INTRODUCTION
The Aburrá Valley Metropolitan Area comprises 10 municipalities from the Antioquia department (Colombia, South America). It is a densely populated area, concentrated in a semi closed and narrow valley with diverse industrial activities and increasing urban development, number of cars and consumption of fossil fuels. All these activities release large quantities of pollutants to the atmosphere such as carbon monoxide, ozone, nitrogen oxides, sulphur oxides, volatile organic compounds and particulate matter that have an adverse effect on the air quality throughout the Aburrá Valley (Bedoya and Martinez 2009). Therefore, air pollution is a major threat to the health and quality of life of the population (Gaviria et al. 2011).
Particulate matter is classified according to its aerodynamic diameter in PM10 respirable particles (<10 μm) and PM2.5 inhalable particles (< 2.5 μm) (WHO 2005). PM2.5 material is smaller and it can easily transport particles of biological origin (bioaerosols) on its surface such as pollen grains, virus, bacteria, and fungal and bacterial spores (Focil et al. 1999). This type of particles can reach the deepest parts of the lungs, sediment in the alveoli, be carried through systemic circulation, and enter other internal organs (Miller et al. 2007). This characteristic favors the development of acute respiratory infections, chronic respiratory diseases, cardiovascular diseases, lung cancer and even reproductive abnormalities (O’Neill et al. 2003, Pope and Dockery 2006). Usually the most affected are the sensitive groups such as children under five years old, immunosuppressed people, pregnant women and the elderly, constituting a major public health problem (Blanco 2003, WHO 2005).
Acceptable conditions for the proliferation and survival of microorganisms can be generated in the atmosphere because of the relative humidity, which provides water, CO2 that provides carbon, and particulate matter that provides a wide variety of nutrient sources and serves as substrate. Bacteria in the atmosphere, especially in aerosol particles, originate from soil, water, and plant surfaces (Jones and Harrison 2004) and once in the air, bacterial dispersion is carried out by air currents (wind speed and direction) (Olaya and Pérez 2005).
Different studies have found a great bacterial diversity associated with particulate matter samples collected in the atmosphere near the surface, some of which are potential pathogens in animals, plants and humans that can have important effects on health (Bowers et al. 2011). They have identified different potentially pathogenic genera in the air, such as Acinetobacter, Bacillus, Corynebacterium, Kocuria, Mycobacterium, Micrococcus, Paenibacillus, Staphylococcus, Streptomyces, Enterobacter and Klebsiella (Griffin et al. 2007, Chen et al. 2012). The short-term effects of PM2.5 particulate matter exposure have shown that an increase in its concentration is associated with higher mortality rates, emergency room visits, increased respiratory symptoms and reduced lung function (Katsouyanni et al. 1997, von Klot et al. 2002, Pope and Dockery 2006). In recent years, several studies have noticed the presence of bacteria in the atmosphere (Després et al. 2007, Barahona 2010, Fahlgren et al. 2010), but Colombia has few air quality studies based on the microbiological component in urban or rural environments since they have been mainly focused on the physical and chemical characterization of the different air pollutants (Menetrez et al. 2007, Rave et al. 2008, Toro et al. 2010). Therefore, the present study aimed to evaluate the bacterial communities associated with PM2.5 particulate matter collected from two urban (Urban-C and Urban-NW) and one rural (Rural-N) locations from the Aburrá Valley, Colombia, South America.
MATERIALS AND METHODS
Site description and sample collection
This study was conducted in three locations from the Aburrá Valley, Antioquia, Colombia, South America. Sampling points in the urban area were selected based on traffic flow and population density: two sampling sites were located in the urban area of Medellín: Robledo neighborhood (Urban-C, 6º16’26.46”N; 75º35’33.19”W), located at the central-west part of the Aburrá Valley and Poblado neighborhood, located south of the Valley (Urban-NW, 6º12’32.06”N; 75º34’40.11”C). A third sampling site was chosen, north of the Aburrá Valley, in the rural area of Barbosa, Antioquia (Rural-N, 6º24’23.24’’N; 75º25’9.08’’W) (Fig. 1) (AMVA 2016).
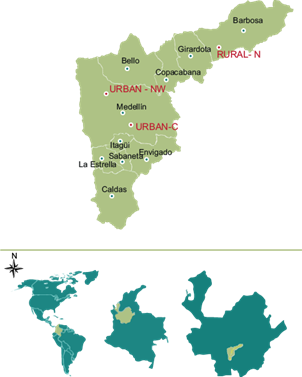
Fig. 1 Location of PM2.5 particulate matter sampling sites. Urban Area: Urban-C, 6º16’26.46”N; 75º35’33.19”W and Urban-NW, 6º12’32.06”N; 75º34’40.11”C. Rural area: Rural-N, 6º24’23.24”N; 75º25’9.08”W. (Metropolitan Area of the Aburrá Valley, Antioquia, Colombia, South America). Scale: 1:10 km. Source: (AMVA 2016)
For Urban-C site, samples were collected in both semesters of the year, from January to May (1st semester) and from July to December (2nd semester), for a total of 20 samples processed. For Rural-N and Urban-NW, 14 filters were processed per site in the months of July and August. During these months, three simultaneous samplings were taken at all sampling points.
Samples were collected in polytetrafluoroethylene filters (PTFE, Ø 47 mm, 0.2 μm pore size, Whatman) with low-volume air samplers: PQ200 semiautomatic (BGI Inc), Partisol 2000 (Thermo Electron Corp., MA, U.S.) and Partisol Plus 2025 (Thermo Electron Corp., MA, U.S.). The equipment absorbs the air at a constant volumetric flow rate of 16.7 L/m. The filter exposure time in was 24 hours (USEPA 1998, Menetrez et al. 2007).
Environmental conditions
Meteorological data such as air temperature, relative humidity and wind speed for each sampling site were taken using Vantage Pro 2 weather stations (Davis Instruments, Hayward, CA, USA). Canonical correspondence analysis (CCA) was implemented to establish a relationship between the species distribution matrix (presence/absence) and environmental conditions of the sampling sites (Legendre and Legendre 2012).
Microbiological characterization
To recover the bacteria from the PM2.5 particulate material, a half portion of the filter was mixed in brain heart infusion (BHI) enrichment broth (Merck) in order to release all the captured particles. It was then incubated at 37±2 ºC for 24 hours and serially diluted (1:100000) to plate 0.1 mL per sample in chocolate agar (Merck), blood agar (Merck), eosine methylene blue (EMB) agar (Merck) and nutrient agar (Merck) (Legendre and Legendre 2012). Chocolate agar and blood agar were chosen as an enriched, bacterial growth medium for the isolation of fastidious organisms and certain opportunistic bacterial species that produce extracellular enzymes that lyse red blood cells in the blood agar (hemolysis). EMB agar is both a selective and differential culture medium for bacteria designed to selectively isolate Gram-negative and is commonly used for the isolation and differentiation of coliforms and fecal coliforms. Nutrient agar was chosen as a non-selective medium to promote growth of a diversity of microbes including nutritionally fastidious bacteria (Merck 2013).
Bacterial colonies obtained from the different culture media were sub-cultured in nutrient agar to obtain pure bacterial cultures and were characterized morphologically (size and shape of colony, elevation, pigmentation) and by Gram staining. In addition, hemolytic activity (alpha, beta or gamma) was determined on blood agar. For long-term preservation, all isolates were stored in liquid culture media with 20 % glycerol and then frozen at -20ºC and -80ºC.
Molecular characterization
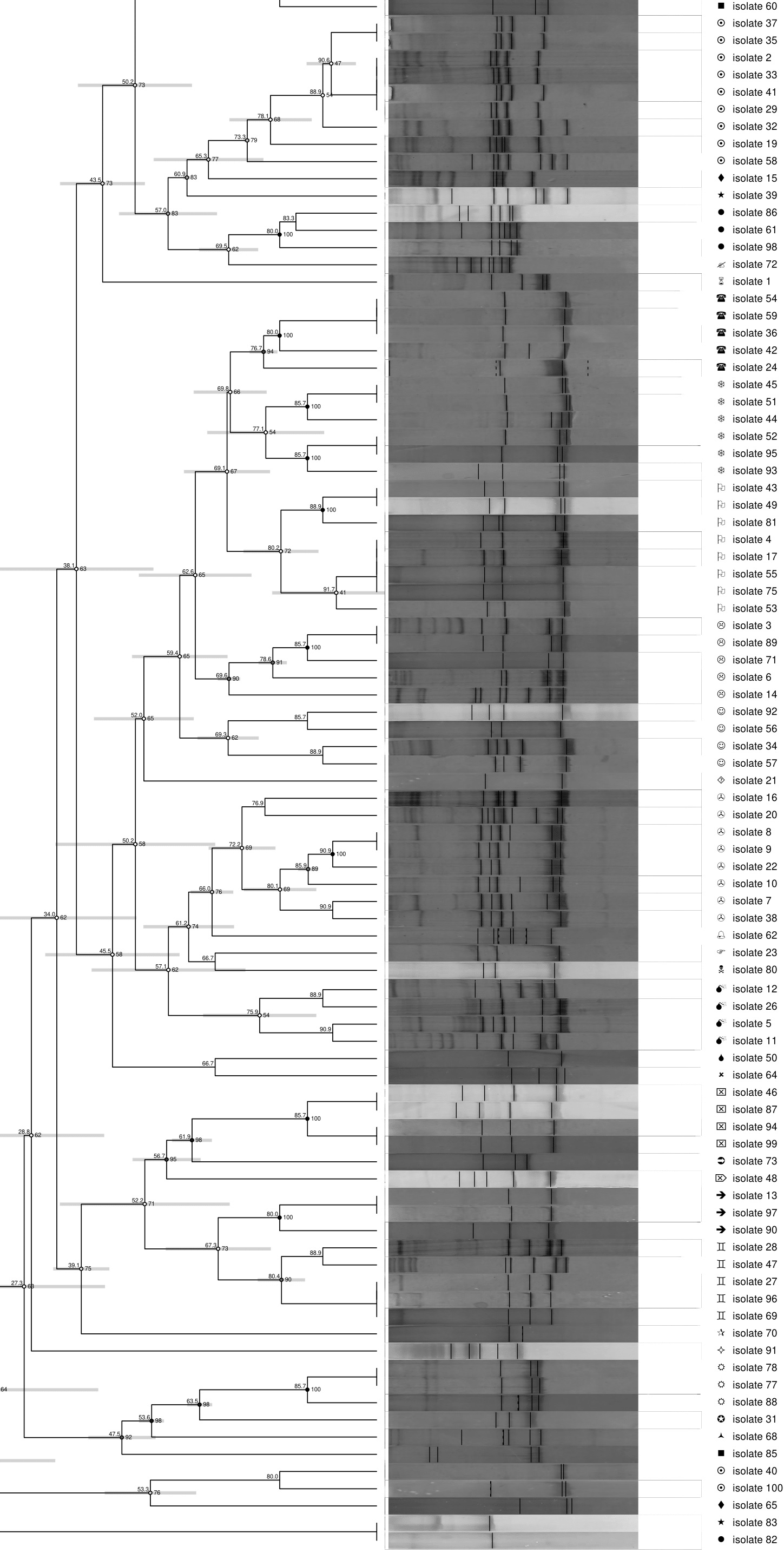
Pure colonies were sub-cultured in nutrient agar at least three times and pure isolates were characterized by ribosomal intergenic spacer analysis (RISA) (Jensen et al. 1993, Moreno et al. 2002). RISA patterns were resolved by polyacrylamide gel electrophoresis (PAGE) and analyzed with GelCompar II software (Applied Biosystems Maths, Belgium) (García et al. 2016). RISA-PAGE was performed in a Mini-Protean Tetra cell electrophoresis unit with 7 % polyacrylamide gels (acrylamide/bis-acrylamide 29:1) for 100 min at 130 V. A clustering analysis by the Dice correlation method and the UPGMA similarity coefficient was elaborated (Nei and Li 1979, Mohammadi and Prasanna 2003).
A cluster was defined by isolates with > 69 % or more similarity percentage on their band patterns according to the dendrogram generated with GelCompar II software. From each cluster one or two isolates with different RISA patterns were identified through 16S rRNA gene sequencing using primers 27F and 1492R (Table I).
TABLE I PRIMERS USED FOR 16S rDNA GENE AMPLIFICATION REACTIONS
| Primer | Sequence (5ʹ-3ʹ) | Amplicon length | Reference |
| 27F | AGAGTTTGATCMTGGCTCAG | 1500 | Moreno et al. 2002 |
| 1492R | TACGGYTACCTTGTTACGACTT | ||
| L1 | CAAGGCATCCACCGT | 300-1000 | Jensen et al. 1993 |
| G1 | GAAGTCGTAACAAGG |
DNA sequence and phylogenetic analysis
All amplification products were purified and sequenced on an ABI Prism 3100 Genetic Analyzer (Applied Biosystems, Carlsbad, CA, USA). Sequences were edited with ChromasPro 1.7.7 ® software (Technelysium) and the presence of chimeric sequences was evaluated with Decipher (Wright et al. 2012). The sequences obtained were compared with the GenBank database through Blast (Altschul et al. 1997) and the rRNA Database Project (RDP II) (Cole et al. 2009). Phylogenetic analyses were performed in Mega 6.1 software using the Neighbor-Joining method (Saitou and Nei 1987). The evolutionary distances were calculated using the Jukes-Cantor method and 1000 bootstrap replicates. (Tamura et al. 2007). All sequences were deposited in the NCBI database under accession numbers KY120748 to KY120773
RESULTS AND DISCUSSION
PM2.5 is a contaminant of interest in public health because it has the ability to penetrate the terminal bronchi and alveoli establishing an effective means of transport for pathogenic bacteria and opportunist microorganisms (Gil et al. 1997, Oyarzún 2010). PM2.5 particulate matter regulation in Colombia tends to be permissive with respect to international values. For the country, the Ministerio de Ambiente, Vivienda y Desarrollo Territorial (Ministry of Environment, Housing and Territorial Development) (now the Ministerio de Ambiente y Desarrollo Sostenible -Ministry of Environment and Sustainable Development-) in 2010 established the daily and annual standards at 50 μg/m3 and 25 μg/m3 respectively while the World Health Organization (WHO) establishes far lower values (daily standard: 25 μg/m3 and annual standard: 10 μg/m3). The short-term effects of PM2.5 particulate matter exposure have shown that an increase in its concentration is associated with higher mortality rates, emergency room visits, increased respiratory symptoms and reduced lung function (Katsouyanni et al. 1997, Von Klot et al. 2002, Pope and Dockery 2006)
In recent years, several studies have noticed the presence of bacteria in the atmosphere (Després et al. 2007, Barahona 2010, Fahlgren et al. 2010) but Colombia has few air quality studies based on the microbiological component in urban or rural environments since they have been mainly focused on the physical and chemical characterization of the different air pollutants (Menetrez et al. 2007, Rave et al. 2008, Toro et al. 2010). Therefore, the present study evaluated the bacterial communities associated with PM2.5 particulate matter collected from two urban (Urban-C and Urban-NW) and one rural (Rural-N) locations from the Aburrá Valley, Colombia, South America, using microbiological (agar plates, hemolytic activity) and molecular techniques (RISA and sequence analysis of the 16S rRNA gene).
Influence of environmental conditions.
Meteorological and environmental factors play an important role in the release and distribution of pollutants and airborne microorganisms (Haas et al. 2013). Average concentration data measured in different sampling days presented higher amounts of PM2.5 for the Urban-C sampling site, followed by Urban-NW and Rural-N (Table II). This pattern is consistent with previous air quality studies which indicate that the PM2.5 concentration levels tend to be higher in densely populated urban areas with high traffic flow (Fang et al. 2007, Burrows et al. 2009, Wang et al. 2013). Likewise, the environmental conditions for each sampling site showed significant differences in wind speed, with the highest average values obtained for the Rural-N sampling site. The average temperature was similar for all sampling sites.
TABLE II AVERAGES FOR PM2.5 PARTICULATE MATTER CONCENTRATION AND ENVIRONMENTAL CONDITIONS
| Sampling site | Concentration (μg/m3) | Wind speed (m/s) | Temperature (ºC) | Relative humidity (%) | |||||
| X̅ | σ | X̅ | σ | X̅ | σ | X̅ | σ | ||
| Urban-C* | 1st semester | 23.21 | 2.4 | 0.41 | 0.2 | 22.44 | 1.8 | 62.81 | 2.7 |
| 2nd semester | 19.47 | 3.4 | 0.28 | 0.2 | 23.70 | 1.38 | 57.01 | 5.83 | |
| Urban-NW** | 2nd semester | 16.10 | 5.3 | 0.90 | 0.1 | 23.40 | 1.3 | 58.70 | 8.3 |
| Rural-N** | 2nd semester | 11.50 | 2.4 | 1.50 | 0.3 | 22.60 | 0.8 | 72.10 | 4.9 |
*Urban-C site, samples were collected from January to May (1st semester); and from July to December (2nd semester), for a total of 20 samples processed. **Urban-NW and Rural-N from July to August (2nd semester,) for 14 samples processed
Microbiological and molecular characterization
Two hundred and sixteen (216) isolates were obtained from the different sampling sites. Sixty-five percent (65 %) were beta hemolytic, indicating a high degree of pathogenicity in the recovered microorganisms since there is a total destruction of the beta hemoglobin (Table III). After morphological characterization (morphotypes, Gram stain) and hemolytic activity, 100 pure colonies were characterized by RISA. The banding pattern analysis of 100 isolates generated a dendrogram with 37 clusters, > 69 % of which were similar (Supplement 1). One or two colonies were selected from each cluster with a total of fifty-seven isolates selected for 16S rDNA sequencing (Fig. 2). Twenty-six (26) different species were identified belonging to the phyla Firmicutes, Proteobacteria and Actinobacteria and all the sequences had high similarity (98-100 %) with sequences reported in the database (Table IV).
TABLE III MICROBIOLOGICAL CHARACTERIZATION OF ISOLATES
| *Code (origin) | Gram stain (100 X) | Colony morphology | Margin (4 X) |
| isolate 61 (N) |
|
|
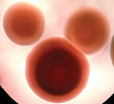
|
| isolate 70 (C) |
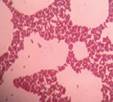
|

|

|
| isolate 73 (C) |
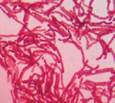
|

|

|
| isolate 88 (NW) |
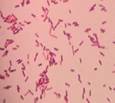
|

|

|
| isolate 96 (N) |
|

|

|
*Code = isolate number and origin site Urban-C (C), Urban-NW (NW) and Rural-N (N)
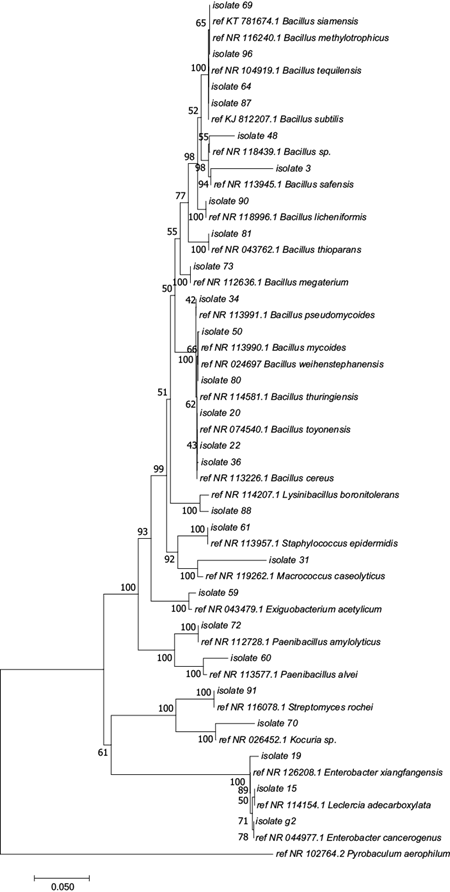
Fig. 2 Phylogenetic analysis based on 16S rRNA gene sequences of isolates from PM2.5 filters. The tree was produced by the Neighbor-Joining method with 1000 bootstrap repetitions, using Mega 6 software and a 16S rRNA gene sequence from Pyrobaculum aerophilum as outgroup to improve the tree’s topology
TABLE IV PHYLOGENETIC AFFILIATION OF MICROBIAL ISOLATES
| *Code (origin) | Accession # | Phylogenetic affiliation | Closest relative accession # (similarity %) |
| isolate 96 (N) | KY120764 | Firmicutes | Bacillus methylotrophicus NR_116240.1 (99 %) |
| isolate 60 (NW) | KY120756 | Paenibacillus alvei NR_113577.1 (97 %) | |
| isolate 61 (N) | KY120755 | Staphylococcus epidermidis NR_113957.1 (99 %) | |
| isolate87 (C) | KY120750 | Bacillus subtilis KJ_812207.1 (99 %) | |
| isolate 36 (C) | KY120758 | Bacillus cereus NR_113266.1 (99 %) | |
| isolate 90 (C) | KY120749 | Bacillus licheniformis NR_118996.1 (99 %) | |
| isolate 31 (C) | KY120763 | Macrococcus caseolyticus NR_119262.1 (99 %) | |
| isolate 88 (NW) | KY120773 | Lysinibacillus sp. NR_114207.1 (99 %) | |
| isolate 48 (C) | KY120760 | Bacillus sp NR_118439.1 (96 %) | |
| isolate 22 (NW) | KY120768 | Bacillus toyonensis NR_074540.1 (99 %) | |
| isolate 34 (C) | KY120761 | Bacillus pseudomycoides NR_113991.1 (99 %) | |
| isolate 3 (C) | KY120766 | Bacillus safensis NR_113945.1 (99 %) | |
| isolate 64 (C) | KY120771 | Bacillus tequilensis NR_104919.1 (99 %) | |
| isolate 20 (C) | KY120759 | Bacillus thuringiensis NR_114581.1 (99 %) | |
| isolate 81 (N) | KY120772 | Bacillus thioparans NR_043762.1 (99 %) | |
| isolate 50 (C) | KY120752 | Bacillus mycoides NR_113990.1 (99 %) | |
| isolate 73 (C) | KY120753 | Bacillus megaterium NR_112636.1 (99 %) | |
| isolate 69 (C) | KY120769 | Bacillus siamensis KT_781674.1 (100 %) | |
| isolate 80 (C) | KY120751 | Bacillus weihenstephanensis NR_024697.1 (100 %) | |
| isolate 59 (C) | KY120757 | Exiguobacterium sp. NR_043479.1 (98 %) | |
| isolate 72 (C) | KY120754 | Paenibacillus amylolyticus NR_112728.1 (98 %) | |
| isolate 2 (C) | KY120767 | Proteobacteria | Enterobacter cancerogenus NR_044977.1 (99 %) |
| isolate 19 (C) | KY120765 | Enterobacter xiangfangensis NR_126208.1 (99 %) | |
| isolate 15 (C) | KY120762 | Leclercia adecarboxylata NR_114154.1 (99 %) | |
| isolate 70 (C) | KY120770 | Actinobacteria | Kocuria sp. NR_026452.1 (79 %) |
| isolate 91 (C) | KY120748 | Streptomyces rochei NR_116078.1 (99 %) |
*Code = isolate number and origin site Urban-C (C), Urban-NW (NW) and Rural-N (N)
According to the molecular identification (Table IV), phylum Firmicutes was the most abundant with genera like Bacillus, Staphylococcus, Paenibacillus, Lysinibacillus, Exiguobacterium and Macrococcus. These Gram positive genera are more resistant to dry or adverse conditions due to a thicker and peptidoglycan-rich cell wall, so they tend to be dominant in the culturable fraction of air samples (De La Rosa et al. 2002).They have also been reported in diverse locations such as forests, coasts, urban and rural areas.
Likewise, the genus Bacillus was the most abundant, possibly because of its ability to form endospores, a structure that allows them to remain dormant for long periods of time under stress conditions, such as the environmental factors found in the atmosphere (Nicholson et al. 2000). Although the vast majority of Firmicutes are non-pathogenic bacteria, some species are well known pathogens such as B. cereus, B. licheniformis, B. thuringiensis, B. weihenstephanensis and S. epidermidis (Kotiranta et al. 2000, Murray et al. 2006, Thorsen et al. 2006) and some have been used for biotechnological applications as B. tequilensis and B. toyonensis (Jeong et al. 2012, Cortés-Camargo et al. 2016). S. epidermidis is part of the normal flora of the skin or mucous membranes of humans and is considered a ubiquitous bacterium in the air. It can remain viable for prolonged periods of time due to its resistance to desiccation and temperature changes. S. epidermidis is usually reported in indoor environments such as hospitals, residential buildings or offices (Bonetta et al. 2010). The appearance of S. epidermidis in all three monitoring points can be due to the proximity of the stations to educational and residential areas with considerable floating population. This could cause S. epidermidis to detach easily from the human body and contaminate the air, other people or inanimate environmental surfaces (Madsen et al. 2018).
Unlike Firmicutes, the phylum Proteobacteria is less resistant to desiccation and is usually present near marine environments where the relative humidity is high. This phylum has been found in air samples processed by culture-independent molecular techniques, as they can be difficult to recover from agar plates (Fahlgren et al. 2010). Something similar happened for the different sampling sites, where the implementation of EMB culture medium was intended for the isolation of rapidly developing Gram-negative bacteria and low nutritional requirements (Merck 2013), but the amount retrieved was quite small and observed only in the Urban-C sampling site. Gram-negative bacteria usually remain and survive in the air for very short periods of time (González 2006), due to its thin peptidoglycan layer (between 10 % - 20 %), this increases the susceptibility to mechanical rupture due to environmental factors such as the desiccation to which they are exposed in the environment and at the time of sampling (Tortora et al. 2007), the choice of recovery media and incubation conditions will also affect the survival of the bacteria. The identified isolates belonged to genera Enterobacter and Leclercia, members of the gamma-Proteobacteria group. These genera, usually found in soil, water, and vegetation, are members of the normal intestinal microbiota of many animals including humans and are common in clinical samples associated with polymicrobial infections (Murray et al. 2006, Correa et al. 2012). Some epidemiological studies in a rural zone of Colombia, show findings of faecal contamination in water probably by deficient sanitation and poor personal hygiene (Botero et al. 1984, Campos-Pinilla et al. 2008, Torres-Bejarano et al. 2018). The faecal contamination has not been reported as an important focus at the cities, however we cannot exclude the possibility that the generated excrement of dogs, cats, and even by humans on public roads can be transformed into dust and may pollute the air.
In the Urban-C sampling site, Actinobacteria from the Kocuria and Streptomyces genera were isolated and identified. This group of microorganisms has been detected mainly in urban environments and the vast majority are associated with land-based sources (Brodie et al. 2007, Hervàs et al. 2009, Fahlgren et al. 2010). Some species have been reported as causative agents of infections (Basaglia et al. 2002, Altuntas et al. 2004, Corti et al. 2012). For Streptomyces, some species are important as a source of secondary metabolites of high industrial value with antibacterial and antifungal applications (Ting et al. 2009, Jeffrey and Halizah 2014).
Additionally, in the Urban-C sampling site, the greatest diversity of bacterial isolates of the three sampling points was observed and as in the Urban-NW sampling site, they had the highest amount of bacteria with potentially harmful effects on human health like B. cereus (Murray et al. 2006), Staphylococcus epidermidis (Murray et al. 2006), Leclercia adecarboxylata (Correa et al. 2012) and B. licheniformis (Veith et al. 2004). These results raise concern since these sampling sites are directly influenced by high traffic flow and are near large educational and residential sectors.
However, in the Rural-N sampling site where green areas predominate and PM2.5 pollution problems are not as severe as in urban areas, most of the identified microorganisms belonged to B. subtilis, B. thioparans, B. safensis and B. methylotrophicus species (Deepa et al. 2010, Yu et al. 2011, Dias et al. 2015), and have been associated as potential agents for biological control of plant diseases or as growth promoters.
Correlation between the bacterial diversity present in the PM2.5 particulate material and the geographical location of the sampling sites
The canonical correspondence analysis for simultaneous samplings at the three sampling sites carried out in the months of July and August 2015 (Fig. 3), displays that the bacterial diversity between both sampling sites located in urban areas at the Aburrá Valley (Urban-C and Urban-NW), were similar. The sampling site located in the rural area (Rural-N) presented bacterial species observed in the urban sampling sites such as B. licheniformis, B. methylotrophicus, B. subtilis and S. epidermidis and in addition to these, unique bacteria for this sampling site were also observed such as B. thioparans, M. boronitolerans and L. caseolyticus. These differences are probably the result of weather changes, combined with anthropogenic influences like changes in land use and concentration of particulate matter, which can alter the atmospheric microbial composition (Fahlgren et al. 2010).
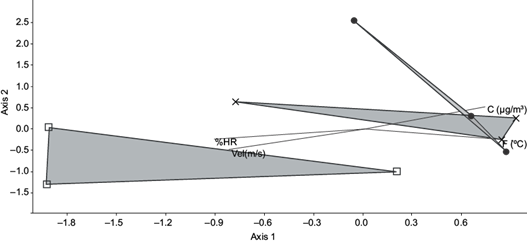
Fig. 3 Canonical correspondence analysis from the presence/absence matrix of bacterial isolates and environmental conditions of three simultaneous samplings: Urban-C = Robledo neighbourhood (●), Urban-NW = Poblado neighbourhood (×), Rural-N = rural area of Barbosa (□), T: = temperature, C = concentration of PM2.5, % RH = Relative Humidity, Vel = wind speed.
This shows that there is a greater presence of opportunistic pathogenic microorganisms in urban areas compared with the sampling site located in a rural area and can probably explain why there is a greater level of contamination in the Urban-C and Urban-NW sampling sites. Although high levels of particulate matter PM2.5 have been clearly linked to adverse health effects, it is speculated that the similarity of airborne bacteria may be caused by anthropogenic activities, including pollution, industrial activity, fuels and fires (Wang et al. 2013, AMVA 2017). PM2.5 does not sediment in short periods but remains suspended in the air due to its size and density (Ginzburg et al. 2015). The level of human exposure depends on the pollutant emission rate of vehicles, the direction of transport, the dispersion rate and the location of the population in relation to the set of mobile sources (Baldauf et al. 2018). Likewise, the presence of pathogenic or opportunistic bacteria associated with this contaminant indicates that there may be a higher risk of disease in people, but the individual reactions to these pollutants depend largely on factors such as health, genetic load and the degree of exposure of people (Sun et al. 2010). Reducing the pollutant emission at origin, toxic emissions from industrial sources and better municipal management would prevent and reduce air pollution.
In summary, this research comprises an initial investigation for the study of bioaerosols present in PM2.5 particles in Colombia. These bioaerosols are a cause for concern because of their potential impact on occupational health as well as on the health of people living near or are continuously exposed to them. Airborne bacteria are clearly an important, but understudied, component of air quality that needs to be better integrated into efforts to measure and model the particles pollutants in the atmosphere and correlate the results with epidemiological information and contaminant dispersion.
CONCLUSIONS
The detection of bacteria associated with particulate matter PM2.5 highlights the relevance of bioaerosols studies and is useful as an indicator of air pollution that had not been taken into account before in Colombia. Results suggest that the presence of specific bacteria can be influenced by particulate matter and environmental factors.











 text new page (beta)
text new page (beta)