INTRODUCTION
Oil-drilling wells generate large volumes of a by-product known as “cuttings”, which present a waste management problem for the petroleum industry (Al-Ansari and Al-Tabbaa 2007, Kogbara et al. 2016). This type of residue is generated when applying drilling fluids which act as a lubricant and coolant during the drilling process. Usually, the drilling cutting is a mixture of clay, quartz, feldspars, carbonates, and other calcareous compounds impregnated with hydrocarbons (Rojas-Avelizapa et al. 2007, Robinson et al. 2009, Bakke et al. 2013). Specifically, the cuttings contain highly toxic and poorly biodegradable contaminants, such as aliphatic hydrocarbons, polycyclic aromatic hydrocarbons (PAHs), and polychlorinated biphenyls (PCBs), as well as heavy metals, such as Pb, Ba, Zn, Hg, Cr, As, and Ni (Neff 2005, Leonard and Stegemann 2010, Junior et al. 2015). This residue represents a high environmental risk; therefore, alternatives for its management and treatment must be found to minimize the environmental threats and negative side effects on the population. Currently, the alternatives that are available for management of cuttings are reuse/recycling, sludge re-injection, and component separation by means of vibrating screens, hydrocyclones, and centrifugal decanters (Njobuenwu and Wobo 2007, Mognonda et al. 2015). Additionally, efforts have been made to treat the cuttings by several techniques such as solidification/stabilization, thermal and microwave treatment, supercritical fluid extraction, and washing processes using synthetic surfactants (Carignan et al. 2007, Robinson et al. 2010, Yan et al. 2011, Ball et al. 2012, Khanpour et al. 2014). This last method is a physicochemical technique that has received special interest because it is relatively fast and has a moderate impact on processing costs because it can be used to treat large volumes of waste (Urum et al. 2006, Han et al. 2009). However, the use of synthetic surfactants can itself be a source of environmental problems due to their toxicity and low biodegradability. The reported toxicity of surfactants on soil microbial cells indicates that they can seriously affect the biodegradation capability of microorganisms and the balance of biological activity (Sandbacka et al. 2000). In washed drill cuttings, the use of natural surfactants is an attractive alternative that appears to be a viable replacement for synthetic surfactants (Perminova and Hatfield 2005, Zacarias et al. 2013). Natural surfactants are molecules that can effectively reduce water surface tension, exhibit excellent surface activity, and display better compatibility with the environment since they are nontoxic and biodegradable (Klavins and Purmalis 2010). One of the most promising natural surfactants are the humic acids (HA), a naturally occurring organic component of soil, water, and sediments. HA are largely composed of aromatic rings and aliphatic structures linked to other structures and have an aliphatic character (Barak and Chen 1992, Liang et al. 2006) because they are a byproduct of organic matter degradation (Ramírez et al. 2013a). In a previous work, Conte et al. (2005) evaluated hydrocarbon removal in highly contaminated soil by washing processes with different synthetic surfactants and HA, experiments were conducted using two polluted soils and solutions of either synthetic or natural surfactants (HA). Their results demonstrated that HA were able to reduce the level of TPH contaminants between 80 to 90 %, which was the first time a natural nontoxic surfactant such as HA was reported to have a similar capacity for removing contaminants from polluted soil as that exhibit by synthetic surfactants. The effectiveness of HA in the washing process may be related to the fact that this compound acts on soil particle surfaces, also it has a high capacity to solubilize a wide variety of hydrophobic species and to develop micellar structures similar to those of synthetic surfactants (Wandruszka 2000).
At the present time, no studies on the application of HA as a surfactant in treating drill cuttings have been reported. Therefore, the aim of this study is to evaluate the use of humic acids as a surfactant for removing hydrocarbons contained in drill cuttings and to compare their efficiency with known synthetic surfactants such as sodium dodecyl sulfate (SDS) and tween-20 (TW20).
MATERIALS AND METHODS
Materials
The surfactants SDS and TW20 used in the experiments were obtained from Sigma-Aldrich. Their chemical properties are given as follow. SDS anionic surfactant: hydrophilic-lipophilic balance (HLB) 40, molecular weight 288.37 g/mol, critical micelle concentration (CMC) 2880 mg/L; TW20 non-ionic surfactant: HLB 16.7, molecular weight 1228 g/mol, density 1.095 g/mL, critical micelle concentration (CMC) 60 mg/L.
The humic acids (HA) were obtained from compost produced by mixing 30 % municipal sewage sludge, 60 % grass and 10 % wooden shavings, its CMC is 2000 mg/L (Ramírez et al. 2013b).
The oil-based drill cuttings (OBDC) used in the experiments were obtained from an installation located in the State of Veracruz, Mexico. TPH concentration in OBDC samples were 114 372 mg/kg.
Washing process with HA solutions
To evaluate the effect of humic acids on the removal of hydrocarbons contained in the OBDC, a 100 g sample was placed in a glass container to which 500 mL of a humic acid solution (2000 mg/L) was added. The container was collocated in a jar test equipment setup (Lovibond® ET 740) and was stirred at 200 rpm for various time periods (10 to 60 min) then left to rest for 30 min. Afterward, the washing solution was separated by decanting and the settled solid was dried at room temperature for three days.
The treated OBDC was milled in a porcelain mortar and sieved through a 0.5 mm mesh to obtain a homogenous particle size. Later, the TPH content was determined by Soxhlet extraction and this value was compared with the initial concentration to determine the rate of removal in the sample.
Washing process with HA solutions and rinsing with water
As a complement to the HA washing process, an additional rinsing step with water was evaluated, with the objective of identifying any potential further increase in TPH removal efficiency. In this case, the OBDC sample was treated as described in previous section and after separation from the humic acid solution, the settled solid was kept in a glass container. Later, 500 mL of distilled water was then added, and the sample was again placed in the jar test equipment setup where it was stirred for 30 min at 200 rpm. Afterward, the TPH concentration in the solid was determined.
TPH removal at different HA concentrations
To determine whether the washing efficiency was influenced by HA concentrations in the washing solutions, concentrations between 500 to 3000 mg/L were prepared with distilled water. The washing experiments were performed according to the procedures and conditions reported in section washing process with HA solutions and rinsing, with an applied washing time between 10 to 60 min.
Comparison between washing with HA and synthetic surfactants
In order to compare the effectiveness of the elimination of TPH with HA and synthetic surfactants, each of these substances were prepared to CMC. This is the concentration at which surfactants are known to form structures known as micelles which have been reported to play an important role in solubilizing compounds. The CMC prepared solutions were: HA (2000 mg/L), SDS (2880 mg/L), and TW20 (60 mg/L).
The OBDC applied treatment, TPH extraction and quantification were performed according to the procedures previously described, applying washing time of 30 min.
Soxhlet extraction
To determine TPH concentration, the standard EPA 9071B and EPA1664A (1998) methodologies were used.
Organic matter content
The organic matter contained in the samples was determined by titration with the Walkley-Black method (Gelman et al. 2012).
RESULTS AND DISCUSSION
Removal of TPH in OBDC, washing with HA solutions and rinsing
Recent studies have reported that when a soil is contaminated, hydrocarbons tend to move into the deeper recesses of soil particles, soil aggregates and the organic matter adsorbed to soil particle surfaces (Prichard et al. 2006, Bezza and Nkhalambayausi 2015). As a result, in the washing process, desorption is commonly considered as a rapid initial release of hydrocarbons that are close to the surface and a very slow release of hydrocarbons that are more deeply adsorbed. For this reason, the fastest and highest removal was carried out in the first 30 min and a slower removal was observed after this time.
In figure 1, the washing experiment results indicate that the HA solutions were able of remove TPH from the solid matrix, with nearly 25 % of TPH in the first 30 min; when the washing times were 40 to 60 min the removal increased to about 30 %. On the other hand, when the samples were rinsed with water after washing, the TPH removal increased near 60 % in the first 30 minutes that is three times greater than the washing with HA.
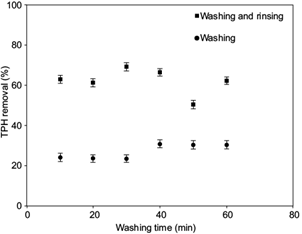
Fig. 1 Removal of total petroleum hydrocarbons (TPH) in perforation cuttings (OBDC) samples using as washing solution 2000 mg/L humic acids (HA) with rinsing and without rinsing
This effect can be explained by the interaction of HA with the OBDC sample, since when the surfactant molecules are present in a heterogeneous soil-water system, they could adsorb on the surface of the soil particles, giving rise to interactions between the hydrophilic groups (or head groups) that enter the aqueous phase and lipophilic groups (or tail groups) that tend to combine with hydrophobic contaminants and with soil particles that reduce the elimination of TPH (Mulligan et al. 2001, Vishnyakov et al. 2013). However, when the rinse is carried out, desorption of HA with the contaminant in the OBDC is facilitated resulting in increased removal.
The results obtained were similar to those reported by Conte et al. (2005) that show rates of hydrocarbon removal between 70 % and 80 % in soil samples with a wash without rinse. However, they used a wash time of 24 h which could facilitate the removal due to a longer contact time between the HA solution and the pollutants. So, it can be noted that the rinse after washing decreases the time of TPH removal which is more convenient for the treatment of these pollutants.
TPH removal as a function of HA concentration in continuous washing processes
The removal rates of TPH in OBDC samples using HA at various concentrations above and below the CMC are shown in figure 2 A and B. The contaminants were mostly removed in the first 10 min of contact, demonstrating that the washing time is not important in the removal of TPH with respect to the AH concentration, so there is no need to use longer treatment times.
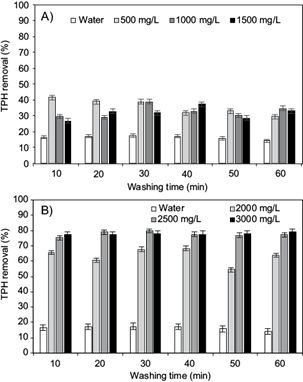
Fig. 2 A) Removal of total petroleum hydrocarbons (TPH) in washing with different humic acids (HA) concentrations below the critical micelle concentration (CMC), B) Removal of TPH in washing with HA at the CMC and above
As can be seen, water alone removes 17 % of the contaminants in the sample, this can be explained by the high hydrophobicity of hydrocarbons. When 500, 1000 and 1500 mg/L HA solutions were used in the washing process, the removal rate increases to values between 30 % and 40 %. It is worth noting that the highest removal rate was attained at a concentration of 500 mg/L HA and 10 min of washing.
The phenomena associated with this result have been explained in studies showing that when surfactants are added into the water-soil system, a certain amount of surfactants will inevitably be adsorbed by soil particles (Paria 2008, Mao et al. 2015). The more the adsorbed surfactants, the less the surfactants contribute to the mobilization of pollutants. Moreover, the hydrophobicity of the soil is increased as the surfactants adsorbed onto soil particles. As a result, removed TPH will be re-adsorbed on soil surface, in such a way that when washing was carried out at concentrations 1000 and 1500 mg/L, re-adsorption of HA-TPH could have occurred in the soil, resulting in lower removal percentages than those obtained in washes at concentrations of 500 mg/L.
When using concentrations equal to or above CMC, the process becomes more efficient, as demonstrated by the significant increase in removal rate (Fig. 2 B). In this case, at 10 min the removal percentages reached values between 66 and 78 %, with an HA concentration of 3000 mg/L yielding the best results. For longer contact times, the removal rate remained near 80 % using HA solutions of 2500 and 3000 mg/L. Otherwise, solutions at the CMC of 2000 mg/L always displayed efficiencies lower than 70 %. The increase in organic pollutant removal agrees well with the results obtained by Pan et al. (2006) for the solubilization of hydrophobic organic contaminants using a series of HA from different sources. The increase in the removal of TPH where the concentration was between 2000 and 2500 mg/L is related with the formation of the pseudomicelles of the humic acids. So, when the CMC is 2000 mg/L, the pseudomicelles could be wrapping the contaminants. According to the obtained results, there is a high removal in the concentration above 2000 mg/L, but there is no significant difference when using 2500 and 3000 mg/L.
As can be seen (Fig. 2 A and B) the highest removal of TPH was obtained with solutions above the CMC of HA. The obtained results are similar to studies carried out on the application of surfactants to improve ex situ soil washing (Urum et al. 2003, Muherei and Junin 2007, Pacwa et al. 2011). These studies have proposed two hydrocarbon removal mechanisms using surfactant solutions: mobilization and solubilization. The mobilization mechanism occurs at concentrations below the CMC, and it is associated to reduction of surface and interfacial tension, reduction of capillary force, wettability and reduction of contact angle. In turn, above the surfactant CMC, the solubilization takes place when these molecules are incorporated into a micelle, resulting in highest removal of TPH when surfactant solutions are used above the CMC.
Determination of OM in washing/rinsing cuttings samples with HA
Figure 3 displays the organic matter content of the samples after washing and rinsing. The original organic matter content in the sample was 6.4 % and decreased to 5.8 % when the sample was washed only with water. When the concentration of HA in the solutions is increased, the OM content in the cuttings decreases so that the organic matter contents were 5.2 %, 2.2 %, and 2.8 % with HA concentrations of 500 mg/L, 2500 mg/L, and 3000 mg/L, respectively.
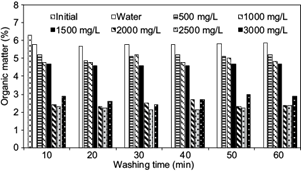
Fig. 3 Percentage of organic matter in cuttings after washing and rinsing with water and different HA concentrations
Fluctuations in the percentage of OM content have a similar trend to the removal of TPH by washing/rinsing using similar concentrations of HA. When HA solutions are used in concentrations higher than CMC the percentage of OM that remained in the sample decreased, indicating that the HA desorb the OM contained in the cuttings. According to this, there is some evidence that most of the organic matter contained in the cuttings are related with the content of hydrocarbons.
The low content of organic matter (6.2 %) compared to the high concentration of TPH in the sample (114 372 mg/kg) could be explained by the fact that the determination of the Walkley-Black method has a limitation, which is that it can only quantify organic matter easily oxidizable by potassium dichromate, Hence, the value obtained is lower than the actual total organic content (TOC), so that the TOC value could be 1.23 to 1.58 higher than the oxidizable organic carbon (De Vos 2007). Then, the total organic carbon in the OBDC sample is greater than 6.2 %.
Comparison of washing using HA and synthetic surfactants at CMC
The results of washing experiments on OBDC samples using either synthetic surfactants or HA solutions are shown in figure 4. It is clear that all of the surfactants removed the contaminants contained in the sample, although the HA solution displayed the best performance reaching a decrease in TPH concentration in the OBDC sample from 114 372 to 34 216 mg/kg (70 % removal). Among the synthetic surfactants, TW20 performed best as indicated by a reduction in TPH concentration to 52 513 mg/kg, while SDS reduced the hydrocarbon content to 80 000 mg/kg of TPH in the sample.
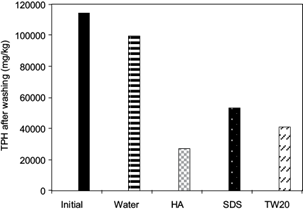
Fig. 4 Removal of total petroleum hydrocarbons (TPH) using synthetic surfactants and a humic acid (HA) solution at the critical micelle concentration (CMC)
The experimental results indicate that under CMC conditions the HA remove efficiently the TPH contained in cuttings, obtaining better results than synthetic surfactants.
Conte et al. (2005) obtained similar results of removal in washes with SDS, Triton-100 and HA, explaining that synthetic or natural surfactants in the washing techniques have the effect of increasing the structural stability of the soil due to the aggregating action that the hydrophobicity of the surfactants can exert on the solid particles of the soil, affecting the solubilization of contaminants. This may explain that the SDS and TW20 at CMC are more easily adsorbed to the OBDC particles, resulting in the HA being less adsorbed, having more micelles that can solubilize TPH by increasing the removal.
CONCLUSIONS
In the present study, HA solutions were used to perform washing processes of an OBDC sample containing 114 372 mg/kg of TPH. The washing/rinsing treatment using HA concentration of 2500 mg/L removed 80 % of the TPH in the cuttings. Removal percentage of TPH depends on the concentration of the HA solution, with the best results found for concentrations above the CMC. A comparison of the efficiency attained with HA solutions and synthetic surfactants indicates that the natural surfactant exhibits better performance, demonstrating its technical viability for use in washing drilling cuttings.











 nueva página del texto (beta)
nueva página del texto (beta)


