIX MODIFIED ZEOLITES AND DIFFERENT INITIAL pH VALUES (GROUNDWATER).
| Source | F* | SS | AS | F | Pr > F |
| Model | 17 | 1507.128 | 88.655 | 3.99 | 0.00024 |
| Error | 36 | 799.876 | 22.219 | ||
| Total | 53 | 2307.004 |
R2 = 0.653, R2 aj = 0.490, RSME (root mean square error) = 4.714.
FD: freedom degrees, SS: sum of squares, AS: average square, F: F value, Pr: probability.
TABLE V TUKEY TEST FOR BORON ADSORPTION TREATMENTS ON SIX MODIFIED ZEOLITES AND DIFFERENT INITIAL pH VALUES (GROUNDWATER SOLUTIONS).
| Modified zeolite | pH | ||
| 7.0 | 8.5 | 10.0 | |
| X-APS | 20.8* abcd | 11.6 bcd | 20.7 abcd |
| X-Fe | 12.03 abcd | 10.7 cd | 13.8 abcd |
| X-Ni | 17.9 abcd | 15.2 abcd | 20.2 abcd |
| LTL-APS | 21.6 abcd | 22.2 abc | 16.2 abcd |
| LTL-Fe | 7.3 d | 10.2 cd | 19.9 abcd |
| LTL-Ni | 26.0 a | 25.3 ab | 18.4 abcd |
X-APS, X-Fe, X-Ni, LTL-APS, LTL-Fe and LTL-Ni: X (FAU X) and LTL (Linde type L) zeolites modified with aminopropyltriethoxysilane (APS), FeCl3 and NiCl2, respectively.
*Averages with different letters are significantly different (Pr ≤ 0.05), Tukey test.
TABLE VI ANOVA FOR BORON ADSORPTION TREATMENTS ON SIX MODIFIED ZEOLITES AND THREE ADSORBENT DOSES (5, 10 AND 20 g/L) USING GROUNDWATER.
| Source | FD | SS | AS | F | Pr > F |
| Model | 17 | 1935.981 | 113.881 | 16.020 | < 0.0001 |
| Error | 36 | 255.918 | 7.109 | ||
| Corrected total | 53 | 2191.899 |
R2 = 0.883, R2 aj = 0.828, RSME (root mean square error) = 2.666
FD: freedom degrees, SS: sum of squares, AS: average square, F: F value, Pr: probability.
TABLE VII TUKEY TEST FOR BORON ADSORPTION (%) IN THREE DOSES OF MODIFIED ZEOLITES AND GROUNDWATER.
| Modified zeolite | Doses of zeolite | ||
| 5 g/L | 10 g/L | 20 g/L | |
| X-APS | 8.9*f | 10.5 ef | 20.7 bc |
| X-Fe | 15.5 bcdef | 15.0 bcdef | 19.9 bcd |
| X-Ni | 17.9 bcde | 15.7 bcdef | 22.5 b |
| LTL- APS | 10.9 ef | 12.3 def | 22.2 bc |
| LTL-Fe | 12.5 def | 14.3 cdef | 19.9 bcd |
| LTL-Ni | 14.8 bcdef | 21.5 bc | 35.2 a |
X-APS, X-Fe, X-Ni, LTL-APS, LTL-Fe and LTL-Ni: X (FAU X) and LTL (Linde type L) zeolites modified with aminopropyltriethoxysilane (APS), FeCl3 and NiCl2, respectively.
*Averages with different letters are significantly different (Pr ≤ 0.05), Tukey test.
Comparison of boron adsorption capacity in the six modified zeolites with other materials
The boron adsorption capacity of the modified LTL-Ni zeolite obtained in this work is moderate compared to other materials reported in the literature (Table VIII). However, low synthesis costs for these materials are an incentive to continue experimental work on the factors affecting their adsorption and determining the feasibility to using them as effective boron adsorbents. The boron adsorbents considered to be most efficient are exchange and chelating resins (Ipek et al. 2008, Nasef et al. 2014), although they are expensive. Adeyemi and Gazi (2016) used of chitosan as a modifier of the Fe3O4 adsorbent in the removal of boron from water, obtaining high values in the adsorption capacity (Table VIII).
TABLE VIII COMPARATIVE OF MATERIALS WITH DIFFERENT BORON ADSORPTION CAPACITY.
| Material | Doses of adsorbent (g/L) | Boron (B) adsorption conditions | Boron adsorption capacity | Reference |
| LTL-Ni (Linde type L zeolite modified with NiCl2) | 20 | pH = 8.49 T = 25 ºC B = 5.5 mg/L | 35.2 % (0.216 mg/g) | This work |
| Fly ash | 100 | pH = 10.0 T = 25 ºC B = 10 mg/L | 94 % (0.094 mg/g) | Yüksel and Yürüm (2009) |
| Demineralized lignite | 50 | pH = 11.0 T = 25 ºC B = 10 mg/L | 18 % (0.036 mg/g) | Yüksel and Yürüm (2009) |
| Clinoptilolite | 50 | pH = 10.0 T = 25 ºC B = 10 mg/L Adsorption time: 24 h | 18 % (0.036 mg/g) | Yüksel and Yürüm (2009) |
| Fe(O)OH | - | pH = 8.0 T = 22 ºC B = 55 mg/L | 0.03 mol/kg (0.324 mg/g) | Demetriu and Pashalidis (2012) |
| HFO (hydrous ferric oxide) | - | pH = 9.4 B = 2.25 mM Reaction time: 48 h | 1.728 mg/g | Peak et al. (2003) |
| Al-Fe-Si oxide | 25 | pH = 8.3 B = 80 mg/L Reaction time: 24 h | 0.980 mg/g | Irawan et al. (2011) |
| Fe3O4-TSPA (bis (trimethoxysilylpropyl)amine) | 60 | pH = 6.0 T = 22 ºC B = 2 M Adsorption time: 2 h | 50 mmol/kg (0.5 mg/g) | Liu et al. (2009) |
| FeO-AC (activated carbon/iron oxide composite) | 5 | pH = 9.0 B = 80 mg/L Adsorption time = 2 h | 97 % (0.485 mg/g) | Chioma et al. (2018) |
| Polymers VBC (4-vinylbenzychloride)/DVB (divinyl benzene) + NMDG (N-methyl-D-glucamine) | 10 | pH = 8.0 T = room temperature B = 350 mg/L Adsorption time = 24 h | 18.15 mg/g | Jinging et al. (2018) |
| Fe3O4-Chitosan-glycidol | 5 | pH = 7.0 B =125 mg/L | 128.5 mg/g | Adeyemi and Gazi (2016) |
| Ion exchange resin Diaion CRB 02 | 2 | pH = 8.4 T = 24 ºC B = 12-13 mg/L E.C. = 1936 µS/cm | 0.31 mmol/g (3.35 mg/g) | Ipek et al. (2008) |
CONCLUSIONS
Two zeolites (FAU X and LTL) modified with NiCl2, FeCl3 and APS were synthesized and tested for boron adsorption in synthetic and groundwater solutions at different pH and EC. The modified zeolites showed a buffer effect, increasing the final pH in equilibrium solutions. The boron adsorption capacity of zeolites was a function of the pH, EC, and the kind of modifier and adsorbent dosage. The greatest boron adsorption capacity was obtained at pH > 8 and < 9 in both solutions, while increased EC in groundwater solutions caused an overall negative effect on boron adsorption. By type of modifier, the best results were obtained with NiCl2 due to its complexing properties with boron species. In groundwater, the zeolitic material with the best response to boron adsorption was the LTL-Ni zeolite at a concentration of 20 g/L, which combines the properties of high surface area, greater volume and mesopore area with the capacity of the NiCl2 modifier to form complexes with boron species. All these characteristics give it the potential use of a selective boron adsorbent. In future research, it is recommended to determine the maximum adsorption capacity of LTL-Ni zeolite, as well as to analyze the ability of NiCl2, FeCl3 and APS modifiers to react with the surface of zeolites, which may be affecting the adsorption of boron in these materials.











 nueva página del texto (beta)
nueva página del texto (beta)




