INTRODUCTION
Mangrove soils are characterized by their high organic matter content (Bouillon et al. 2003, Kristensen et al. 2008) and represent very important organic carbon sinks (Alongi 2007, 2012, McLeod et al. 2011). The need to understand the evolution of organic carbon and its reservoir in mangrove soils makes it necessary to identify the natural organic matter (Mater et al. 2004, Dittmar et al. 2006, Alongi 2007, Kristensen et al. 2009) and the one coming from contamination (Farias et al. 2008, García et al. 2008, Ranjan et al. 2012, Ranjan et al. 2015). Due to in many cases, these environments are close to urban areas, industrial facilities and additionally are areas of tourism, may be subject to possible sources of pollution by PAHs.
Polycyclic aromatic hydrocarbons (PAHs) are mainly a source of contamination of organic matter in the atmosphere (Marchand et al. 2004, Ravindra et al. 2008a, 2008b), as well as in water, soils and sediments (Walker et al. 2005, Silva et al. 2007, Itoh et al. 2010, Kafilzadeh et al. 2011, Bhupander et al. 2012, Bayen 2012, Dong et al. 2012, Tsibart and Gennadiev 2013, Kaiser et al. 2016, Wang et al. 2017). The presence of PAHs, which are absorbed by fine particles and transported through the atmosphere, is indicative of anthropogenic combustion sources (Giger and Schaffner 1978, La Flamme and Hites 1978, Garrigues and Ewald 1983, Killops and Al-Joboori 1990, Brown and Maher 1992, Leeming and Maher 1992). These compounds can have other anthropogenic sources such as oil spills, waste oils, or outboard motors (Simpson et al. 1996). Compounds such as fluorene, phenanthrene, and anthracene have been found in estuarine sediments in areas with a high degree of hydrocarbon contamination (Killops and Readman 1985). They have also been used as oil spill-related pollution markers in mangrove ecosystems (Silva et al. 2007, Farias et al. 2008, Evans et al. 2016). Based on the PAHs detected, Leeming and Maher (1992) have described two possible anthropogenic sources: non-substituted PAHs associated with vehicular traffic, and alkyl-substituted PAHs associated with lubricants and oils used in the automotive industry. Ravindra et al. (2008a) present a review of the main PAHs sources in the atmosphere, with five major emission sources of PAHs, i.e. domestic, mobile, industrial, agricultural, and natural. The geochemical criteria to differentiate HAPs origin is based on the classification on: a) pyrolytic, represented by the incomplete combustion of recent or fossilized organic matter; b) petroleum source, from the formation of petroleum during catagenesis of organic matter, process at low temperature (50-150 ºC), and c) diagenetic, from direct biosynthesis of microorganisms or vegetation (Bouchez et al. 1996a).
For identifying pollution emission sources of PAHs some diagnostic ratios are used (Ravindra et al. 2008a, Tobiszewski and Namiesnik 2012). These diagnostic ratios are based on parent PAHs, others on the proportion of alkyl-substituted for non-substituted molecules. These ratios distinguish PAHs pollution originating from petroleum products, petroleum combustion and biomass or coal burning. The compounds involved in each ratio have the same molar mass, so it is assumed they have similar physical and chemical properties (Tobiszewski and Namieśnik 2012). In this study PAHs and their diagnostic ratios were used to identify the degree of contamination and the possible emission sources in soils of mangrove from the Cuare Inlet and Morrocoy National Park, located in Venezuela´s central-western coast (Fig. 1).
SAMPLES AND METHODS
Study area
The Wildlife Refuge of Cuare and the National Park of Morrocoy are located on the central-western coast of Venezuelan Caribbean Coast (Fig. 1); they represent one of the most important marine coastal sectors because of their tourism attractions. Both protected areas comprise muddy coastal plains with mangroves and extensive salt pans, swamps and inlets, several carbonate islands of flat topography and partially covered by mangroves, and some other littoral communities (Barreto 2008).
The mangroves of Punta La Matica (PLM), are located in the northwest end of the Cuare Inlet. The low intertidal zone of mangroves is dominated by Rhizophora mangle. The sampling sites at National Park (NP) and Morrocoy (ETU), located in muddy coastal plains, are influenced by a supply of freshwater from the continent. These watercourses have short trajectories and originate in micro-basins located near the coast. They are permanent, but their flow is highly variable and increases substantially during the wet season. These watercourses have been modified by human activities (Barreto 2008).
Sample collection
Superficial (0-20 cm) soil samples were collected at two locations throughout the Cuare Inlet and the NP Morrocoy: Punta La Matica (PLM) and Tucacas Bay (ETU) (Fig. 1). Samples sites location was chosen taking into account the higher level of anthropogenic influence present in ETU (García et al. 2008) compared to PLM. In PLM two soil samples were collected, located in the low intertidal zone, and characterized by the presence of Rhizpophora-Laguncularia and mixed Rhizophora-Laguncularia-Avicennia forest. In Tucacas Bay (ETU) seven soil samples were collected along the edge of the bay, in a transect of 70 m in length, characterized by a mixed forest dominated by Rhizophora and a monospecific Avicennia forest present after the first 50 m of the transect (Fig. 1). The locations were georeferenced using a model GPSmap 76Cx Garmin global positioning system (GPS) receiver with ± 3 m precision. The soil samples were collected using a steel soil corer 10.2 cm in diameter. In each sampling area, the mangrove species and stand conditions (live, deteriorated, or dead mangrove) were identified. The samples were stored in plastic bags until they were transported to the lab.
Sample handling
Samples were placed in plastic containers and left to dry at room temperature. The physical description of the samples was done with the dry material and took into account Munsell´s color chart (Munsell 1973), granulometry, the presence or absence of roots, and other significant characteristics. After, the samples were ground crushing and sieved (2 mm mesh size) into medium sand and clay size fractions for later chemical analyses. Prior to the chemical analyses, the samples were washed with distilled water to eliminate any salts (mainly sodium chloride) precipitated as a result of the drying process. Two techniques were used: filtration by means of long stem funnels and filter paper (Whatman No. 3) for samples with a high root content, and centrifugation and decantation of remaining water for samples with a high content of fine sand, silt, and clay size fractions. To corroborate that excess salts were removed, the chloride test was performed using silver nitrate. After washing, the samples were placed in an oven at 40 ºC until they were completely dry (Cerqueira et al. 2019).
Determination of total carbon (TC), total organic carbon (TOC), and inorganic carbon (IC)
TC concentration was analyzed in samples using a Leco (C-144) carbon analyzer, and carbonatic or inorganic carbon (IC) was determined using the Bernard calcimeter method (Hesse 1971). Calibration curves were constructed to determine sample concentration of organic and inorganic carbon. Certified Leco patterns were used to determine TC, and patterns with different CaCO3 concentrations for IC. TOC concentration was obtained by the difference between TC and IC
Extraction of soluble organic matter (SOM)
The extraction of SOM corresponding to the lipid fraction was performed. Approximately 20 g of soil were weighed in cellulose thimbles and the SOM was extracted with dichloromethane in a Soxhlet extractor. The extracts were then vacuum evaporated and quantified.
Analysis of polycyclic aromatic hydrocarbons (PAHs)
To determine the PAHs, a fraction of the extracted SOM was separated by absorption column chromatography using packed columns (20 cm long and 1.5 cm in diameter) with alumina as the stationary phase (20 g). The saturated hydrocarbons were eluted with n-hexane (30 mL), and the aromatic hydrocarbons with toluene (20 mL).
The following PAHs were analyzed: naphthalene, acenaphthalene, acenaphthene, fluorene, phenanthrene, anthracene, fluoranthene, pyrene, benzo(a)anthracene, chrysene, benzo(k)fluoranthene, benzo(b)fluoranthene, benzo(a)pyrene, benzo(ghi)perylene, dibenzo(ah)anthracene and indene. The internal perdeuterated PAHs standard mix [naphthalene d8 (99%), acenaphthene d10 (98%), phenanthrene d10 (98%) chrysene d12 (98%) and perylene d12 (98%)] was purchased from Cambridge Isotope Laboratories and the 16 PAHs USEPA priority standard [naphthalene, acenaphthylene, acenaphthene, fluorene, phenanthrene, anthracene, fluoranthene, pyrene, benzo(a)anthracene, chrysene, benzo(b)fluoranthene, benzo(k)fluoranthene, benzo(a)pyrene, indene(1,2,3-c,d)pyrene, benzo(g,h,i)perylene and dibenzo(a,h)anthracene] was purchased from Supelco.
The quantitative analysis of regular PAHs and alkylated homologues was carried out using the internal standard method using as standards the perdeuterated naphthalene d8, acenaphthene d10, phenanthrene d10, chrisene d12 and perylene d12. The calibration curves were established using a standard mixture of 16 priory PAHs and the perdeuterated standards above mentioned.
The identification and quantification of the 16 polycyclic aromatic compounds (alkylated and non alkylated) was carried out using a gas chromatograph Agilent, model 6890 with automatic injector and fused silica capillary column (30 m x 0.25 mm x 0.25 mm) with 5 % phenyl - 95 % dimethylpolysiloxane stationary phase. He, with a flow of 1 mL/min, was used as carrier gas. A selective mass detector Agilent 5976 operating in the electronic impact ionization mode (70 eV) and single ion monitoring (SIM) was employed for data acquisition (Peters et al. 2005). An amount of 1 mL of the extract was injected in splitless mode using the following chromatographic conditions: injector temperature = 290 ºC; initial column temperature = 40 ºC isothermal for 1 min, heating rate 6 ºC/min until 290 ºC and isothermal for 20 min. The monitored ions for polyaromatic analysis were: d8 naphthalene (m/z = 136), benz(a)anthracene (m/z = 228), naphthalene (m/z = 128), crysene (m/z = 228), d10 acenaphthene (m/z = 164), d12 perylene (m/z = 264), acenaphthylene (m/z = 152), benz(k)fluoranthene (m/z = 252), acenaphthene (m/z = 154), benzo(a)pyrene (m/z = 252), fluorine (m/z = 166), benzo(ghi)perylene (m/z = 276), d10 fenantrene (m/z = 188), indeno(123-cd)pyrene(m/z = 276), fenantreno (m/z = 178), dibenzo(ah)anthracene (m/z = 278), anthracene (m/z = 178), benzo(b)fluoranthene (m/z = 252), fluoranthene (m/z = 202) and pyrene (m/z = 202).
RESULTS AND DISCUSSION
In samples IC is below the detection limit of the method (Bernard calcimeter ≤ 4). Additionally the soils are characterized by the absence of carbonates and their mineralogy is siliciclastic with the presence of quartz, kaolinite, illite and pyrite (Barreto et al. 2016). Therefore, the total carbon determined by Leco represents the total organic carbon (TOC) in the soils. TOC have concentrations between 9.7 % to 14.9 % in ETU and 11.3 to 19.5 % in PLM; however, the amount of lipid fraction, represented by soluble organic matter (SOM), is very low (< 1%) and the soluble organic matter/total organic carbon ratio is also very low (SOM/TOC <0.01) (Table I) compared to other mangrove soils (Barreto et al. 2016).
TABLE I TOTAL ORGANIC CARBON (TOC wt %), SOLUBLE ORGANIC MATTER (SOM wt %) AND SOM/TOC RATIO
| Site | Samples | TOC (wt %) | SOM (wt %) | SOM/TOC |
| Tucacas Bay | ETU-1 | 12.5 | 0.52 | 0.04 |
| ETU-2 | 12.3 | 0.54 | 0.04 | |
| ETU-3 | 11.4 | 0.52 | 0.05 | |
| ETU-4 | 14.9 | 0.84 | 0.06 | |
| ETU-5 | 10.1 | 0.82 | 0.08 | |
| ETU-6 | 13.7 | 0.99 | 0.07 | |
| ETU-7 | 9.7 | 0.53 | 0.05 | |
| Punta La Matica | PLM-1 | 19.5 | 0.68 | 0.04 |
| PLM-2 | 11.3 | 0.86 | 0.08 |
ETU = Tucacas Bay, PLM = Punta La Matica, inorganic carbon in all samples ≤ 4 %. TOC = total organic carbon, SOM = soluble organic matter, SOM/TOC = soluble organic matter/total organic carbon.
Individual PAH concentrations are shown in table II, their concentration was ≤ 967 ng/g; in some cases they were not detected as they were lower than the detection threshold for the technique employed (≤ 20ng/g). This was true for naphthalene, acenaphthene, acenaphthalene, benzo(ghi)perylene, dibenzo(a,h)anthracene, and indene. None of the PAHs analyzed were detected in the ETU-1 sample; only fluorene was detected in the ETU-2 sample; and ETU-5 was the only sample characterized by the presence of benzo(k)fluoranthene, benzo(b)fluoranthene, and benzo(a)pyrene. On the other hand, phenanthrene was detected in most of the samples and is the PAH found in highest concentration, followed by fluoranthene, pyrene, and chrysene. The rest of the PAHs analyzed (fluorene, anthracene, and benzo(a)anthracene) had concentrations of < 100 ng/g. In the PLM soils, the PAHs detected (phenanthrene, fluoranthene, and pyrene) were present in smaller concentrations that in the ETU soils. Table II also shows the concentration of PAHs in ETU and PLM samples, indicating that only phenanthrene, fluoranthene and pyrene have appreciable concentrations in both locations. In PLM the other analyzed PAHs are in concentrations lower than the detection limit (≤ 20 ng/g), but in ETU chrysene, benzo(a)anthracene and benzo(k)fluoranthene present concentrations greater than 20 ng/g, but not exceeding 300 ng/g. These results reveal that the soils PAHs content is relatively lower in two locations, but their concentration is higher than ETU to PLM.
TABLE II INDIVIDUAL POLYCYCLIC AROMATIC HYDROCARBONS (PAHs), TOTAL PAHs CONCENTRATION (ng/g), AND CALCULATED RATIOS FROM SOIL SAMPLES OF TUCACAS BAY (ETU) AND PUNTA LA MATICA (PLM).
| ETU | PLM | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PAH | 2 | 3 | 4 | 5 | 6 | 7 | ∑PAHs | 1 | 2 | ∑PAHs |
| Fluorene | 44 | ≤ 20 | ≤ 20 | ≤ 20 | ≤ 20 | ≤ 20 | 44 | ≤ 20 | ≤ 20 | ≤ 20 |
| Phenanthrene | 967 | 707 | 301 | ≤ 20 | 718 | 197 | 2891 | 98 | 80 | 177 |
| Anthracene | 37 | 26 | 24 | ≤ 20 | 41 | ≤ 20 | 128 | ≤ 20 | ≤ 20 | ≤ 20 |
| Fluoranthene | 506 | 253 | 68 | 110 | 354 | 75 | 1366 | 83 | ≤ 20 | 83 |
| Pyrene | 570 | 245 | 68 | 133 | 354 | 76 | 1446 | 89 | ≤ 20 | 89 |
| Benzo(a)anthracene | 42 | 27 | ≤ 20 | 97 | 47 | ≤ 20 | 213 | ≤ 20 | ≤ 20 | ≤ 20 |
| Chrysene | 73 | 38 | ≤ 20 | 282 | 156 | 51 | 600 | ≤ 20 | ≤ 20 | ≤ 20 |
| Benzo(k)fluoranthene | ≤ 20 | ≤ 20 | ≤ 20 | 169 | ≤ 20 | ≤ 20 | 169 | ≤ 20 | ≤ 20 | ≤ 20 |
| Benzo(b)fluoranthene | ≤ 20 | ≤ 20 | ≤ 20 | 80 | ≤ 20 | ≤ 20 | 80 | ≤ 20 | ≤ 20 | ≤ 20 |
| Benzo(a)pyrene | ≤ 20 | ≤ 20 | ≤ 20 | 100 | ≤ 20 | ≤ 20 | 100 | ≤ 20 | ≤ 20 | ≤ 20 |
| An/(An+Phe) | 0.04 | 0.04 | 0.07 | NC | 0.05 | NC | --- | NC | NC | --- |
| Fl/(Fl+Py) | 0.47 | 0.51 | 0.50 | 0.45 | 0.50 | 0.50 | --- | 0.48 | NC | --- |
| b(a)An/b(a)An/Ch | 0.37 | 0.42 | NC | 0.26 | 0.23 | NC | --- | NC | NC | --- |
| Total PAHs | 2239 | 1296 | 461 | 971 | 1670 | 399 | --- | 270 | 80 | --- |
| Total PAHs/TOC | 182 | 114 | 31 | 96 | 122 | 41 | --- | 14 | 7 | --- |
| Total PAHs/SOM | 4146 | 2492 | 549 | 1184 | 1687 | 753 | --- | 397 | 93 | --- |
ETU = Tucacas Bay, PLM = Punta La Matica, naphthalene, acenaphthene, acenaphthylene, benzo(ghi)perylene, dibenzo(ah)anthracene and indene ≤ 20 ng/g, in ETU-1 = all PAHs ≤ 20 ng/g, NC = not calculated, ΣPAHs = individual PAH total concentration, An/(An+Phe) = anthracene/(anthracene+phenanthrene), Fl/(Fl+Py) = fluoranthene/(fluoranthene+pyrene), b(a)An/b(a)An/Ch = benzo(a)anthracene/(benzo(a)anthracene + chrysene), total PAHs = ΣPAHs detected in sample site, total PAHs/TOC = ΣPAHs detected in sample site/total organic carbon,, total PAHs/SOM = ΣPAHs detected in sample site/soluble organic matter.
The total aromatic concentrations calculated by adding the PAHs concentrations detected by samples site (total PAHs = SPAH detected in sample site), are in the range between 399-2239 ng/g and 80-270 ng/g to ETU and PLM, respectively (Table II). The graphic representation between the total PAHs and TOC or SOM content in soil (Fig. 2) shows that there is no relationship between these variables. This is a consequence of the fact that both TOC and SOM represent the organic compounds of anthropogenic origin (PAHs and others do not identify in this study), the supply or organic matter of the mangroves vegetation (autochthonous organic matter) and that coming from other natural sources (autochthonous or allochthonous organic matter). Therefore, variations in the concentration of PAHs in the different sampling points can be masked by the content of organic matter of natural origin (TOC and SOM); this may avoid determining any relationship between these parameters. As consequence, the concentration of total PAHs was normalized relate to TOC (total PAHs/TOC = SPAHs detected in sample site/Total Organic Carbon) and SOM (total PAHs/SOM = SPAHs detected in sample site/Soluble Organic Matter) according to López et al. (2000) (Table II). In figure 3, the graphical relationship between total PAHs with PAHs/TOC (r = 0.9769) and PAHs/SOM (r = 0.8877) is represented, where a linear relationship with a high correlation coefficient between these variables is observed. These results suggest that the PAHs detected come from the same source. In this graphic, the sampling point where the concentration of all PAHs was below the detection limit (≤ 20 ng/g) was included (ETU-1), as a measure of the minimum value of contamination determined. On the other hand, according to values of these ratios in the sampling sites and the location in the graph of figure 3, PAHs/TOC and PAHs/SOM are in the order: a) ETU-2 > ETU-6 > ETU-3 > ETU-5 > ETU 7 > ETU-4 > PLM-0 > PLM-1 >>>> ETU-1 and b) ETU-2 > ETU-3 > ETU-6 > ETU-5 > ETU 7 > ETU-4 > PLM-0 > PLM-1 >>>> ETU-1 respectively. Again, these graphs show a higher level of PAHs in ETU relate to PLM.
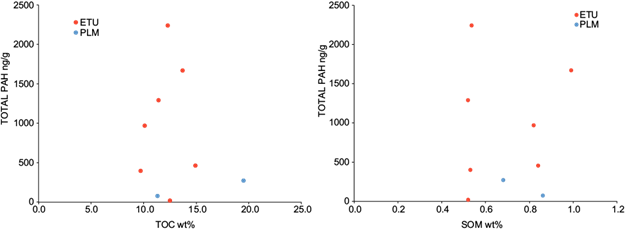
Fig. 2 Relationship between total polycyclic aromatic hydrocarbons (PAHs) with total organic carbon (TOC) and soluble organic matter (SOM) in soils from mangrove forest from Tucacas Bay (ETU) and Punta La Matica (PLM).
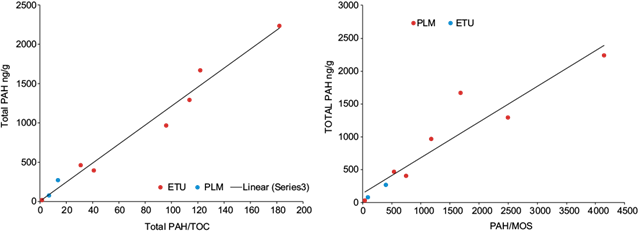
Fig. 3 Relationship between total polycyclic aromatic hydrocarbons (PAHs) with total polycyclic aromatic hydrocarbons normalized to total organic carbon (TOC) and soluble organic matter (SOM) in soils from mangrove forest from Tucacas Bay (ETU) and Punta La Matica (PLM).
Moreover, samples were classified according to the number of PAHs rings observed and yielded the following results: a) indene, the six-ring PAH analyzed, was not detected; b) no five-ring PAHs were detected in the PLM samples and only one was detected in the ETU samples (ETU-5); c) ETU samples are characterized by the presence of PAHs with three to five-membered rings with total individual PAHs total concentration (ΣPAHs) between 44 and 2891 ng/g, the least abundant of which is fluorene and the most abundant phenanthrene; d) three and four-ring PAHs were observed in the PLM samples, with concentration levels (ΣPAHs) of 83 ng/g for fluoranthene and 177 ng/g for phenanthrene and e) PAHs concentration levels were higher in ETU samples than in PLM samples. In general, the prevalence of three and four-ring PAHs reflects the origin of combustion products, products of low-temperature pyrolytic processes, or petrogenetic sources (Dong et al. 2012). Additionally, the PAHs identified in the soils of the Cuare Inlet and the Morrocoy National Park were of the non-substituted type, which suggests petroleum combustion, as the main source, due to PAHs from pyrolytic source are characterized by high molecular weight non-alkylated aromatic compounds (Volkman et al. 1997). The products of combustion are probably transported in particulated form to the soils or sediments in this area.
On the other hand, the ratios used to identify pollution emission sources are based on the proportion of non-substituted PAHs. Sources are classified into: 1) petrogenetic sources from oil spills, 2) pyrolytic sources from incomplete combustion or pyrolysis of fossil fuels, and 3) sources derived from the burning of biomass or carbon (Boumar et al. 1998, Ravindra et al.2008a, 2008b, Tobiszewski and Namiesnik 2012, Tobiszewski 2014, Zhang et al. 2014, Evans et al. 2016). These ratios can have some limitations depending on the changes in the concentration of the PAHs involved, for example, the accumulation and biodegradation of phenanthrene and fluoranthene by algae present in mangrove ecosystems (Hong et al. 2008), their alteration in the atmosphere due to photodegradation, or to the reaction of PAHs to other atmospheric elements such as ozone and/or nitrogen oxides (Yunker et al. 2002, Rivandra et al. 2008a, 2008b, Tobiszewski and Namiesnik 2012). All of these could limit the usefulness of the values obtained with these ratios as indicators of source. Nevertheless, PAHs ratios are widely used in the literature to identify pollution emission sources and many of them are biodegradation-recalcitrant compounds (Khalili et al. 1995, Bouchez et al. 1996b, Hwang et al. 2003, Ravindra et al. 2008a, 2008b, Zhang et al. 2014).
From results, only three ratios could be calculated for six of the ETU samples and one PLM sample (Table II) with the following results: a) anthracene/(anthracene+phenanthrene), with values between 0.04 and 0.07, related to a petrogenetic source; b) fluoranthene/(fluoranthene+pyrene), with values between 0.45 and 0.51, related to pyrolytic sources or diesel fuel; and c) benzo(a)anthracene/(benzo(a)anthracene+chrysene), with values between 0.23 and 0.37, related to the combustion of plants or a carbon source (,Hong et al. 2008, Ravindra et al. 2008a, b, Tobiszewski and Namiesnik 2012; Tobiszewski 2014). The graph of figure 4 (Dong et al. 2012), represents the relationships anthracene/(anthracene+phenanthrene) vs. fluoranthene/(fluoranthene+pyrene) ratios in soils samples from ETU location. Results show that ratios of An/(An + Phe) and Fl/(Fl + Py) are in the range from 0.0 to < 0.1 and 0.45 to 0.55, in samples to ETU location, suggesting petroleum combustion sources, and also indicate a single anthropogenic source for these PAHs, from as suggested by the normalization of total PAHs to TOC or SOM. Thus, these results indicate a prevalence of three and four-ring PAHs, which together with the diagnostic ratios obtained, suggest that the source of combustion of organic matter are anthropogenic industrial activities and/or natural fires.

Fig. 4 Polycyclic aromatic hydrocarbons cross plots for fluoranthene/(fluoranthene+pyrene) [Fl/(Fl+Py)] vs. anthracene/(anthracene+phenanthrene) [An/(An+Phe)] ratios (Dong et al. 2012).
Relate to contamination level by PAHs, Baurmard et al. (1998) assigned the following pollution levels in sediments of the western Mediterranean sea (French Riviera, Corsica, Sardinia): low (0-199 ng/g), moderate (100-1000 ng/g), high (1000-5000 ng/g), and very high (> 5000 ng/g). This suggests a moderate to high contamination level in the case of ETU (total PAHs 399-2239 ng/g) with the presence of three (phenanthrene) and four ring (fluoranthene and pyrene) PAHs, and a low contamination level for PLM (total PAHs 80-270 ng/g) and the presence of only three PAHs (phenanthrene, anthracene and pyrene) detected in some samples (Table II). This confirms that the anthropogenic input is lower in PLM than in ETU.
Mangrove ecosystems in coastal zones, are under crescent anthropogenic pressures from tourism, industry and other activities (Paez-Osuna 2001, Molnar 2013), in particular in the Wildlife Refuge of Cuare and the National Park of Morrocoy (Barreto 2008). However, soils samples at ETU are most exposed to possible sources of PAHs due to the intense traffic of boats with gasoline engines than mangroves soils from PLM. Also, the National Park Morrocoy, is closer to the town of Tucacas, with very high urban and tourist activity. In this area, the high vehicular traffic produces higher densities of PAHs emissions into the atmosphere from motor vehicles that use fossil fuels. Additionally, the location of a refinery in the east of the study area (El Palito), as well as petrochemical and other industries related to the production of petroleum derivatives are an important source of PHAs in the area.
CONCLUSIONS
In mangrove soils located in Cuare Inlet and Morrocoy National Park from Venezuela the PAHs detected are non-substituted, with three (phenanthrene, anthracene) and four-ring (fluoranthene, pyrene, benzo(a)anthracene, chrysene). The other PAHs (naphthalene, acenaphthene, acenaphthalene, benzo(ghi)perylene, dibenzo(a,h)anthracene, and indene) were not detected (≤ 20 ng/g).
The three PAHs diagnostic ratios calculated a) anthracene/(anthracene+phenanthrene), (b) fluoranthene/(fluoranthene+pyrene); and (c) benzo(a)anthracene/(benzo(a)anthracene+chrysene), suggest a combustion source.
The PAH/TOC and PAH/SOM ratios present a linear relationship with total PAHs, and suggest that the PAHs detected come from the same source, that is, the combustion of organic matter, whose possible source is industrial activity or natural fires.
The sample sites are classified according to thefollowing pollution levels: a) moderate to high in ETU (total PAHs 399-2239 ng/g) with the presence of three (phenanthrene) and four ring (fluoranthene and pyrene) PAHs, b) low for PLM (total PAHs 80-270 ng/g) with the presence of only three PAHs (phenanthrene, anthracene and pyrene). This result indicates more anthropic contribution to soils from ETU compared to soils from PLM.











 nueva página del texto (beta)
nueva página del texto (beta)



